Abstract
Pseudomonas aeruginosa secretes copious amounts of the redox-active phenazine, pyocyanin (PCN), during cystic fibrosis lung infection. PCN has been shown to interfere with a variety of cellular processes in cultured lung epithelial cells. Here, by using two respiratory tract models of infection, we demonstrate that PCN mediates tissue damage and necrosis during lung infection.
Pseudomonas aeruginosa is the most common pathogen associated with airway infections of cystic fibrosis (CF) patients. The ability of P. aeruginosa to cause disease is due, in part, to its ability to produce a large repertoire of virulence factors. In addition to delivering cytotoxic type III secretion effectors such as ExoU and ExoT into eukaryotic cells to disrupt the host immune responses and cause cytoskeletal reorganization (5, 12), P. aeruginosa also produces a large number of exoproducts, including proteases, hemolysin, rhamnolipids, and the blue redox-active phenazine, pyocyanin (PCN) (2, 6, 10, 20). Previous studies have shown that the PCN-deficient mvfR-phnAB and phzB1 mutants, which produce no or reduced levels of PCN, cause less mortality than the wild-type strain PA14 in the P. aeruginosa-mediated burn sepsis model in mice (2, 9). In vitro studies have shown that PCN has multiple deleterious effects on mammalian cells, such as inhibition of cell respiration, ciliary function, epidermal cell growth (16, 17, 20), and prostacyclin release (7), disruption of calcium homeostasis (4), and inactivation of catalase (14). PCN also induces apoptosis in neutrophils (19) and modulates the glutathione redox cycle (13) in lung epithelial and endothelial cells. PCN also inactivates α1 protease inhibitor and contributes to the imbalance of protease-antiprotease activity, which is readily detected in the airways of patients with CF lung disease (1). More recently, it was shown that PCN inactivates the vacuolar ATPase of lung epithelial cells (15). Although the toxic effects of PCN are widespread, whether it is required for infection of airways in patients with CF has not been confirmed experimentally.
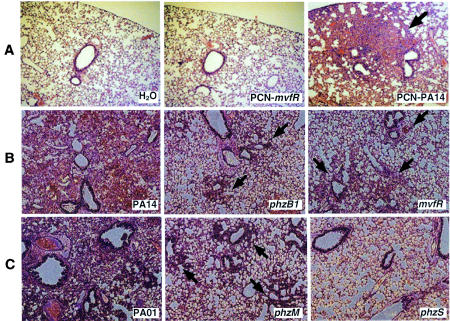
In this study, we examined whether PCN causes a pathological effect in the lung by directly instilling PCN into the lungs of adult (∼20-g) CD-1 mice. PCN was purified from a late stationary-phase culture of wild-type P. aeruginosa strain PA14 and its isogenic PCN-deficient mvfR mutant (Table 1) with successive rounds of CHCl3-0.2 N HCl extraction as previously described (15). The final PCN preparations had no detectable levels of Pseudomonas lipopolysaccharide, as determined by the E-TOXATE assay (Sigma), or of autoinducer, as measured by high-performance liquid chromatography (15). The PCN extracts (50 μg) were instilled into the lungs of mice for 3, 6, 12, 16, and 24 h. Sterile water was used as a control. Lungs were recovered from two mice at each time point, embedded and sectioned in paraffin, stained with hematoxylin and eosin, and examined by light microscopy. As shown in Fig. 1A, PCN induced bronchopneumonia with an influx of neutrophils as early as 3 h after exposure, causing congestion of the small airways surrounding some bronchi. The influx of neutrophils persisted for up to 24 h. In contrast, in the lungs treated with water or with extracts from the PCN-deficient mvfR strain, the small airways remained clear (Fig. 1A). These results suggest that PCN is proinflammatory and plays a role in mediating pneumonia development. Considering that PCN levels as high as 27 μg/ml have been detected in the sputum of CF patients chronically infected with P. aeruginosa (20), it is likely that PCN plays an important role in the pathogenesis of CF in airways.
TABLE 1.
