Abstract
The Ami-AliA/AliB oligopeptide permease is an ATP-binding cassette transporter which is found in Streptococcus pneumoniae and which is involved in nutrient uptake. We investigated the role of the three paralogous oligopeptide-binding lipoproteins AmiA, AliA, and AliB by using murine models of pneumococcal colonization and invasive disease. A series of mutants lacking aliA, aliB, and amiA either alone or in combination as double or triple mutations were used. Inoculation of the nasopharynx with a mixture of the obl (oligopeptide-binding lipoprotein-negative) triple-mutant and wild-type (D39) bacteria resulted in significantly smaller numbers of obl bacteria colonizing the nasopharynx. The use of a mixture of individual mutants and wild-type pneumococci revealed that AmiA, AliA, and AliB were all required for successful colonization of the nasopharynx. The obl mutant was more attenuated than the aliB mutant but not the aliA or amiA mutant. Therefore, there is some redundancy in the Ami-AliA/AliB complex in terms of nasopharyngeal colonization, with AliA and AmiA being able to compensate for the removal of AliB. Animals with invasive disease caused by these mutants had survival times, bacterial loads, and inflammatory cytokine production levels similar to those of animals infected with wild-type pneumococci. Our results show that although the Ami-AliA/AliB complex is not required for virulence during pneumococcal pneumonia, it does play a role in colonization of the nasopharynx.
Streptococcus pneumoniae remains a human pathogen of significant importance despite the availability of adequate antibiotics and the current polysaccharide conjugate vaccine. The worldwide increase in pneumococcal drug resistance and the limited vaccine coverage of pneumococcal serotypes highlight the need for the identification of pneumococcal proteins for use as vaccine candidates.
The use of genome-based technologies allows the large-scale detection of surface-expressed pneumococcal proteins that have homology to virulence factors from other microbes. These proteins may be used as new drug targets or may be included in vaccine preparations. Recent studies highlighted the importance of ATP-binding cassette (ABC) transporters in the virulence of S. pneumoniae. Mutations in pneumococcal ABC transporters result in the attenuation of pneumococcal virulence in models of both pneumonia and bacteremia (11, 13).
The Ami-AliA/AliB oligopeptide permease was the first ABC permease identified in a gram-positive bacterium (3). This permease comprises three oligopeptide-binding lipoproteins, called AmiA (The Institute for Genomic Research designation SP1891), AliA (SP0366), and AliB (SP1527); the latter two are paralogues of AmiA (2), two transmembrane proteins, AmiC (SP1890) and AmiD (SP1889), and two ATPases, the ABC proteins AmiE (SP1888) and AmiF (SP1887). The genes encoding AmiA and AmiC to AmiF are all part of a single operon (3), whereas aliA and aliB are separated on the pneumococcal genome.
The Ami-AliA/AliB oligopeptide permease is involved in the uptake of nutrients from the environment in order to overcome auxotrophy for various amino acids (6). Ami-AliA/AliB may also act as an environmental signal allowing the organism to adapt accordingly. Mutations in either amiA or aliA have been suggested to decrease adherence to pulmonary epithelial cells in vitro (7) due to reduced binding to the GalNAc-β-1-4Gal glycoconjugate receptor on resting lung epithelial or vascular endothelial cells. It has also been proposed that Ami-AliA/AliB may be involved in triggering competence, since the cell-density-dependent control of competence was altered in an obl mutant (6). Little is known about the effects of Ami-AliA/AliB in vivo.
We have constructed a series of mutations in aliA, aliB, and amiA either alone or in combination as double or triple mutations (2). Using a well-characterized murine model of pneumococcal infection (9), we have now investigated the relevance of these genes in colonization and invasive disease.
MATERIALS AND METHODS
Bacteria.
