Abstract
Dectin 1 is a mammalian cell surface receptor for (1→3)-β-d-glucans. Since (1→3)-β-d-glucans are commonly present on fungal cell walls, it has been suggested that dectin 1 is important for recognizing fungal invasion. In this study we tried to deduce the amino acid residues in dectin 1 responsible for β-glucan recognition. HEK293 cells transfected with mouse dectin 1 cDNA could bind to a gel-forming (1→3)-β-d-glucan, schizophyllan (SPG). The binding of SPG to a dectin 1 transfectant was inhibited by pretreatment with other β-glucans having a (1→3)-β-d-glucosyl linkage but not by pretreatment with α-glucans. Dectin 1 has a carbohydrate recognition domain (CRD) consisting of six cysteine residues that are highly conserved in C-type lectins. We prepared 32 point mutants with mutations in the CRD and analyzed their binding to SPG. Mutations at Trp221 and His223 resulted in decreased binding to β-glucan. Monoclonal antibody 4B2, a dectin- 1 monoclonal antibody which had a blocking effect on the β-glucan interaction, completely failed to bind the dectin-1 mutant W221A. A mutant with mutations in Trp221 and His223 did not have a collaborative effect on Toll-like receptor 2-mediated cellular activation in response to zymosan. These amino acid residues are distinct from residues in other sugar-recognizing peptide sequences of typical C-type lectins. These results suggest that the amino acid sequence W221-I222-H223 is critical for formation of a β-glucan binding site in the CRD of dectin 1.
Fungi are some of the typical causal microorganisms in opportunistic infections (4). Human immunodeficiency virus patients with lower immunological potentials are frequently infected with Pneumocystis carinii and Candida, which cause P. carinii pneumonia and systemic candidiasis (20, 57). Since these fungi generally contain (1→3)-β-d-glucans in their cell walls (22, 34), it is assumed that the host defense system has certain receptors for (1→3)-β-d-glucans in order to recognize and eliminate fungal cells. Leukocytes, including neutrophils, macrophages, and dendritic cells (DC), possess a specific receptor, dectin 1, for (1→3)-β-d-glucans (8, 53). Dectin 1 is a type II transmembrane protein and has the typical amino acid sequence of C-type lectins (5, 48, 58). The cytoplasmic domain of dectin 1 also has three consecutive acidic amino acid residues that are a putative internalizing signal sequence for the lysosomal endosome (5, 17), and it also has a putative immunoreceptor tyrosine-based activating motif (ITAM)-like region consisting of a YXXL amino acid sequence (5). This ITAM can be phosphorylated by stimulation with particulate β-glucan (24). It has been reported that this phosphorylation can be involved in superoxide production by macrophages (24). Therefore, dectin 1 may contribute not only to phagocytosis of fungal cells but also to induction of fungicidal effector molecules.
(1→3)-β-d-Glucan recognition proteins also have been isolated from invertebrates, including silkworms (41), crayfish (15, 16), earthworms (6), and horseshoe crabs (50, 51, 52), and some of their properties have been reported previously (41, 50). All these recognition proteins participate in triggering a proteolytic cascade by which the host system for defense against microbes may be accelerated (35, 42, 51). However, the binding domains and their (1→3)-β-d-glucan structures have not been fully characterized.
C-type lectins play important roles in the innate immune response by recognizing microbial saccharides (10). The C-type lectins recognize sugar ligands through the carbohydrate recognition domain (CRD) with Ca2+ dependence (19, 38). For instance, mannose binding protein A interacts with a single terminal nonreducing mannose or GlcNAc residue in an oligosaccharide ligand (11, 30). In contrast, DC-SIGN, a well-characterized C-type lectin molecule, binds to an internal mannose residue of the oligosaccharide, and the external saccharides also interact with the surface of DC-SIGN (18). Some C-type lectins expressed by DC have specificity for mannose- and galactose-containing carbohydrates (18, 55). Within the CRD, the highly conserved Glu-Pro-Asn (EPN) and Gln-Pro-Asp (QPD) sequences are essential for recognizing mannose- and galactose-containing ligands (13). Although mouse dectin 1 is also expressed on DC and macrophages, it has no EPN or QPD sequence in the CRD and does not require Ca2+ for the interaction (5, 8). Therefore, it has been suggested that dectin 1 has a recognition mode that is distinct from that of other C-type lectins.
