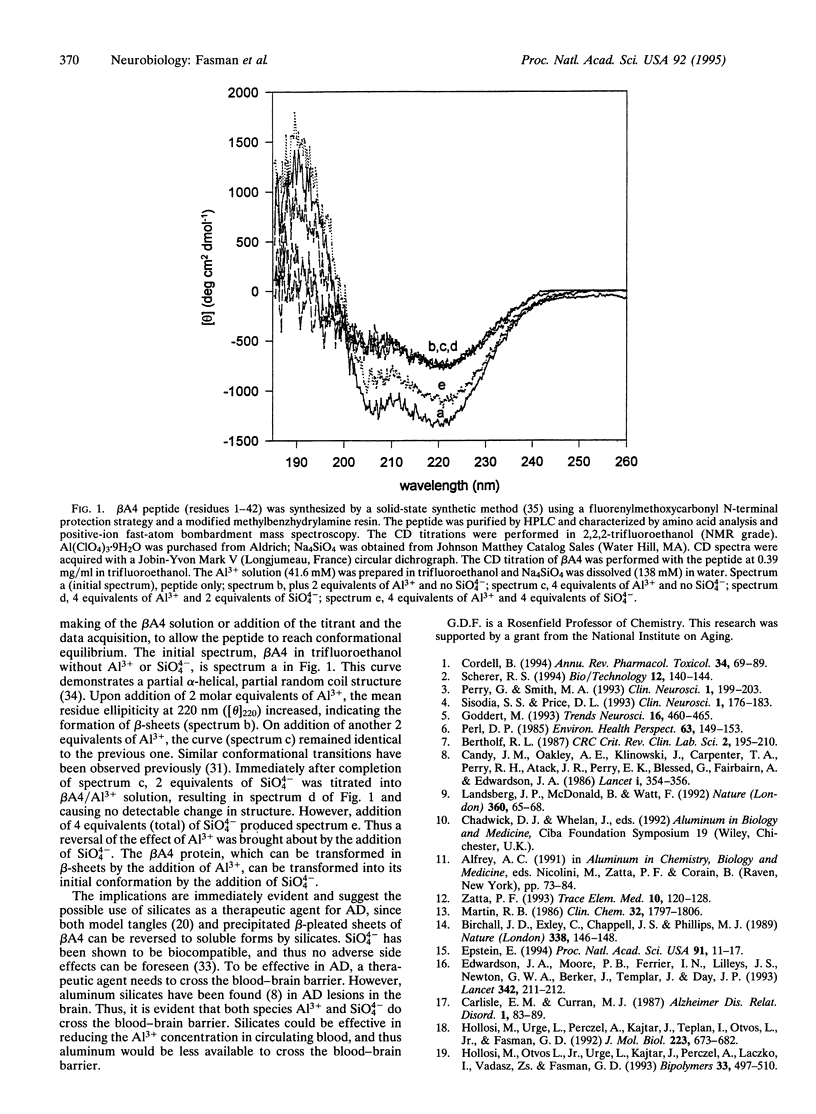
Abstract
Plaques are one of the two lesions found in the brain of patients with Alzheimer disease. Using a synthetic peptide corresponding to rat beta-amyloid-(1-42) (beta A4), circular dichroism (CD) analyses were performed to examine the effect of Na4SiO4 on the conformational state produced by Al3+. A previous study on fragments of neuronal proteins involved in tangle formation had shown a conformational transition from a beta-pleated sheet to a soluble random coil upon addition of Na4SiO4. In the present study, CD measurements showed that the beta-pleated sheet conformation of beta A4 induced by Al3+ was reversed to the random coil soluble form by the addition of Na4SiO4. The tight binding of SiO4(4-) with Al3+ provides the mechanism for this transition. These results provide insight into the role of aluminum in the Alzheimer diseased brain and suggests that investigation of the use of silicates as a therapeutic agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow C. J., Yasuda A., Kenny P. T., Zagorski M. G. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- Barrow C. J., Zagorski M. G. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 1991 Jul 12;253(5016):179–182. doi: 10.1126/science.1853202. [DOI] [PubMed] [Google Scholar]
- Bertholf R. L. Aluminum and Alzheimer's disease: perspectives for a cytoskeletal mechanism. Crit Rev Clin Lab Sci. 1987;25(3):195–210. doi: 10.3109/10408368709105882. [DOI] [PubMed] [Google Scholar]
- Candy J. M., Oakley A. E., Klinowski J., Carpenter T. A., Perry R. H., Atack J. R., Perry E. K., Blessed G., Fairbairn A., Edwardson J. A. Aluminosilicates and senile plaque formation in Alzheimer's disease. Lancet. 1986 Feb 15;1(8477):354–357. doi: 10.1016/s0140-6736(86)92319-6. [DOI] [PubMed] [Google Scholar]
- Carlisle E. M., Curran M. J. Effect of dietary silicon and aluminum on silicon and aluminum levels in rat brain. Alzheimer Dis Assoc Disord. 1987;1(2):83–89. doi: 10.1097/00002093-198701020-00003. [DOI] [PubMed] [Google Scholar]
- Cordell B. beta-Amyloid formation as a potential therapeutic target for Alzheimer's disease. Annu Rev Pharmacol Toxicol. 1994;34:69–89. doi: 10.1146/annurev.pa.34.040194.000441. [DOI] [PubMed] [Google Scholar]
- Edwardson J. A., Moore P. B., Ferrier I. N., Lilley J. S., Newton G. W., Barker J., Templar J., Day J. P. Effect of silicon on gastrointestinal absorption of aluminium. Lancet. 1993 Jul 24;342(8865):211–212. doi: 10.1016/0140-6736(93)92301-9. [DOI] [PubMed] [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):11–17. doi: 10.1073/pnas.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley C., Price N. C., Kelly S. M., Birchall J. D. An interaction of beta-amyloid with aluminium in vitro. FEBS Lett. 1993 Jun 21;324(3):293–295. doi: 10.1016/0014-5793(93)80137-j. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Moore C. D. The solubilization of model Alzheimer tangles: reversing the beta-sheet conformation induced by aluminum with silicates. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11232–11235. doi: 10.1073/pnas.91.23.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer's disease. Trends Neurosci. 1993 Nov;16(11):460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Halverson K., Fraser P. E., Kirschner D. A., Lansbury P. T., Jr Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 1990 Mar 20;29(11):2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J Mol Biol. 1991 Mar 5;218(1):149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- Hollósi M., Otvös L., Jr, Urge L., Kajtár J., Perczel A., Laczkó I., Vadász Z., Fasman G. D. Ca(2+)-induced conformational transitions of phosphorylated peptides. Biopolymers. 1993 Mar;33(3):497–510. doi: 10.1002/bip.360330316. [DOI] [PubMed] [Google Scholar]
- Hollósi M., Urge L., Perczel A., Kajtár J., Teplán I., Otvös L., Jr, Fasman G. D. Metal ion-induced conformational changes of phosphorylated fragments of human neurofilament (NF-M) protein. J Mol Biol. 1992 Feb 5;223(3):673–682. doi: 10.1016/0022-2836(92)90983-q. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kawahara M., Muramoto K., Kobayashi K., Mori H., Kuroda Y. Aluminum promotes the aggregation of Alzheimer's amyloid beta-protein in vitro. Biochem Biophys Res Commun. 1994 Jan 28;198(2):531–535. doi: 10.1006/bbrc.1994.1078. [DOI] [PubMed] [Google Scholar]
- Landsberg J. P., McDonald B., Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer's disease. Nature. 1992 Nov 5;360(6399):65–68. doi: 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- Martin R. B. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986 Oct;32(10):1797–1806. [PubMed] [Google Scholar]
- Perl D. P. Relationship of aluminum to Alzheimer's disease. Environ Health Perspect. 1985 Nov;63:149–153. doi: 10.1289/ehp.8563149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schehr R. S. Therapeutic approaches to Alzheimer's disease. An informal survey of promising drug discovery strategies. Biotechnology (N Y) 1994 Feb;12(2):140–144. doi: 10.1038/nbt0294-140. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Terzi E., Hölzemann G., Seelig J. Reversible random coil-beta-sheet transition of the Alzheimer beta-amyloid fragment (25-35). Biochemistry. 1994 Feb 15;33(6):1345–1350. doi: 10.1021/bi00172a009. [DOI] [PubMed] [Google Scholar]
- Tomski S. J., Murphy R. M. Kinetics of aggregation of synthetic beta-amyloid peptide. Arch Biochem Biophys. 1992 May 1;294(2):630–638. doi: 10.1016/0003-9861(92)90735-f. [DOI] [PubMed] [Google Scholar]