Serotype 2 D39 pneumococci were used as the parental strain for the production of mutants and as the wild-type strain for infection. Construction of the individual, double, and triple ami-aliA/aliB mutants was described previously (2). Bacteria were cultured on blood agar base number 2 (Oxoid, Basingstoke, United Kingdom) plus 5% (vol/vol) defibrinated horse blood (E&O Laboratories, Bonnybridge, United Kingdom), in brain heart infusion broth (Oxoid; for invasive disease studies), or in Todd-Hewitt broth plus 0.5% yeast extract (Oxoid; for colonization studies). Strain validation was based on optochin sensitivity (Difco, Detroit, Mich.) and the Quellung reaction. During competitive colonization studies, amiA mutants were selected on 10 μg of tetracycline/ml, aliB mutants were selected on 2 μg of chloramphenicol/ml, and both aliA and amiA aliA aliB (obl [oligopeptide-binding lipoprotein-negative]) mutants were selected on 1 μg of erythromycin/ml. The rpsL gene, encoding a streptomycin-resistant mutant of ribosomal protein S12, was used to transform D39 for competitive colonization studies. Streptomycin-resistant D39 transformants were selected in Todd-Hewitt broth plus 0.5% yeast extract and 100 μg of streptomycin/ml, and a single transformant clone was used in our animal experiments.
In order to maintain virulence, bacteria were animal passaged as previously described (1) prior to use for infection.
Infection of mice.
Eight- to 15-week-old female MF1 mice (Harlan Olac, Bicester, United Kingdom) were used in all studies. To study invasive disease, mice were lightly anesthetized with 1.5% (vol/vol) halothane, and 106 CFU of D39 were introduced intranasally in a 50-μl volume of phosphate-buffered saline. For nasopharyngeal colonization assays, mice were anesthetized as described above and inoculated intranasally with 2.5 × 105 CFU of each bacterial strain either alone or in combination in a 10-μl final volume. The combinations of bacterial strains were D39 and obl or D39, amiA, aliA, and aliB.
Signs of disease were closely monitored until the animals were deemed moribund (10), when they were humanely sacrificed. Mice displaying no signs of illness by 336 h were considered to have survived the infection. All experiments were carried out in accordance with the UK Animals Scientific Procedures Act of 1986.
Bacteriologic sample collection and processing.
At predetermined times following infection, mice were sacrificed and blood samples were removed by cardiac puncture. The nasopharynx was washed by using a modification of the method of Wu et al. (15). The trachea was exposed and clamped, and 2 ml of sterile phosphate-buffered saline was passed through the nasopharynx via a 16-gauge nonpyrogenic Angiocath (F. Baker Scientific, Runcorn, United Kingdom). Bronchoalveolar lavage and lung tissue sampling were carried out as previously described (10).
Bacterial loads were determined by plating 10-fold dilutions on blood agar plates plus 5% (vol/vol) defibrinated horse blood.
Cytokine assay sample collection and processing.
Bronchoalveolar lavage fluid (BALF) and lung tissue samples were snap-frozen on collection and processed by the method of van der Poll et al. (14). Both BALF and lung tissue homogenates were stored at −80°C prior to use. Serum was prepared from blood samples after 30 min at room temperature by centrifugation at 3,800 × g. Serum was stored at −80°C until use.
Tumor necrosis factor (TNF) activity was measured by using an L929 cytotoxicity bioassay as previously described (10) with methylthiotetrazole to measure cell viability. Interleukin-6 (IL-6) levels were measured by using an enzyme-linked immunosorbent assay with commercially available antibodies (10).
Statistics.
Survival times, expressed as medians, were compared by using Mann-Whitney U analysis. Bacteriologic assay results were expressed as means and standard errors of the means (SEMs). Results from bacteriologic time course experiments were compared by using one-way analysis of variance with Scheffe's post hoc test. Bacteriologic assay results below the detection limits of the viable count assays (log 1.92 units per ml of blood and log 0.92 unit per ml of lung or nasopharyngeal samples) were ascribed values just below the detection limits (log 1.91 and 0.91, respectively). Comparisons of bacterial loads between bacterial strains were carried out by using multiple unpaired Student's t tests with Bonferroni correction.
In in vitro and in vivo competition studies with D39 and obl bacteria, the data were mathematically corrected to account for the higher obl count in the initial inoculum.