Phenotypes of wild-type P. aeruginosa strains PA14 and PA01 and their respective isogenic PCN mutants
| Strain | % PCN production | Resulting adult mouse lung infectiona | Reference(s) |
|---|---|---|---|
| PA14 (wild type) | 100 | Lobar pneumonia | 2, 9, 15 |
| phzB1 mutant | 15 | Mild bronchopneumonia | 9 |
| mvfR mutant | <10 | Mild bronchopneumonia | 2 |
| PA01 (wild type) | 100 | Lobar pneumonia | 10 |
| phzM mutant | 0 | Mild bronchopneumonia | 10 |
| phzS mutant | 0 | Alveolitis | 10 |
See the text and the legend to Fig. 1 for a more detailed description of the lung pathology studies.
FIG. 1.
PCN contributes to pneumonia development in adult mouse lungs. (A) PCN causes neutrophil influx and small-airway congestion. Histological sections from 16 h postinfection are shown. (B and C) PCN-deficient P. aeruginosa mutants are less able to cause pneumonia. Adult CD-1 mice were infected with 107 cells of PA14 and its isogenic PCN mutants, the phzB1 and mvfR mutants (B), or PAO1 and its isogenic PCN-deficient mutants, the phzM and phzS mutants (C). Infected lungs were collected at 16 h postinfection, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy. Original magnification, ×10.
To confirm whether PCN is important in lung infection, we compared the levels of virulence of wild-type P. aeruginosa PA14 and PAO1 and those of the PCN-deficient isogenic mutants of PA14, the phzB1 and mvfR mutants, and those of the PCN-deficient isogenic mutants of PAO1, the phzM and phzS mutants (Table 1), by using an acute mouse pneumonia model of infection (18). The phzB1, phzM, and phzS genes encode enzymes involved in the final steps of the PCN biosynthetic cascade (10), whereas mvfR encodes a LysR-like transcription activator that controls the phnAB and pqs genes that synthesize quorum-sensing quinolones to regulate PCN biosynthesis (2, 6). Adult CD-1 mice were infected intranasally with 107 bacteria. Infected lungs were harvested at 16 h postinoculation, sectioned, and stained with hematoxylin and eosin for histological analysis.
Mice infected with strains PA14 and PAO1 showed hunched backs, distress, and ruffled furs, signs that are associated with an ongoing infection. Histological analyses indicated that wild-type strains PA14 and PAO1 induced acute pulmonary responses consistent with lobar pneumonia involving either entire lobes of the lungs or segments of lobes. The pathological appearance of the infected areas is consistent with the stage of red hepatization characterized by a massive exudation of red blood cells and neutrophils filling the alveolar spaces (Fig. 1B and C). Within these areas, tissue necrosis, thrombosis of the blood vessels, and obstruction of small airways by lymphocytic infiltrates were evident (Fig. 1B and C). In contrast, the PCN-deficient phzB1 and mvfR mutant strains as well as the phzM mutant induced a less severe pneumonia, which presented as a bronchopneumonia, than the lobar pneumonia caused by PA14 or PAO1 (Fig. 1B and C). The mutant strains, however, still resulted in areas of consolidation and acute inflammation in the lungs. These areas were patchy and spread throughout the lungs. We detected masses of bacteria that caused necrosis, thrombosis, and obstruction of the small airways. Abundant fibrin deposits were also observed. These features were present for at least 48 h. The mice were subsequently able to clear the infection and recovered. The phzS strain caused a diffuse inflammatory process consistent with alveolitis (Fig. 1C). Accumulation of few inflammatory cells within the alveolar walls and spaces could be observed. There was also slight congestion but no exudate in the alveolar spaces. No systemic spread of bacteria to the rest of the parenchymal organs was observed. The ability of PCN to contribute to P. aeruginosa-mediated pneumonia arises presumably through its ability to mediate damage through oxidation.