In this study, we prepared a dectin-1 transfectant in order to examine its ability to bind a gel-forming (1→3)-β-d-glucan, schizophyllan (SPG) from Schizophyllum commune. SPG is a fungal (1→3)-β-d-glucan that has a 1,6-β-monoglucosyl branch in every three 1,3-β-glucosyl residues on the main chain (40). SPG is used clinically in Japan for cervical cancer patients in combination with irradiation to enhance the immunological surveillance system (37). It has been reported that SPG augments the cytokine-related immunomodulating activities of lymphocytes and myeloid cells in mice (54).
Binding of biotin-conjugated SPG (SPG-biotin) to the dectin 1 transfectant was inhibited by unlabeled (1→3)-β-d-glucan, suggesting that there is a specific interaction with the (1→3)-β-d-glucosyl structure. One or two amino acid residues of wild-type dectin-1 were replaced with alanine by a PCR-assisted cDNA mutation procedure. Examination of glucan binding to dectin 1 mutants revealed that two amino acids, Trp221 and His223, are important for this specific interaction. A neutralizing monoclonal antibody (MAb) for dectin 1 also failed to interact with the mutant. These critical amino acids are in the third β-sheet strand of a typical C-type lectin structure and are quite different from other amino acids necessary for mannose or galactose interactions. The results of this study suggest that there is a novel sugar interaction moiety of C-type lectins on macrophages and DC.
MATERIALS AND METHODS
Polysaccharides.
SPG (40), a 1,6-branched 1,3-β-glucan from S. commune that has a triple-helix conformation, was purchased from Kaken Pharmaceutical Co. (Tokyo, Japan). Alkali-treated SPG (SPG-OH), which had a single-helix conformation, was prepared by diluting an SPG solution with an equal volume of a 1 M sodium hydroxide solution, followed by dialysis against phosphate-buffered saline (PBS) (43). Grifolan (GRN) from Grifola frondosa (1) and Sclerotinia sclerotiorum glucan (SSG) (45) are also 1,6-branched 1,3-β-glucans. SSG is a water-soluble highly branched 1,3-β-glucan. Solublized GRN was prepared by heating GRN at 150°C as described by Ishibashi et al. (31). CSBG is a soluble part of the NaClO-oxidized cell wall of Candida albicans obtained by dimethyl sulfoxide extraction and was dialyzed against PBS. CSBG has a (1→3)-β-d-glucan with a long 1,6-linked glucosyl side chain (44). Pullulan was purchased from Wako Pure Chemicals (Tokyo, Japan) (26). Curdlan (28), a linear (1→3)-β-d-glucan without a 1,6-glucosyl branch, was purchased from Wako Pure Chemicals and was dissolved in 0.5 M sodium hydroxide, neutralized with 0.5 M HCl, and diluted with 0.05 M Tris-buffered saline (pH 7.2). Laminarin was purchased from Sigma (St. Louis, Mo.) (46). Dextran T-500 was purchased from Pharmacia (Uppsala, Sweden) (47). The physicochemical characteristics of the β-glucans and α-glucans used in this study are shown in Table 1.
TABLE 1.
Physicochemical properties of the polysaccharides used in this study
| Glucan | Source | Primary structurea | Reference |
|---|---|---|---|
| Curdlan | Alcaligenes faecalis | Linear 1,3-β-glucan | 39 |
| Laminarin | Laminaria digitata | Linear 1,3- and 1,6-β-glucan | 40 |
| SPG | Schizophyllum commune | 1,6-Monoglucosyl-branched 1,3-β-glucan | 30 |
| SPG-OH | Schizophyllum commune | 1,6-Monoglucosyl-branched 1,3-β-glucanb | 33 |
| GRN | Grifola frondosa | 1,6-Monoglucosyl-branched 1,3-β-glucanb | 34 |
| SSG | Sclerotinia sclerotiorum | 1,6-Monoglucosyl-branched 1,3-β-glucanb | 35 |
| CSBG | Candida albicans | 1,3-β-glucan with 1,6 long glucosyl side chain | 37 |
| Pullulan | Pullularia pullulans | 1,4- and 1,6-α-glucan | 38 |
| Dextran | Leuconostoc dextranium | 1,3-branched 1,6-α-glucan | 41 |
The primary structure of SPG is as follows: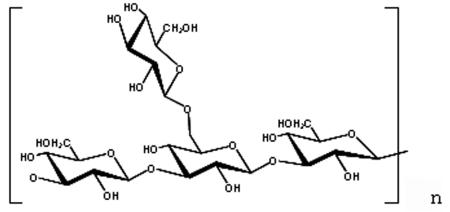
Branching ratio (1,6-glucan/1,3-glucan) of 2/6 for SPG-OH and GRN and 3/6 for SSG.
