Abstract
Nucleoside reverse transcriptase inhibitors (NRTIs) are mainstay therapeutics for HIV that block retrovirus replication. Alu (an endogenous retroelement that also requires reverse transcriptase for its life cycle)-derived RNAs activate P2X7 and the NLRP3 inflammasome to cause cell death of the retinal pigment epithelium (RPE) in geographic atrophy, a type of age-related macular degeneration. We found that NRTIs inhibit P2X7-mediated NLRP3 inflammasome activation independent of reverse transcriptase inhibition. Multiple approved and clinically relevant NRTIs prevented caspase-1 activation, the effector of the NLRP3 inflammasome, induced by Alu RNA. NRTIs were efficacious in mouse models of geographic atrophy, choroidal neovascularization, graft-versus-host disease (GVHD), and sterile liver inflammation. Our findings suggest that NRTIs are ripe for drug repurposing in P2X7-driven diseases.
NRTIs are widely used to treat HIV. Age-related macular degeneration (AMD) is a leading cause of blindness in the elderly population worldwide (1, 2). In geographic atrophy, the late stage of the prevalent and untreatable dry form of AMD, overabundance of non-coding Alu RNAs is implicated in cell death of the retinal pigment epithelium (RPE) (3–5). Since Alu sequences are non-coding retrotransposons that, like HIV, rely on reverse transcriptase for their life cycle (6), we hypothesized that NRTIs could block Alu RNA-induced cytotoxicity. Multiple NRTIs are typically administered orally to HIV patients at a total NRTI dose of up to 15 mg/kg/day (7) (equivalent dose in mice: 185 mg/kg/day; (8)). A clinically relevant dose (single daily oral administration of 150 mg/kg) of the NRTI stavudine, (d4T), which is U.S. FDA-approved for the treatment of HIV infection, prevented Alu-induced RPE degeneration (4, 5) in wild-type mice (Fig. 1A). A lower dose of oral d4T (50 mg/kg/day) also prevented Alu-induced degeneration, as did twice daily intraperitoneal administration of 50 mg/kg of the NRTI azidothymidine (AZT) (Fig. 1B and fig. S1).
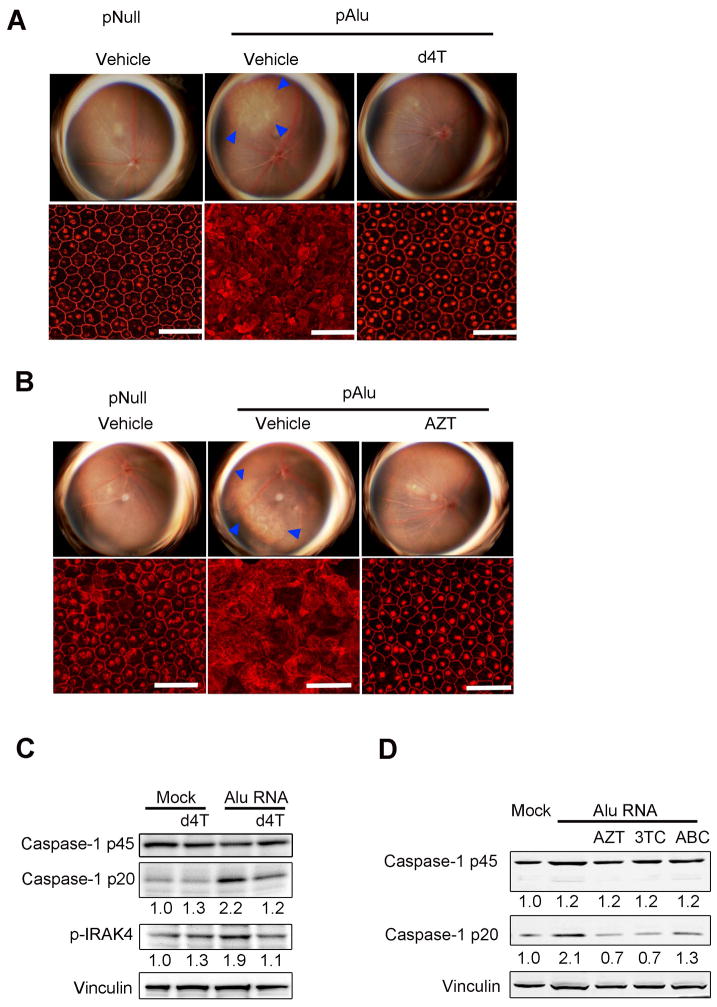
Fig. 1. NRTIs block Alu-induced RPE degeneration and Caspase-1 activation.