TNF and IL-6 levels, expressed as medians (minimum-maximum), were compared by Mann-Whitney U analysis with Bonferroni correction for multiple analyses.
Statistical analyses were carried out with StatView 4.1 (Abacus Concepts, Berkeley, Calif.). A P value of <0.05 was considered statistically significant for all analyses.
RESULTS
obl bacteria are less able to colonize the nasopharynx than D39 bacteria.
In order to compare the abilities of the obl mutant and D39 to colonize the nasopharynx, we carried out competition infections in which the bacterial strains were inoculated together (Fig. 1).
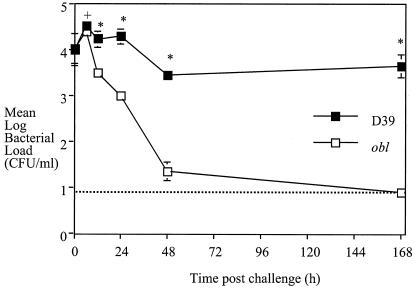
FIG. 1.
Mean and SEM viability of obl and D39 bacteria within the nasopharynx following inoculation of a mixture of 2.5 × 105 CFU of each strain into each of five mice. The broken line represents the detection limit of the assay. A plus sign indicates a P value of <0.05 and an asterisk indicates a P value of <0.01 in a comparison of the results for the two strains.
Immediately following intranasal inoculation of 2.5 × 105 CFU of each strain, equal numbers of both obl and D39 bacteria were recovered from the nasopharynx (104 CFU/ml). The viability of D39 was not altered significantly from the 0-h value during the experiment. At 1 week after inoculation, D39 was found to have colonized the nasopharynx at ca. 104 CFU/ml. In parallel, the numbers of obl bacteria recovered at 24 and 48 h were significantly lower than the number recovered at 0 h (P < 0.05 and P < 0.01, respectively). In addition, at 6 h, the viable counts of obl bacteria within the nasopharynx were significantly lower than those of D39 bacteria (P < 0.05), and the reduction from 12 h on was even more significant (P < 0.01).
An experiment then was carried out with D39 or obl bacteria administered intranasally to separate mice in order to investigate whether the reduced colonization of obl bacteria (Fig. 1) was due to the inability of obl bacteria to colonize or rather to the failure of obl bacteria to compete in the presence of D39 bacteria. Even when obl bacteria were given alone, their viability within the nasopharynx at 48 h (101 CFU/ml) (Fig. 2) was significantly lower than that of D39 bacteria given alone (more than 103 CFU/ml). Bacterial loads recovered for each bacterial strain given alone (Fig. 2) were similar to those recovered for the strains given in combination (Fig. 1).
FIG. 2.
Mean and SEM viability of obl and D39 bacteria within the nasopharynx following inoculation of 2.5 × 105 CFU of each strain into each of five mice. The broken line represents the detection limit of the assay. An asterisk indicates a P value of <0.01 in a comparison of the results for the two strains.
The amiA, aliA, and aliB genes are all involved in nasopharyngeal colonization.
In order to identify which of the oligopeptide-binding lipoproteins were required for the ability to colonize the nasopharynx, a mixed culture of 2.5 × 105 CFU each of D39 and the amiA, aliA, and aliB single mutants was used to inoculate mice. Similar numbers of the individual bacterial strains were recovered immediately (Fig. 3). By 48 h, significantly fewer amiA, aliA, and aliB pneumococci than D39 pneumococci were recovered (P < 0.01, P < 0.05, and P < 0.01, respectively). The numbers of amiA and aliA mutants recovered were also significantly lower than the numbers of the aliB mutant recovered.
FIG. 3.
Mean and SEM bacterial load within the nasopharynx of MF1 mice following inoculation of a mixture of 2.5 × 105 CFU each of D39, amiA, aliA, and aliB bacteria into each of five mice. The broken line represents the detection limit of the assay. A plus sign indicates a P value of <0.05 and an asterisk indicates a P value of <0.01 in a comparison of the results for the mutant strains and strain D39.