Preparation of dectin-1 transfectant.
Plasmids containing mouse dectin 1 cDNA were amplified by reverse transcriptase PCR from total RNA prepared from a murine macrophage cell line, RAW264 (RIKEN Cell Bank, Tsukuba, Japan). The coding region for mouse dectin-1 isoform A was inserted into the mammalian expression vector p3xFLAG CMV-14 (Sigma). A stable transfectant was prepared by electroporation of HEK293 (Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan) with a linearized expression vector and was selected from a culture containing Geneticin (Invitrogen). Plasmids for single-amino-acid dectin-1 mutants were constructed by PCR-mediated mutagenesis by using KOD-Plus DNA polymerase (TOYOBO, Osaka, Japan) and DpnI nuclease (Roche). The gene encoding wild-type dectin 1 and mutant cDNAs in the p3xFLAG CMV-14 vector were transduced into HEK293 cells (105 cells/well on six-well plates) by lipofection by using FuGene6 (Roche).
Preparation of SPG-biotin.
The 1,6-monoglucosyl-branched (1→3)-β-d-glucan SPG (Japanese name, Sonifilan) was purchased from Kaken Pharmaceutical Co. SPG was originally manufactured from mycelium culture fluids of S. commune, and it is used clinically as a biological response modifier in Japan. SPG-biotin was prepared as previously described (3). Briefly, SPG was partially periodate oxidized from its side-branched glucose residues, and the resulting aldehyde groups were conjugated with biotin hydrazide (Vector Laboratories) by reductive amination by using sodium cyanoborohydride (Sigma). The biotinylated SPG (SPG-biotin) was dialyzed against PBS containing 0.05% NaN3 and stored at 4°C until it was used.
Analysis of SPG-biotin binding to transfectants.
Dectin-1 transfectants (5 × 105 cells) suspended in 100 μl of 50 mM Tris-buffered saline (pH 7.2) containing 0.5% bovine serum albumin (Sigma) were incubated for 30 min on ice with 50 μg of SPG-biotin per ml and 5 μg of streptavidin-Alexa 488 (Molecular Probes) per ml. The cells were washed and fixed for 10 min on ice with neutral buffer (pH 7.0) containing 4% formaldehyde (Nakarai, Kyoto, Japan), and the cells were subjected to fluorescence-activated cell sorting (FACS) analysis with a FACSCalibur (BD Bioscience). The expression of dectin 1 and mutant molecules on the cell surface was also monitored by immunochemical staining with anti-FLAG BIO-M2 MAb (Sigma) and streptavidin-Alexa 488 (Molecular Probes).
Mutation of dectin 1.
Forward and reverse mutation oligonucleotide primers composed of 30 bases (Sigma Genosys, Tokyo, Japan) were designed with the 14th and/or 15th nucleotide residue replacing code Ala. An expression plasmid vector with wild-type dectin-1 cDNA inserted was replicated by high-fidelity PCR by using KOD-Plus DNA polymerase (TOYOBO) and a mutation primer. The template plasmid in the resultant PCR mixture was incubated for 3 h at 37°C with DpnI (Roche) to digest wild-type cDNA. Sense and antisense DNA were mixed and transformed into Escherichia coli DH5α competent cells. The DNA sequence of the plasmid with the mutation was confirmed with a DNA sequencer (ABI PRISM 310).
Establishment of hybridoma producing an anti-mouse dectin-1 MAb.