(A, B) Fundus photographs (top row) and flat mounts stained for zonula occludens-1 (ZO-1; red) (bottom row) of mice injected sub-retinally with control (pNull) or Alu RNA-expressing (pAlu) plasmids and (A) once daily oral administration of d4T (150 mg/kg/day) or (B) twice daily intraperitoneal administration of AZT (100 mg/kg/day). In fundus photographs degeneration outlined by blue arrowheads. RPE degeneration prevented in (A) 5/6 (d4T) vs. 0/6 (vehicle) eyes; P = 0.015 and (B) 8/9 (AZT) vs. 0/8 (vehicle) eyes; P = 0.0004 by Fisher’s exact test (pAlu vs. pAlu + d4T or AZT). Scale bars, 50 μm. See also fig. S1. (C) Western blot of Caspase-1 activation (p20 subunit) or p45 pro-form and IRAK4 phosphorylation in primary human RPE cells transfected with Alu RNA ± d4T (100 μM). Fold change in densitometry compared to mock. (D) Western blot of Caspase-1 pro (p45) and active (p20) forms in human RPE cells transfected with Alu RNA ± NRTIs (3TC, AZT, ABC) (100 μM). Fold change in densitometry compared to mock. Images representative of n = 3–4 (A, B), n = 6–9 (C, D).
Alu RNA induces RPE cell death via activation of Caspase-1 by the innate immune complex known as the NLRP3 inflammasome (5, 9). Western blotting of RPE lysates from d4T-treated mice confirmed that d4T blocked Caspase-1 activation (fig. S2A). Caspase-1, in turn, cleaves pro-interleukin (IL)-18 into its mature form, which induces RPE degeneration via IRAK4 phosphorylation (5); d4T also blocked IL-18 maturation and IRAK4 phosphorylation in vivo (fig. S2A). Consistent with the concept that d4T prevents RPE degeneration upstream of IL-18 by blocking Caspase-1 activation, the protective effect of d4T in Alu-treated mice was overridden by subretinal injection of recombinant mature mouse IL-18 (fig. S2B). d4T also prevented Alu-induced Caspase-1 activation (10) and IRAK4 phosphorylation in primary human (Fig. 1C) and wild-type mouse RPE cells (fig. S2C) without reducing Alu RNA levels (fig. S2D). Other clinically relevant NRTIs, including lamivudine (3TC) and abacavir (ABC), similarly blocked Caspase-1 cleavage induced by Alu RNA (Fig. 1D).