Of note is the fact that none of the animals used in the colonization studies showed signs of invasive disease. Immediately following inoculation of either D39 or obl bacteria only, 100 CFU could be recovered from the total lung samples. Lung homogenates and blood samples taken at 48 h revealed no viable pneumococci, proving that the model used represents purely nasopharyngeal colonization.
Invasive disease caused by Ami-AliA/AliB mutants and that caused by D39 result in similar survival times.
Having established that the Ami-AliA/AliB oligopeptide-binding lipoproteins play a role in nasopharyngeal colonization, we next investigated their role in invasive disease.
Intranasal infection with 106 CFU of any of the bacteria resulted in clinical signs of illness at about 24 h. Initially, a mild hunched stance was noted, followed by progression through piloerection and lethargy. The median survival time of mice infected with D39, amiA, aliA aliB, aliB amiA, aliA amiA, or obl bacteria was 34 h, while mice infected with either aliA or aliB bacteria survived to 46.5 h (Table 1). There were no significant differences in the survival times of mice infected with any of the amiA, aliA, and aliB single mutants and mice infected with D39.
TABLE 1.
Survival times and levels of bacteremia in mice infected intranasally
| Bacterial strain | Survival time (h)a | Mean ± SEM log CFU/ml at the following h postchallengeb:
|
|||
|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | ||
| D39 | 34 | NF | NF | 3.20 ± 0.70 | 5.76 ± 1.02 |
| aliA | 46.5 | ||||
| aliB | 46.5 | ||||
| amiA | 34 | ||||
| aliA aliB | 34 | ||||
| aliB amiA | 34 | ||||
| aliA amiA | 34 | ||||
| obl | 34 | NF | 2.01 ± 0.98 | 4.21 ± 0.62 | 4.66 ± 0.87 |
Median survival times of 5 or 10 mice intranasally infected with 106 CFU of D39 or various mutants.
Levels of bacteremia following intranasal infection of five mice with either D39 or the obl mutant were similar. NF, none found.
As intranasal infection with aliA, aliB, or amiA single mutants or with aliA aliB, aliB amiA, or aliA amiA double mutants did not significantly alter mouse survival times, the remaining experiments were carried out only with obl bacteria.
Comparable levels of obl and D39 bacteria are recovered within the nasopharynges, lung airways, and lung tissues during pneumococcal pneumonia.
Although intranasal infection with the ami-aliA/aliB mutants did not result in significantly different survival times, it was possible that there would be differences in nasopharyngeal, lung, or blood bacteriologic assay results following infection. We therefore carried out basic bacteriologic assays with D39 and obl bacteria separately.
Immediately following intranasal infection with either obl or D39 bacteria, ca. 104 CFU/ml were recovered from the nasopharynx (Fig. 4A). The level of each strain then declined so that by 24 h, 103 CFU/ml were recovered for each strain; this level was significantly lower than that recovered at 0 h for both groups (P < 0.01).
FIG. 4.
Mean and SEM bacterial loads within the nasopharynges (A), lung airways (B), and lung tissues (C) following intranasal inoculation of 106 CFU of D39 or obl bacteria into each of five mice.
Similar numbers of viable pneumococci were detected in the lung airways of mice following infection with either obl or D39 bacteria (Fig. 4B). Between 103 and 104 CFU/ml of lung homogenate were found directly following infection with either obl or D39 bacteria (Fig. 4C). The numbers of obl bacteria increased during the experiment so that there were significantly more at 24 h than at 0 h (P < 0.01). The greater variation in the D39 group prevented detection of statistical significance.
At no time were there significant differences in bacterial loads between mice infected with obl bacteria and those infected with D39 bacteria.
Bacteremia develops with similar kinetics following intranasal inoculation of obl bacteria and of D39 bacteria.