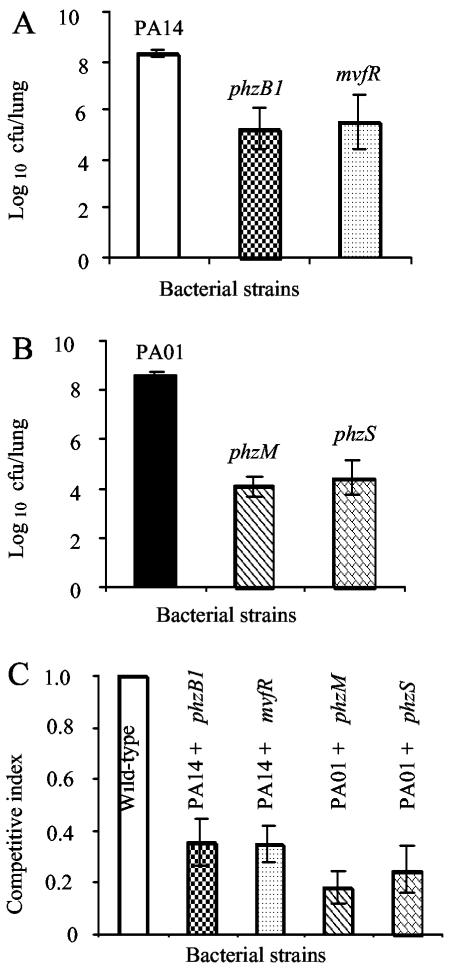
A true measure of in vivo virulence attenuation can be demonstrated by infection with individual bacterial strains. When infected singly with 107 bacteria, CD-1 mice were unable to clear the wild-type strains PAO1 and PA14. By 16 h postinfection, the viable counts of PA14 and PAO1 increased by ∼1.4 logs (Fig. 2A and B). In contrast, the viable counts of the PCN-deficient mvfR and phzB1 mutant strains decreased by 2.83 and 3.08 logs, respectively, compared to that of wild-type strain PA14 (Fig. 2A). Similarly, the viable counts of the PCN-deficient phzM and phzS mutant strains were attenuated by 4.47 and 4.15 logs, respectively, compared to that of wild-type strain PAO1 (Fig. 2B). These results suggest that full virulence of P. aeruginosa in murine lungs requires PCN biosynthesis.
FIG. 2.
PCN-deficient P. aeruginosa mutants have impaired virulence in an acute pneumonia model of mouse infection. (A and B) PCN-deficient mutants of P. aeruginosa are highly attenuated in single respiratory tract infections. CD-1 mice (six per group) were infected intranasally with wild-type PA14 or the mvfR or phzB1 isogenic PCN-deficient mutant (A) and with wild-type PAO1 or the phzM or phzS isogenic PCN-deficient mutant (B). Attenuation is defined as the log10 difference in CFU between the wild type and mutant bacteria recovered from lung tissue 16 h after inoculation. The means ± standard errors of results from six mice are shown. P values for results: mvfR mutant, 0.040; phzB1 mutant, 0.036; phzM mutant, 0.003; phzS mutant, 0.013. (C) The competitive index is defined as the output ratio of the numbers of mutant to wild-type bacteria divided by the input ratio of the numbers of mutant to wild-type bacteria. Thus, if a mutant strain is as competitive as its isogenic wild-type parent, a value of 1 will be achieved, indicating that the mutant is not attenuated. The means ± standard errors of results from five mice are shown. P values for results: phzB1 mutant, 0.002; mvfR mutant, 0.006; phzM mutant, 0.007; phzS mutant, 0.007.
Competitive mixed-infection assays have been widely used to assess the fitness of individual mutants versus that of their parental strains during in vivo infection (3, 8). We assessed the competitiveness of four PCN-deficient mutants against wild-type strains in the lungs after 16 h. In vivo competition assays between the wild types and individual PCN mutants were carried out by infecting adult CD-1 mice (five per group) intranasally with 107 cells (1:1 ratio) of wild-type PA14 and one of its isogenic PCN mutants, either the phzB1 or mvfR mutant, or with wild-type PAO1 and one of its isogenic PCN-deficient mutants, either the phzM or phzS mutant. Infected lungs were recovered 16 h after infection for bacterial load determination. As shown in Fig. 2C, the phzB1 and mvfR mutant strains were only 36 and 35% as competitive, respectively, as their parent strain, PA14. The phzM and phzS mutant strains were only 18 and 25% as competitive, respectively, as their parent, PAO1, during the competitive index infection assays.