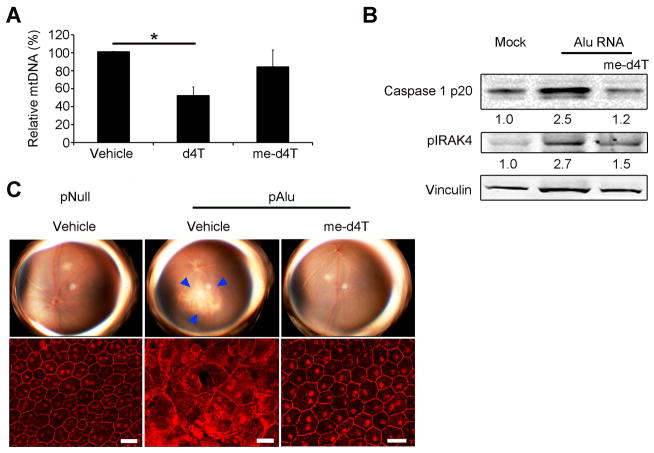
To test whether reverse transcriptase inhibition was required for inflammasome blockade by d4T, we synthesized a novel 5′-O-methyl-modified version of d4T (me-d4T) (figs. S3–S5). Only the triphosphate forms of nucleoside analogs inhibit reverse transcriptase; the methyl modification at the 5′ position prevents phosphorylation and thus formation of nucleoside triphosphate (11). As predicted, me-d4T, unlike d4T, did not inhibit lentiviral vector transduction of GFP (fig. S6, A and B). Moreover, the triphosphate metabolite of some dideoxy nucleoside analogs causes depletion of mitochondrial DNA (12); we found that d4T, but not me-d4T, reduced mtDNA levels in RPE cells (Fig. 2A). Remarkably, despite its inability to inhibit polymerases, me-d4T still blocked Caspase-1 activation and IRAK4 phosphorylation by Alu RNA in RPE cells (Fig. 2B). We confirmed that me-d4T also prevented Alu-induced RPE degeneration in wild-type mice (Fig. 2C). These data suggest that d4T can block Caspase-1 activation and RPE degeneration independent of reverse transcriptase inhibition.
Fig. 2. Methoxy-d4T blocks Alu-induced RPE degeneration and Caspase-1 activation independent of reverse transcriptase inhibition. (A) (B, C).
(A) Real-time quantitative PCR for mitochondrial DNA normalized to chromosomal DNA exon-intron junction sequence of primary mouse RPE cells treated with unmodified d4T or me-d4T (100 μM both drugs). n = 4, * P < 0.05 by one-way ANOVA and Tukey’s post-hoc test. (B) Western blot of Caspase-1 activation (p20 subunit) and phosphorylated IRAK4 in primary human RPE cells transfected with Alu RNA ± me-d4T (100 μM). Fold change in densitometry compared to mock. (C) Fundus photographs (top row) and flat mounts stained for zonula occludens-1 (ZO-1; red) (bottom row) from mice treated with me-d4T (twice daily intraperitoneal injection; 50 mg/kg/day) (P = 0.029). In fundus photographs degeneration outlined by blue arrowheads. Representative images of n = 4 (B, C) shown. Scale bars, (C): 20 μm.
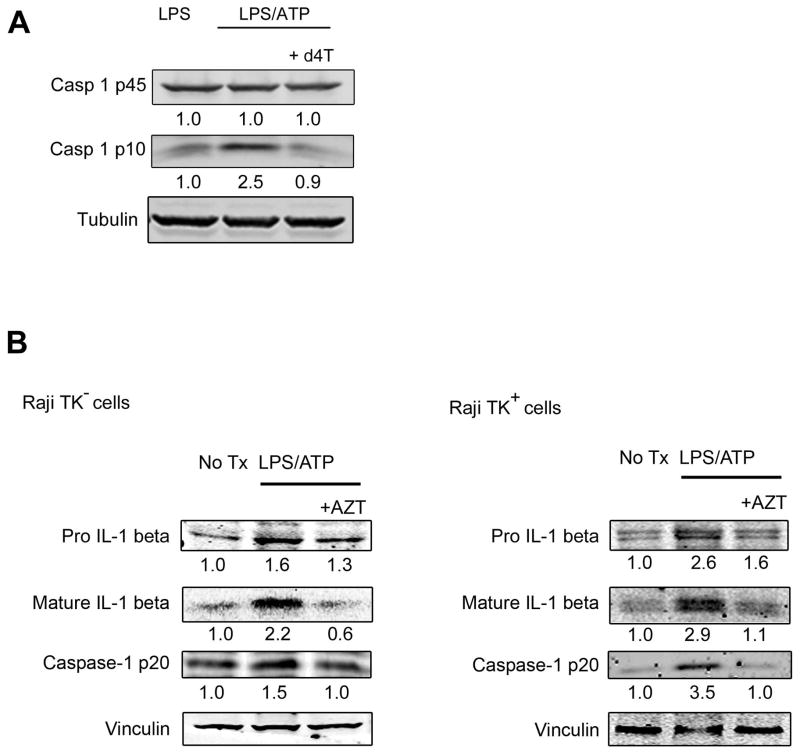
We also tested whether NRTIs blocked NLRP3 inflammasome activation by LPS/ATP, which is not known to signal via reverse transcriptase (13). d4T inhibited LPS/ATP-induced Caspase-1 maturation in primary mouse bone marrow-derived macrophages (Fig. 3A). To demonstrate that LPS/ATP-induced inflammasome activation can be inhibited without reverse transcriptase inhibition, we utilized a