The soluble CRD (sCRD) of dectin 1 was obtained from the culture supernatant of a CHO-dhfr− transfectant (American Type Culture Collection), which was prepared by electroporation with an expression vector containing cDNA coding for the CRD of dectin 1 and murine dehydrofolate reductase. Freund complete adjuvant (Difco) and sCRD were emulsified and injected into the footpads of BALB/c mice (Japan SLC, Hamamatsu, Japan). After the third immunization, the draining lymph nodes were teased, and lymphocytes were fused with P3 X63.Ag8.653 myeloma (Cell Resource Center for Biomedical Research, Tohoku University) and cultured with hypoxanthine, aminopterin, and thymidine selection. An antibody-producing hybridoma was screened by an enzyme-linked immunosorbent assay (ELISA) by using 5 μg of sCRD per ml as a coating antigen. 4B2, a hybridoma clone producing rat immunoglobulin G2a/κ (IgG2a/κ), was selected on the basis of the blocking effect of the culture supernatant on the binding of 5 μg of SPG-biotin per ml and 2,000-fold-diluted streptavidin-conjugated peroxidase (Pharmingen) to the sCRD on an ELISA plate. The epitope sharing of MAb 4B2 and SPG on dectin 1 was also confirmed by FACS. Briefly, the FLAG-tagged dectin-1 transfectant was preincubated with 1 mg of SPG per ml for 30 min on ice, and then 4B2 culture supernatant or anti-FLAG BIO-M2 was added and the preparation was incubated for 30 min on ice. After incubation, the cells were washed and stained with anti-rat IgG-biotin and streptavidin-Alexa 488. The cells were washed, fixed, and analyzed by FACS as described above. The binding of 4B2 to the SPG-treated transfectant was significantly decreased. However, staining with a FLAG antibody was not decreased by pretreatment with SPG (data not shown).
Reactivity of an anti-dectin-1 MAb with a dectin-1 mutant.
Transient transfectants (3 × 105 cells) with mutated dectin 1 on HEK293 cells were incubated with dectin-1 MAb 4B2 or anti-FLAG BIO-M2 (10 μg/ml) for 30 min on ice. After washing, anti-rat IgG-biotin (2.5 μg/ml) for 4B2 staining and streptavidin-Alexa 488 (5 μg/ml) were added and incubated for 30 min on ice. The cells were washed, fixed, and subjected to FACS analysis.
Luciferase assays.
The plasmids used for transfection were purified by using an Endo-free plasmid kit (QIAGEN, Chatsworth, Calif.). HEK293 cells were plated (5 × 104 cells/well) in 48-well plates on the day prior to transfection. Transfection was performed by using the FuGene6 reagent (Roche) according to the manufacturer's recommendations for 48-well plates with 150 ng of DNA per well. Each DNA mixture consisted of 50 ng of a reporter plasmid mixture and 100 ng of an expression plasmid for Toll-like receptor (TLR2) and dectin 1. The reporter plasmid mixture consisted of 10 volumes of the pELAM NF-κB-firefly luciferase plasmid and 1 volume of plasmid pRL-TK (Promega) as an internal control for transfection efficiency. The pELAM luciferase vector was a gift from D. Golenbock (University of Massachusetts, Amherst). The transfection mixture was added drop-wise to the cells and incubated for 24 h. The culture was grown in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal calf serum. At 24 h after transfection, the cells were stimulated with various stimulants for 6 h. They were then washed once with PBS and lysed by using passive lysis buffer (Promega). Luciferase activity was measured by using a dual-luciferase reporter gene assay system (Promega). Cells were lysed in lysis buffer (100 μl per well), and 20 μl of lysate was used for each assay. The luciferase assay reagents were added to the lysate with an injector, and the results were immediately read with a luminometer (EG&G Berthold Microlumat Plus). Data are presented below as means ± standard deviations for duplicate samples.
RESULTS
Binding of soluble (1→3)-β-d-glucan to dectin-1 transfectant.
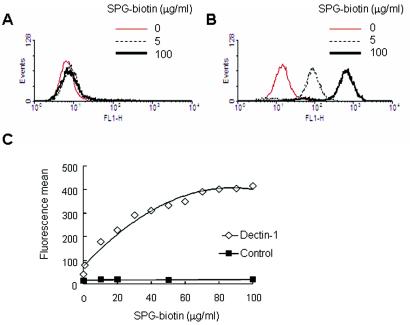
To test whether the dectin-1 transfectant could bind to soluble (1→3)-β-d-glucan, the stable dectin-1 transfectant was incubated with various concentrations of SPG-biotin, and the binding was analyzed by FACS by using streptavidin-Alexa 488 for fluorescence staining. The dectin-1 transfectant showed increased fluorescence intensity with a higher concentration of SPG-biotin (Fig. 1B), and a plateau was reached at a concentration around 70 μg/ml (Fig. 1C); however, control HEK293 cells did not show increased binding of SPG-biotin (Fig. 1A). This suggests that SPG-biotin does indeed bind to dectin 1 in a saturation dose-dependent manner.
FIG. 1.