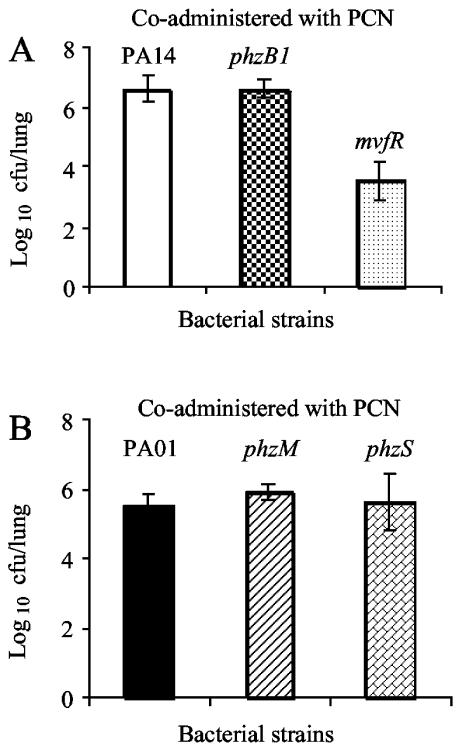
The degree of virulence attenuation in PCN-deficient mutants appeared to be more severe during single infections than during mixed infections, suggesting that PCN-deficient strains might have benefited from PCN secretion by the wild-type strains during mixed infection. To test this possibility, we coadministered PCN and PCN-deficient bacteria and investigated whether exogenously supplied PCN could overcome PCN deficiency in the mutant strains. We used 106 bacterial cells for infection, because higher bacterial titers (i.e., 107 cells) caused death when coadministered with 50 μg of PCN (data not shown). As shown in Fig. 3, exogenously supplied PCN restored virulence to the phzB1, phzM, and phzS mutants but not to the mvfR mutant. Our results again suggest that PCN is important for lung infection and that the primary functions of PhzB1, PhzM, and PhzS are in PCN biosynthesis. Because MvfR is the transcription activator for biosynthesis of quorum-sensing molecule quinolones (2, 6), its mutation causes pleiotropic phenotypes, among them the decreased secretion of multiple exoproducts (2). Thus, it is not surprising that the exogenously supplied PCN did not rescue the loss of virulence of this mutant strain (Fig. 3A).
FIG. 3.
Coadministration of PCN rescues the virulence of PCN-deficient mutants in mouse lungs. CD-1 mice (five per group) were coinstilled intranasally with 50 μg of PCN and 106 bacterial cells of wild-type PA14 or its mvfR or phzB1 isogenic PCN-deficient mutant (A) or with the wild-type PAO1 or its isogenic phzM or phzS PCN-deficient mutant (B). Attenuation is defined as the log10 difference in CFU between wild-type and mutant bacteria recovered from lung tissue 16 h after inoculation. The means ± standard errors of results from five mice are shown. P values for results: mvfR mutant, 0.002; phzB1 mutant, 0.167; phzM mutant, 0.618; phzS mutant, 0.876.
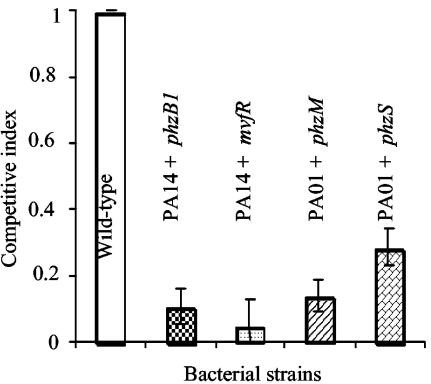
We further tested the requirement for PCN biosynthesis in P. aeruginosa virulence by using a previously described mouse chronic lung infection model with P. aeruginosa cells entrapped in agar beads (11). Mixed-infection assays were carried out as described above. As shown in Fig. 4, the phzB1 and mvfR mutant strains were only 10.8 and 4.8% as competitive, respectively, as wild-type strain PA14, whereas the phzM and phzS mutant strains were only 13.8 and 28.7% as competitive, respectively, as wild-type PAO1 during mixed-infection assays in the mouse lung.
FIG. 4.