cell system that is incapable of NRTI phosphorylation, which is required for reverse transcription inhibition. Although d4T metabolism is largely unaffected in thymidine kinase-deficient (Raji/TK–) cells, AZT phosphorylation is severely impaired compared to thymidine kinase-expressing (TK+) parental cells)(figs. S7–S10) (14). One consequence of LPS/ATP-induced Caspase-1 activation is the proteolytic cleavage and maturation of interleukin-1β (IL-1β). Even though AZT was not phosphorylated in TK– cells, it still inhibited LPS/ATP-induced Caspase-1 and IL-1β maturation (Fig. 3B), indicating that AZT did not inhibit IL-1β and Caspase-1 maturation via reverse transcriptase inhibition.
Fig. 3. NRTIs block LPS/ATP-induced inflammasome activation.
(A) Western blot of pro (p45) and active (p10) Caspase-1 from cell lysate of wild-type BMDM treated with LPS alone or LPS/ATP with or without d4T (50 μM). Fold change in densitometry compared to LPS alone. (B) Western blot of pro- and mature IL-1beta and mature Caspase-1 in cell lysates of Raji TK– and TK+ cells untreated or with LPS ATP with or without AZT (100 μM). Fold change in densitometry compared to no treatment. Representative images of n = 3–4 experiments (A–B). See also figs. S7–S10.
In support of the idea that NRTIs specifically impaired Caspase-1 activation, levels of pro-Caspase-1/IL-18 were not considerably changed as shown in (Figs. 1, C and D, fig. S2A, and Fig. 3, A and B), although AZT did diminish pro-IL-1β protein levels (Fig. 3B). Moreover, d4T did not reduce expression of NLRP3 or IL1B mRNAs in RPE cells treated with Alu RNA (fig. S11). d4T also did not affect mRNA priming of non-inflammasome genes (Il6, Il12a, Tnf) by LPS (fig. S12) in BMDMs. Consistent with this finding, and that LPS signaling occurs via IRAK4 phosphorylation, d4T did not reduce IRAK4 phosphorylation after LPS stimulation (fig. S13).
Alu RNA (9) and LPS/ATP (15) activate the NLRP3 inflammasome via the ATP receptor P2X7. d4T did not block extracellular release of ATP induced by Alu RNA in primary human RPE cells (fig. S14A). We therefore hypothesized that d4T blocks P2X7 or a P2X7-dependent pathway. Upon ATP binding, cell-surface P2X7 forms non-selective cation channels that can mediate inflammasome activation (16). However, d4T did not significantly modulate P2X7 cation channel function as monitored by patch clamp analysis of HEK293 cells expressing either the mouse or rat P2X7 receptor (fig. S14, B and C).