SPG-biotin can bind to dectin-1-transduced HEK293 cells. (A and B) Non-dectin-1A-transduced cells (A) and dectin-1-transduced cells (B) were treated with 0, 5, and 100 μg of SPG-biotin per ml. (C) Dose dependence of SPG-biotin binding to dectin-1-transduced cells or control cells, as shown by the fluorescence means from a FACS analysis. Dectin-1-transduced cells were incubated with various concentrations of SPG-biotin for 30 min on ice. After preincubation and washing, the amount of SPG-biotin bound to the cells was determined by staining with streptavidin-Alexa 488 conjugate and using a flow cytometer.
Specificity of SPG binding to dectin-1.
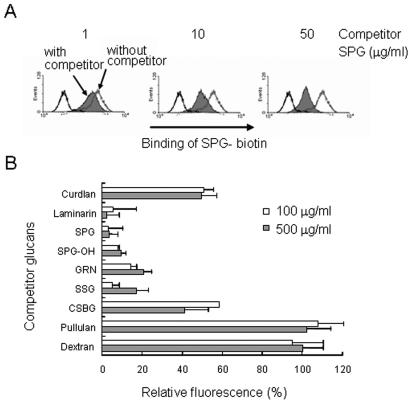
To examine whether the binding of SPG-biotin to the dectin-1 transfectant involved specific binding to the (1→3)-β-d-glucan structure, the cells were pretreated with various polysaccharides and incubated with SPG-biotin (Table 1). The binding of SPG-biotin was monitored by FACS as described above. The fluorescence intensity of the cells significantly decreased during coincubation with laminarin, SPG (Fig. 2A), SPG-OH, GRN, SSG, curdlan, and CSBG, suggesting that the binding of SPG-biotin was specific for the (1→3)-β-d-glucosyl linkage (Fig. 2B). However, preincubation of the transfectant with the α-glucans pullulan and dextran did not alter the binding of SPG-biotin (Fig. 2B). Therefore, the data suggest that this binding assay measured the specific interaction of dectin 1 with (1→3)-β-d-glucans.
FIG. 2.
Binding of SPG-biotin to dectin-1-transduced cells can be inhibited by 1,3-β-glucans. Dectin-1A-transduced HEK293 cells were pretreated with 1, 10, and 50 μg of SPG per ml (A) or with 100 and 500 μg of various competitor glucans per ml (B) for 30 min on ice. After washing, the cells were treated with 50 μg of SPG-biotin per ml. (A) Amount of SPG binding to the cells, as shown by a histogram of flow cytometry data. (B) Amount of SPG binding quantitated by flow cytometry and expressed as the mean fluorescence relative to that of an uninhibited control (100%). The background value (0%) was obtained with nontransduced HEK293 cells treated with 50 μg of SPG-biotin per ml. The data are the averages of the means of two independent experiments, and the error bars indicate the standard errors of the means.
Examination of the binding of dectin-1 mutants to SPG.
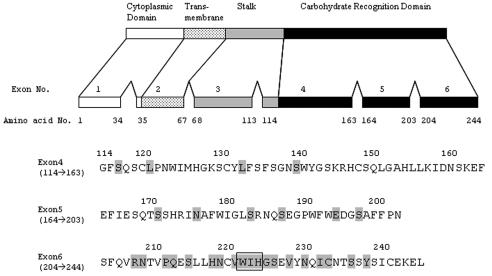
In human dectin 1, there are several splice variants in the CRD (58). Only two splice variants, isoforms A and B, can bind zymosan particles containing β-glucans, mannan, and proteins. As determined by a comparison of the amino acid sequences of the variants, exons 5 and 6 may be critical for the recognition of β-glucan. Therefore, we tried to identify the amino acid in the CRD that is important for glucan binding by replacing a single amino acid residue, especially around exon 6. The mutated residues used are shown in Fig. 3.
FIG. 3.
Schematic representation of murine dectin 1A. The residues are numbered beginning with the initial codon. The numbers 114 to 244 in the upper panel indicate positions in the CRD in the complete molecule. The sequence of amino acid residues 114 to 244, which include exons 4, 5, and 6 of murine dectin 1A, is shown in the lower panel. Mutations of single amino acid residues by Ala are indicated by shading. The boxed amino acid residues are the double-amino-acid replacement to Ala mutation (Trp/Ile, Ile/His, and Trp/His).