PCN-deficient mutants are less competitive than wild-type P. aeruginosa in a mouse chronic lung infection model. In vivo competition assays between the wild type and individual PCN mutants embedded in agar beads were carried out by infecting CD-1 mice (five per group) intranasally with a 1:1 ratio of wild-type PA14 and one of its isogenic PCN mutants, either the phzB1 or the mvfR mutant, or PAO1 and one of its isogenic PCN-deficient mutants, either the phzM or the phzS mutant. Infected lungs were recovered 60 h after infection for bacterial load determination. The means ± standard errors of results from five mice are shown. P values for results: mvfR mutant, 1.9 × 10−5; phzB1 mutant, 0.002; phzM mutant, 0.007; phzS mutant, 0.007.
In summary, our results provide further support to the view that the full virulence of P. aeruginosa during airway infections requires PCN biosynthesis and strengthen the credibility of previous in vitro observations about PCN-mediated injuries and killing of lung epithelial cells (1, 4, 7, 13-17, 19, 20). Furthermore, strategies to inhibit PCN biosynthesis and to neutralize its toxicity by antioxidant therapies may be incorporated into existing regimens for the treatment of CF.
Acknowledgments
This work was partially funded by a research grant from the American Lung Association to G.W.L.
Editor: J. T. Barbieri
REFERENCES
- 1.Britigan, B. E., M. A. Railsback, and C. D. Cox. 1999. The Pseudomonas aeruginosa secretory product pyocyanin inactivates α1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease. Infect. Immun. 67:1207-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, H., G. Krishnan, B. Goumnerov, J. Tsongalis, R. Tompkins, and L. G. Rahme. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:14613-14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang, S. L., and J. J. Mekalanos. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797-805. [DOI] [PubMed] [Google Scholar]
- 4.Denning, G. M., M. A. Railsback, G. T. Rasmussen, C. D. Cox, and B. E. Britigan. 1998. Pseudomonas pyocyanine alters calcium signaling in human airway epithelial cells. Am. J. Physiol. 274:L893-L900. [DOI] [PubMed] [Google Scholar]
- 5.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 184:6472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath, J. M., B. E. Britigan, C. D. Cox, and D. M. Shasby. 1995. Pyocyanin from Pseudomonas aeruginosa inhibits prostacyclin release from endothelial cells. Infect. Immun. 63:4921-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555-571. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan-Miklos, S., M.-W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 10.Mavrodi, D. V., R. F. Bonsall, S. M. Delaney, M. J. Soule, G. Phillips, and L. S. Thomashow. 2001. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183:6454-6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMorran, B. J., J. S. Palmer, D. P. Lunn, D. Oceandy, E. O. Costelloe, G. R. Thomas, D. A. Hume, and B. J. Wainwright. 2001. G551D CF mice display an abnormal host response and have impaired clearance of Pseudomonas lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L740-L747. [DOI] [PubMed] [Google Scholar]
- 12.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muller, M. 2002. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic. Biol. Med. 33:1527-1533. [DOI] [PubMed] [Google Scholar]
- 14.O'Malley, Y. Q., K. J. Reszka, G. T. Rasmussen, M. Y. Abdalla, G. M. Denning, and B. E. Britigan. 2003. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L1077-L1086. [DOI] [PubMed] [Google Scholar]
- 15.Ran, H.-M., D. J. Hassett, and G. W. Lau. 2003. Human targets of Pseudomonas aeruginosa pyocyanin. Proc. Natl. Acad. Sci. USA 100:14315-14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen, R. U., and J. D. Klinger. 1987. Biological effects of Pseudomonas aeruginosa phenazine pigments. Antibiot. Chemother. 39:113-124. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen, R. U., and F. Joseph, Jr. 1993. Phenazine pigments in P. aeruginosa infection, p. 43-57. In M. Campa, M. Bendinelli, and H. Friedman (ed.), Pseudomonas aeruginosa as an opportunistic pathogen. Kluwer Academic/Plenum Publishers, Hingham, Mass.
- 18.Tang, H., M. Kays, and A. Prince. 1995. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 63:1278-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usher, L. R., R. A. Lawson, I. Geary, C. J. Taylor, C. D. Bingle, G. W. Taylor, and M. K. Whyte. 2002. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J. Immunol. 168:1861-1868. [DOI] [PubMed] [Google Scholar]
- 20.Wilson, R., D. A. Sykes, D. Watson, A. Rutman, G. W. Taylor, and P. J. Cole. 1988. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 56:2515-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]