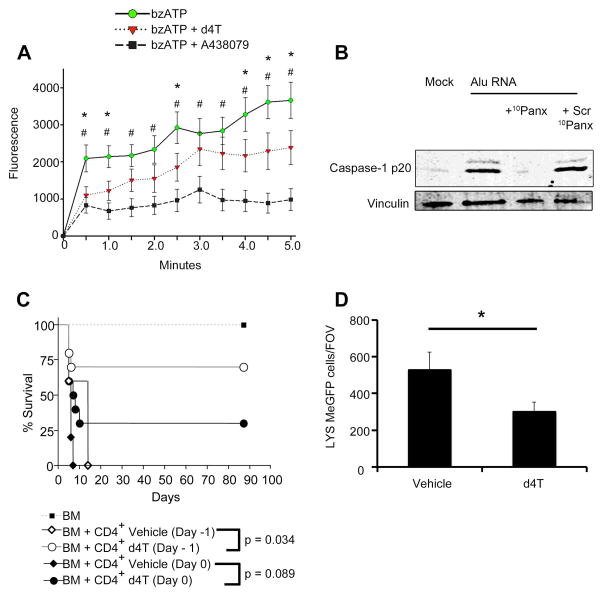
P2X7 activation also increases cell permeability to molecules of up to ~ 1,000 Da (17). We found that d4T and the known P2X7 antagonist A438079 inhibited P2X7-dependent uptake of YO-PRO-1 iodide (M.W. 629 Da) induced by the selective P2X7 agonist bzATP in human P2X7-expressing HEK293 cells (Fig. 4A and fig. S15). Interestingly, d4T only partially inhibited YO-PRO-1 uptake, whereas Caspase-1 activation by Alu RNA was completely blocked by a peptide (10Panx) targeting a P2X7-associated channel that inhibits P2X7-dependent dye uptake and LPS/ATP-induced inflammasome activation, but not cation flux (18) (Fig. 4B). These data are consistent with the concept that P2X7 activation leads to activation of multiple cell permeabilization pathways (19), and that inhibition of some but not all of these pathways by d4T is sufficient to fully block Caspase-1 activation. Although a high concentration of 10Panx peptide (20) was reported to induce cytotoxicity, the lower concentration used here did not (fig. S16A). Moreover, 10Panx-treated human RPE cells were still capable of synthesizing other cytokines such as IL-6 (fig. S16B). Interestingly, Alu RNA activation of Caspase-1 was unimpaired in Panx1–/– mouse RPE cells (fig. S16C), which parallels a previous report that Pannexin-1 is not required for Caspase-1 activation by LPS/ATP (15). The 10Panx peptide can have non-specific steric effects that impact cell permeability (21), possibly via overlapping mechanisms with P2X7-dependent pathways. Collectively, our data indicate that Caspase-1 activation in RPE cells by Alu RNA occurs via a P2X7-dependent, Pannexin-1-independent pathway.
Fig. 4. NRTIs selectively block P2X7 pore function and P2X7-driven models of graft rejection and sterile liver inflammation.
(A) P2X7-dependent YO-PRO-1 dye uptake induced by bzATP (100 μM) in HEK293 cells stably expressing the human P2X7 receptor and treated with d4T or A438079 (64 μM for both drugs). Fluorescence values are baseline subtracted from cells without bzATP treatment. * bzATP vs. d4T; # bzATP vs. A438079, P < 0.05 by two-way ANOVA and Student-Newman Keuls post-hoc test (n = 12). See also fig. S15. (B) Western blot of Caspase-1 activation (p20 subunit) in primary human RPE cells transfected with Alu RNA ± (A) Short peptide (10Panx; 240 μM) that blocks P2X7 pore function but not cation flux (vs. scrambled peptide: Scr 10Panx; 240 μM). (C) Administration of d4T starting From Day –1 pre-transplant protects murine recipients from graft versus host disease (GVHD). BALB/c mice were subjected to TBI (950 cGY) and then reconstituted with T depleted bone marrow (TDBM) from B6 donors. Cohorts (n = 10 per cohort) received either BM alone or BM plus bulk CD4+ T cells (2.5M) on the same day as the irradiation. Cohorts that received BM and bulk CD4+ T cells were additionally treated with either vehicle (saline) or drug (d4T) from day –1 or day 0 for a period of 10 days. Clinical manifestations of GVHD and overall survival were monitored post-BMT. Survival analysis was performed according to the Kaplan-Meier method and survival between groups was compared using the log-rank test. Results were pooled from two independent experiments. See also fig. S19. (D) LysMeGFP+ cells/FOV around a sterile focal hepatic lesion. d4T (400 mg/kg) or PBS administered via jugular vein 20 minutes prior to injury. Intravenous d4T inhibits neutrophil recruitment 60 minutes after injury. n = 4, error bars S.E.M., P < 0.05 by two-way ANOVA and Student-Newman Keuls post-hoc test. See also fig. S20.