The development of systemic disease following intranasal inoculation of either D39 or obl bacteria was investigated. Bacteremia was first detected in mice infected intranasally with 106 CFU of obl bacteria at 6 h postchallenge (Table 1). At this time, only one of five mice was bacteremic. No mice infected with D39 were bacteremic until 12 h postchallenge. At this time, three of five mice infected with D39 were bacteremic, while four of five mice infected with obl bacteria had positive blood cultures. Pneumococcal viable counts in the bloodstream continued to increase in both groups of mice until the end of the experiment. Significantly higher levels of pneumococci were recovered from the bloodstream of mice infected with D39 and obl bacteria at 24 h than at 0 h (P < 0.01 and P < 0.05, respectively).
Similar levels of proinflammatory cytokines are produced within the lung airways of mice infected with obl bacteria and with D39 bacteria.
To investigate whether the lack of expression of Ami-AliA/AliB affected proinflammatory cytokine expression, TNF activity and IL-6 levels in BALF from mice infected intranasally with either D39 or obl were measured.
TNF activity increased transiently in BALF from mice infected with either bacterial strain, with levels at 6 and 12 h being significantly higher than those at 0 h (P < 0.01) (data not shown). By 24 h, levels were no longer significantly different from the baseline.
IL-6 production within the lung airways increased gradually following infection with either D39 or obl bacteria (data not shown). Levels in both groups were higher at 24 h than at 0 h (P < 0.05).
At no time were there significant differences in TNF activity or IL-6 levels between mice infected with D39 bacteria and those infected with obl bacteria.
DISCUSSION
By using pneumococcal strains with targeted mutations in the Ami-AliA/AliB permease, we have investigated the role of this system in colonization and invasive disease.
Mutation of aliA, aliB, or amiA, either alone or in combination as double or triple mutants, does not significantly alter survival times of mice following intranasal infection with 106 CFU in a 50-μl volume (Table 1).
This lack of effect in invasive disease was also observed when pneumococcal viability was measured. Similar bacterial loads were recovered from the nasopharynges, lung airways, lung tissues, and bloodstreams of mice infected with either D39 or the obl mutant (Fig. 4).
Blue et al. previously found that in vivo proinflammatory cytokine production can still be altered by mutations in S. pneumoniae that do not alter bacterial loads (5). This was not the case for obl bacteria. Both TNF activity and IL-6 levels increased with typical kinetics and to similar extents during invasive disease due to either wild-type or mutant bacteria.
Therefore, the Ami-AliA/AliB oligopeptide permease does not appear to affect the virulence of serotype 2 S. pneumoniae D39 in our model of bacteremic pneumonia. Previously, mutations in aliB or amiA (as well as in amiC and amiD) were found to result in attenuation in two separate models of pneumococcal infection (8, 11). These studies identified aliB and amiA via high-throughput screening (signature-tagged mutagenesis) and, as Lau et al. (11) stated, this method of mutagenesis may also result in unrelated changes elsewhere on the genome. Alternatively, these differences may be attributed to the use of different pneumococcal strains, since it has been shown that the effect of a mutation depends on the strain of S. pneumoniae used (4).
In contrast to our results for invasive disease, our results for nasopharyngeal colonization do agree with those of another signature-tagged mutagenesis screen that identified amiA (as well as amiC and amiD) as being required for lung infection (8). In our study, the coadministration of obl and D39 bacteria to mice revealed that pneumococci deficient in amiA, aliA, and aliB were less able to survive and colonize the nasopharynx than were D39 pneumococci (Fig. 1). The same phenotype was seen when the bacteria were administered separately (Fig. 2).
Therefore, by infecting mice with mixtures of mutant and wild-type bacteria, we were able to study the effects of the mutations but to use significantly fewer animals; the results were similar to those obtained with individual infections. This method allowed us to investigate the contribution of each of the individual mutations in obl bacteria. The results revealed that aliA, aliB, and amiA all play a role in nasopharyngeal colonization. Depletion of any of the three encoded oligopeptide-binding lipoproteins resulted in pneumococci that were less able to colonize the nasopharynx (Fig. 3). The aliA and amiA genes appeared to play the most important role; a deficiency in aliB had a less significant effect. It was previously shown that AliB is the most important component of Ami-AliA/AliB for oligopeptides containing arginine, whereas AliA and AmiA are more vital for leucine-containing oligopeptides (2). Our results may therefore reflect the nutrients available within the nasopharynx. It remains a possibility that we would have seen different results if we had investigated nasopharyngeal colonization with the individual mutants alone and over a longer period of time.
