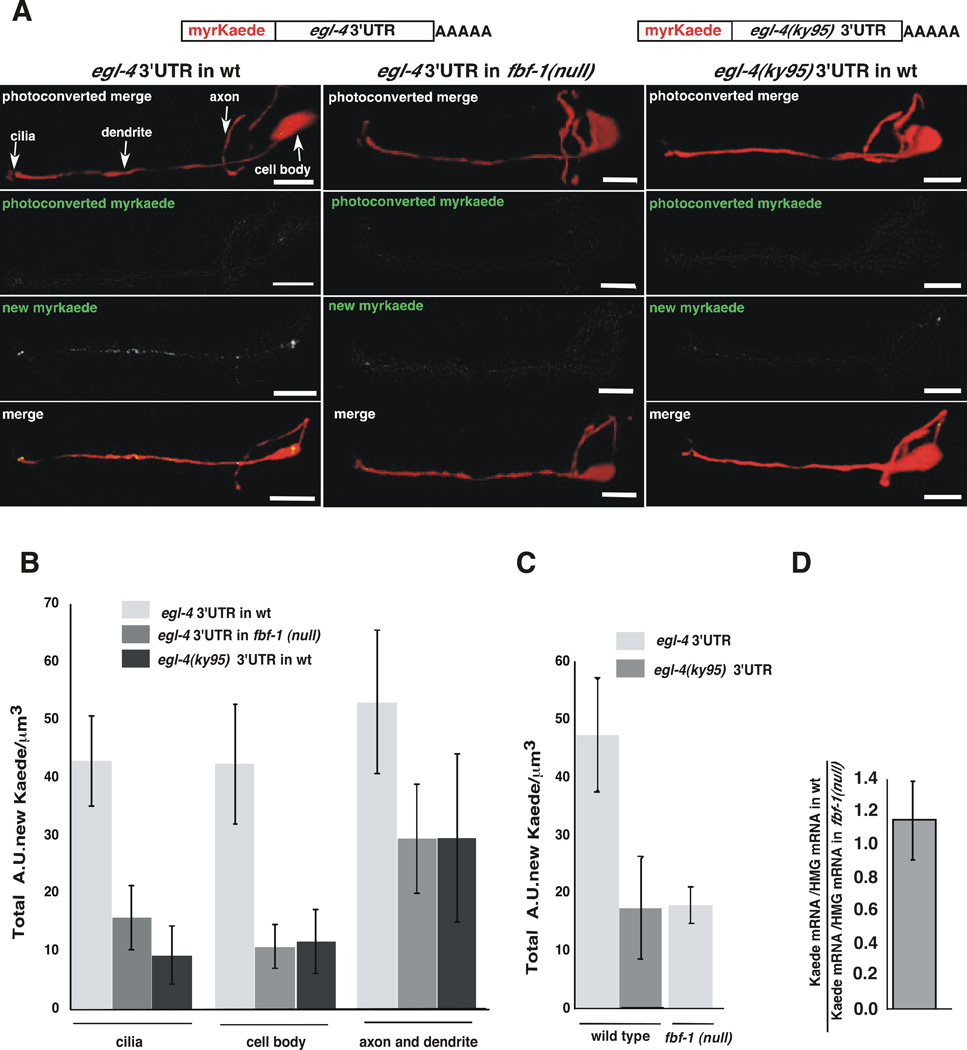
Figure 4. The egl-4 NR/FBE promotes translation within the AWC neuron.
(A) Top, cartoons of myristoylated Kaede expression reporters under the control of the str-2 promoter were flanked with the wild type egl-4 3’UTR or the egl-4(ky95) 3’UTR. These were expressed in either wild type (left and right columns of images) or fbf-1(ok91) animals (middle column). pstr-2::RFP (red) delimits the AWC neuron (top and bottom rows of images). The parts of the AWC neuron are marked with arrows (upper left). The top row of panels: red and green channels immediately after photoconversion. The second row panels: residual unconverted Kaede in the green channel immediately after photoconversion. The third row of panels: the green channel reveals the subcellular location of accumulated Kaede of the same animal after 3 hours of recovery on food. The fourth row of panels: the merged red and green channels of the same animal.
(B) Kaede synthesis near the sensory cilia and cell body depends on the 3’UTR and FBF-1. Newly synthesized Kaede under the control of the wild type 3’UTR expressed in the fbf-1(ok91) mutant animals was 2.7 fold lower in the cilia (*P=0.0233 n=5) and 3.9 fold lower in the cell body (*P=0.028) than when the same reporter transgene is expressed in wild type animals. In wild type animals expressing Kaede under the control of the egl-4(ky95) 3’UTR, newly synthesized Kaede was 4.6 fold lower in the cilia (**P=0.0032 n=6) and 3.6 fold lower in the cell body (*P=0.0210 n≥6) than in wild type animals expressing the reporter under the control of the wild-type 3’UTR. In the axon and dendrite, the Kaede expression was not significantly different in animals that bear the ky95 mutation (p=0.25) or in animals that lack fbf-1 (p=0.17). The copy number of the wildtype and ky95 3’UTR reporter was the same in each line (Supplemental Materials and Figure S3). P values were obtained using a two-tailed unpaired T test. Error bars denote s.e.m.
(C) AWC-wide Kaede expression was determined by integrating Kaede levels over the whole cell using the images analyzed in (B). Over-all Kaede levels were 2.6 fold higher in wild type worms expressing Kaede flanked with the wild-type egl-4 3’UTR than in fbf-1(ok91) mutants expressing the same transgene (*P=0.0165 n=6). Kaede recovery was 2.8 fold higher in wild type worms expressing Kaede flanked with the wild-type egl-4 3’UTR than in wild type worms expressing Kaede with the ky95 3’UTR (*P= 0.0443 n= 6). Newly synthesized Kaede was quantified from confocal images using Volocity software (see Supplemental Materials). (D) Kaede mRNA levels are the same in both wild-type 3’UTR and fbf-1(ok91) mutant worms. Kaede mRNA levels did not differ significantly between these strains (P= 0.416). Real time PCR was performed on cDNA made from worms of each genotype. Units of Kaede mRNA/unit of HMG CoA mRNA were determined for each and the ratio of this value is shown.