Conversely, Alu RNA-induced Caspase-1 activation in RPE cells was not inhibited by calmidazolium (fig. S16C), which has been shown to inhibit rat P2X7-mediated cation flux but not dye uptake (22), and to inhibit mouse P2X7-mediated cation flux but not cell death (23). We also found that calmidazolium blocked Caspase-1 activation by the cation-specific inflammasome agonist nigericin in these cells (fig. S16D), which supports the idea that the mode of action of calmidazolium involves the inhibition of cation flux (necessary for nigericin-induced inflammasome activation, but not for P2X7-induced inflammasome activation). Furthermore, the intracellular C-terminus of P2X7 governs P2X7-associated dye uptake, and a version of d4T that is not cell permeable (24) did not block Caspase-1 activation by Alu RNA in RPE cells (fig. S16, F and G). Consistent with antagonism downstream of P2X7 but preceding inflammasome activation, d4T blocked Alu-induced mitochondrial ROS (mtROS) production (fig. S17, A and B) (5).
Supportive of the idea that d4T inhibits NLRP3 inflammasome activation via P2X7, d4T did not prevent Caspase-1 activation in primary mouse BMDMs treated with nigericin or crystalline monosodium urate, NLRP3 agonists that do not signal via P2X7 (fig. S18, A and B) (25). Furthermore, d4T did not inhibit AIM2 inflammasome activation by poly dA:dT or NLRC4 inflammasome activation by flagellin (fig. S18, C and D). These findings suggest that d4T specifically inhibits P2X7-dependent inflammasome activation, since cytosolic flagellin and poly dA:dT activate these other inflammasomes independent of P2X7 (26, 27).
To explore the therapeutic relevance of NRTIs beyond geographic atrophy, we hypothesized that NRTIs might be broadly useful in other P2X7-driven animal models of disease. Murine graft-versus-host disease (GVHD) is mediated by P2X7 (28); consistent with these results, irradiated BALB/c mice reconstituted with allogeneic (C57/Bl6) bone marrow and T cells showed improved survival when treated with d4T compared to saline-treated controls (Fig. 4C). We also found that at day 3 after transplant, d4T-treated mice had lower serum levels of IFN-γ, TNF-α, and IL-6 proteins compared to saline treatment (fig. S19). The increased abundance of these cytokines in serum is characteristic of allogeneic T cell transfer in murine models and thought to play a role in acute GVHD pathogenesis (29–31). Supporting the idea that d4T targets P2X7, serum levels of these three cytokines were also decreased in an acute GVHD model employing P2X7-deficient host mice (28). Moreover, studies in a variety of other systems indicate that P2X7 regulates the expression of these cytokines (32–34). Further supporting the activity of d4T at the level of P2X7, in the P2X7-driven model of liver inflammation in which neutrophils are recruited from the circulation to a site of sterile injury (35), intravenous d4T reduced early neutrophil migration to the focus of hepatic necrosis (Fig. 4D and fig. S20). Finally, since P2X7 activation is known to increase tumor angiogenesis (36), we investigated whether NRTIs reduced choroidal neovascularization (CNV), which characterizes the “wet” form of AMD. In the laser-induced mouse model of CNV we found that d4T and me-d4T reduced CNV volume in wild-type mice (fig. S21A), but not in P2X7-deficient mice (fig. S21B). These data suggest that NRTIs might be therapeutic for both dry and wet AMD, and provide further evidence that these drugs work at the level of P2X7 in these systems.
NRTIs are a diverse, widely used, inexpensive class of small molecules, with extensive pharmacokinetic and safety data collected over several decades of human use. Our work, by illustrating a novel mechanism of action of NRTIs, paves a clear path for the broad repurposing of this drug class to address major unmet medical needs. Our data indicate that NRTIs could have dual therapeutic use in AMD in treating both geographic atrophy and neovascular AMD.