That the levels of D39 in the nasopharynx did not remain at ca. 104 CFU/ml during invasive disease studies (Fig. 4A), unlike during specific nasopharynx colonization studies (Fig. 1), might be explained by the bacterial inoculum and the host response initiated.
It remains unclear whether the Ami-AliA/AliB oligopeptide-binding lipoproteins are directly involved in binding to cells. It is possible that some indirect effect of an ami-aliA/aliB deficiency reduces adherence, perhaps by altering the expression of distinct proteins involved in adhesion. Such an effect is known for the regulation of the S. gordonii adhesin CshA by the HppA oligopeptide-binding protein (12). An alternative explanation for the lower bacterial loads of Ami-AliA/AliB mutants within the nasopharynx may be that they are impaired in a signaling mechanism that informs them of their environment so that they can adapt accordingly (6). Finally, they may be unable to compete with the normal commensal flora for nutrients. It would be interesting to repeat the amiA, aliA, and aliB mutant colonization studies with other bacteria, for example, Haemophilus influenzae, in this model.
In order to cause disease, the pneumococcus must first colonize the nasopharynx. It is from here that the organism is spread to the lungs via aspiration. We have identified the Ami-AliA/AliB oligopeptide-binding lipoproteins as novel pneumococcal factors responsible for the initial colonization step. It may be possible to target antibacterial agents against the Ami-AliA/AliB proteins to prevent colonization and thus subsequent infection.
Acknowledgments
This study was financially supported by ZonMw-STIGON, The Hague, The Netherlands (grant 014-81-114 to P.V.A. and S.E.).
Editor: J. N. Weiser
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansmann, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alloing, G., P. de Philip, and J.-P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the Gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. [DOI] [PubMed] [Google Scholar]
- 3.Alloing, G., M. C. Trombe, and J.-P. Claverys. 1990. The ami locus of the gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of gram-negative bacteria. Mol. Microbiol. 4:633-644. [DOI] [PubMed] [Google Scholar]
- 4.Blue, C. E., and T. J. Mitchell. 2003. Contribution of a response regulator to the virulence of Streptococcus pneumoniae is strain dependent. Infect. Immun. 71:4405-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blue, C. E., G. K. Paterson, A. R. Kerr, M. Bergé, J.-P. Claverys, and T. J. Mitchell. 2003. ZmpB, a novel virulence factor of Streptococcus pneumoniae that induces tumor necrosis factor alpha production in the respiratory tract. Infect. Immun. 71:4925-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claverys, J.-P., B. Grossiord, and G. Alloing. 2000. Is the Ami-AliA/B oligopeptide permease of Streptococcus pneumoniae involved in sensing environmental conditions? Res. Microbiol. 151:457-463. [DOI] [PubMed] [Google Scholar]
- 7.Cundell, D. R., B. J. Pearce, J. Sandros, A. M. Naughton, and H. R. Masure. 1995. Peptide permeases from Streptococcus pneumoniae affect adherence to eucaryotic cells. Infect. Immun. 63:2493-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1405. [PMC free article] [PubMed] [Google Scholar]
- 9.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. The role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555-571. [DOI] [PubMed] [Google Scholar]
- 12.McNab, R., and J. H. F. 1998. Altered adherence properties of Streptococcus gordonii hppA (oligopeptide permease) mutant result from transcriptional effects on cshA adhesin gene expression. Microbiology 144:127-136. [DOI] [PubMed] [Google Scholar]
- 13.Polissi, A., A. Pontiggia, G. Feger, M. Altieri, H. Mottl, L. Ferrari, and D. Simon. 1998. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 66:5620-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174:994-1000. [DOI] [PubMed] [Google Scholar]
- 15.Wu, H.-Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]