Since inflammasome inhibition by NRTIs can be achieved without their phosphorylation, the use of me-d4T or other phosphorylation-incompetent nucleoside analogs to treat disease could avoid dose-limiting toxicities associated with NRTI-triphosphate-mediated polymerase inhibition. It is not known whether long-term NRTI use is protective against developing AMD; however, as the population of aging HIV-positive individuals continues to grow, it might be possible to determine this predicted effect.
Interestingly, recent work has shown caspase-1 activation by HIV in abortively infected T-cells (37) and a role for NLRP3 in sensing HIV in macrophages and monocytes (38); such studies support the importance of inflammasome regulation by NRTIs. Also of note, HIV patients have increased plasma levels of the inflammasome effector IL-18 (39), which decreases after treatment with NRTI-containing highly active anti-retroviral therapy (40). Thus, while it is unclear whether suppression of viral replication by NRTIs or other components of HAART leads to the reduction of plasma IL-18 levels in these patients, our findings raise the possibility that inflammasome inhibition by NRTIs independent of reverse transcriptase inhibition could be responsible, at least in part, for modulation of HIV-induced cytokine expression.
Supplementary Material
Acknowledgments
We thank B.K. Ambati, K. Ambati, A.M. Adams, A.M. Rao, and G.S. Rao for discussions; L. Toll, G.R. Pattison R. King, L. Xu, M. McConnell, C. Payne, D. Robertson, G. Botzet, and J. May for technical assistance; G. Dubyak for discussions and providing the HEK293-P2X7 cell line; University of Kentucky Viral Core (COBRE) for providing lentivirus-GFP. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. J.A. and B.J.F. are listed as inventors on a patent application for the therapeutic use of NRTIs and chemical derivatives filed by their employer, the University of Kentucky. J.A. was supported by NIH grants DP1GM114862, R01EY018350, R01EY018836, R01EY020672, R01EY022238, and R01EY024068, Doris Duke Distinguished Clinical Scientist Award, Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, Ellison Medical Foundation Senior Scholar in Aging Award, Foundation Fighting Blindness Individual Investigator Research Award, Harrington Discovery Institute Scholar-Innovator Award, Dr. E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, and Research to Prevent Blindness departmental unrestricted grant; B.J.F. by NIH T32HL091812 and UL1RR033173; A.B.C. by the Programme for Advanced Medical Education (sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal) and Bayer Global Ophthalmology Research Award; Y.H. by Alcon Japan Research award; N.K. by Beckman Initiative for Macular Research and NIH K99EY024336; B.D.G. by American Heart Association and International Retinal Research Foundation (IRRF); T.Y. by Fight for Sight postdoctoral award; C.B.R. by The Loris and David Rich Postdoctoral Scholar Award (IRRF). D.H.F and S.A. are supported by the Center for Cancer Research, National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.T.Y. and M.R. are supported by BBSRC BB/J017345/1. D.R.H. is supported by NIH P30EY003040 and R01EY001545, and Arnold and Mabel Beckman Foundation.
Footnotes
Supplementary figures and references 41–45 can be found in Supporting Online Material.
References and Notes
- 1.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, Ambati BK, Bogdanovich S, Chiodo VA, Hauswirth WW, Kugel JF, Goodrich JA, Ponicsan SL, Hinton DR, Kleinman ME, Baffi JZ, Gelfand BD, Ambati J. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci U S A. 2012;109:13781–13786. doi: 10.1073/pnas.1206494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee DK, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, Zhang Q, Grossniklaus HE, Provis JM, Madigan MC, Milam AH, Justice NL, Albuquerque RJ, Blandford AD, Bogdanovich S, Hirano Y, Witta J, Fuchs E, Littman DR, Ambati BK, Rudin CM, Chong MM, Provost P, Kugel JF, Goodrich JA, Dunaief JL, Baffi JZ, Ambati J. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, Albuquerque RJ, Hauswirth WW, Chiodo VA, Kugel JF, Goodrich JA, Ponicsan SL, Chaudhuri G, Murphy MP, Dunaief JL, Ambati BK, Ogura Y, Yoo JW, Lee DK, Provost P, Hinton DR, Nunez G, Baffi JZ, Kleinman ME, Ambati J. DICER1 Loss and Alu RNA Induce Age-Related Macular Degeneration via the NLRP3 Inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35:41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2014. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 8.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. U.S. Food and Drug Administration; Rockville, Maryland, USA: 2002. [Google Scholar]
- 9.Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A, Yasuma T, Yasuma R, Kim Y, Hinton DR, Kirschning CJ, Gelfand BD, Ambati J. TLR-Independent and P2X7-Dependent Signaling Mediate Alu RNA-Induced NLRP3 Inflammasome Activation in Geographic Atrophy. Invest Ophthalmol Vis Sci. 2013;54:7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamin TT, Ayala JM, Miller DK. Activation of the native 45-kDa precursor form of interleukin-1-converting enzyme. J Biol Chem. 1996;271:13273–13282. doi: 10.1074/jbc.271.22.13273. [DOI] [PubMed] [Google Scholar]
- 11.Nykanen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 12.Lim SE, Copeland WC. Differential incorporation and removal of antiviral deoxynucleotides by human DNA polymerase gamma. J Biol Chem. 2001;276:23616–23623. doi: 10.1074/jbc.M101114200. [DOI] [PubMed] [Google Scholar]
- 13.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 14.Balzarini J, Herdewijn P, De Clercq E. Differential patterns of intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J Biol Chem. 1989;264:6127–6133. [PubMed] [Google Scholar]
- 15.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol. 2011;186:6553–6561. doi: 10.4049/jimmunol.1100478. [DOI] [PubMed] [Google Scholar]
- 16.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 17.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 18.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 22.Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 23.Salas E, Carrasquero LM, Olivos-Ore LA, Bustillo D, Artalejo AR, Miras-Portugal MT, Delicado EG. Purinergic P2X7 receptors mediate cell death in mouse cerebellar astrocytes in culture. The Journal of pharmacology and experimental therapeutics. 2013;347:802–815. doi: 10.1124/jpet.113.209452. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal HK, Loethan K, Mandal D, Doncel GF, Parang K. Synthesis and biological evaluation of fatty acyl ester derivatives of 2′,3′-didehydro-2′,3′-dideoxythymidine. Bioorg Med Chem Lett. 2011;21:1917–1921. doi: 10.1016/j.bmcl.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 25.Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012;3:e403. doi: 10.1038/cddis.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 27.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Juttner E, Zerweck A, Gartner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HZ, Li GL, Lim YK, Chan SH, Yap EH. Kinetics of interferon-gamma secretion and its regulatory factors in the early phase of acute graft-versus-host disease. Immunology. 1999;98:379–385. doi: 10.1046/j.1365-2567.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmaltz C, Alpdogan O, Muriglan SJ, Kappel BJ, Rotolo JA, Ricchetti ET, Greenberg AS, Willis LM, Murphy GF, Crawford JM, van den Brink MR. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 32.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, Gourine VN. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. Journal of cell science. 1999;112(Pt 3):297–305. doi: 10.1242/jcs.112.3.297. [DOI] [PubMed] [Google Scholar]
- 35.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 36.Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957–2969. doi: 10.1158/0008-5472.CAN-11-1947. [DOI] [PubMed] [Google Scholar]
- 37.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, 3rd, Blankson JN, Pardoll D, Cox AL. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS pathogens. 2014;10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad R, Sindhu ST, Toma E, Morisset R, Ahmad A. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J Virol. 2002;76:12448–12456. doi: 10.1128/JVI.76.24.12448-12456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stylianou E, Bjerkeli V, Yndestad A, Heggelund L, Waehre T, Damas JK, Aukrust P, Froland SS. Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clin Exp Immunol. 2003;132:462–466. doi: 10.1046/j.1365-2249.2003.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 42.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 43.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young MT, Pelegrin P, Surprenant A. Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol. 2007;71:92–100. doi: 10.1124/mol.106.030163. [DOI] [PubMed] [Google Scholar]
- 45.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.