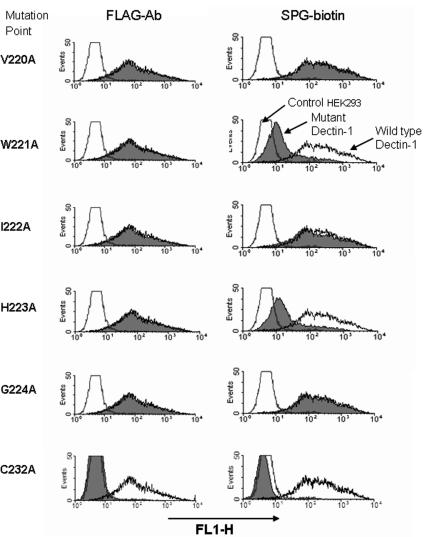
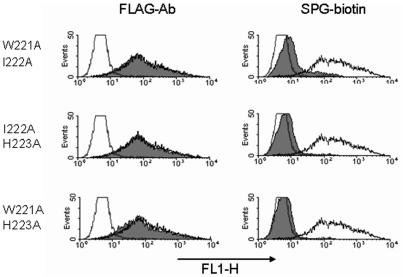
We confirmed that all mutants were expressed on the cell surface by performing FACS with an anti-FLAG-tag antibody. Only one mutant, C232A, did not bind to the anti-FLAG antibody (Fig. 4), implying that the C232 residue is necessary for consistent expression of the molecule on the cell surface. The abilities of mutants to bind SPG-biotin were extensively examined by FACS. As shown in Fig. 4, the W221A or H223A mutation resulted in less binding to SPG-biotin; however, the V220A, I222A, and V224A mutants exhibited binding similar to that of the wild type. In addition to W221A and H223A, the W221A-I222A and W221A-H223A double mutants exhibited no binding to SPG-biotin (Fig. 5). These results indicate that the Trp221 or His223 residue after the fourth cysteine residue of the CRD is important for formation of the β-glucan binding domain.
FIG. 4.
Effects of dectin-1 point mutations on binding to SPG-biotin. Mutant dectin 1A cDNA inserted into a FLAG-tagged expression vector was transiently transduced into HEK293 cells by using FuGene 6. The levels of expression of the FLAG tag in various dectin-1 mutants were monitored by using anti-FLAG MAb. The cells were also tested for the ability to bind SPG-biotin. The histograms show representative results for single point mutations for 29 mutants tested in this study. Reproducible results were obtained in three independent experiments. The shaded histograms show the results for mutations in dectin 1. The open histograms show the results for wild-type dectin 1 (high fluorescence) and the control vector (low fluorescence).
FIG. 5.
Double-amino-acid replacement in dectin 1 reduced binding to SPG-biotin. The levels of expression of various dectin-1 mutants were monitored by using anti-FLAG MAb. Binding of SPG-biotin to the mutants was also examined. The shaded histograms show the results for dectin-1 mutants. The open histograms show the results for wild-type dectin-1-transduced cells (high fluorescence) and control vector-transduced cells (low fluorescence).
Reactivity of dectin-1 MAb 4B2 with dectin-1 mutants.
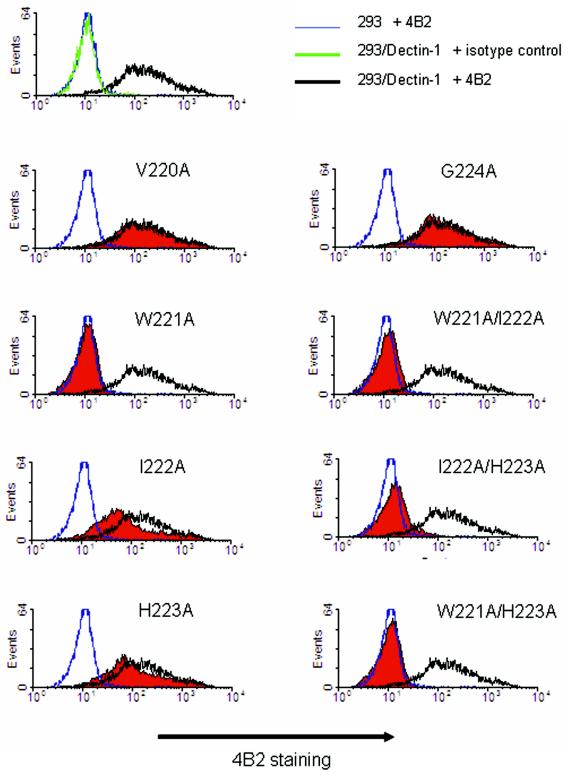
The reactivity of dectin-1 mutants with the neutralizing the antibody for murine dectin-1 was also examined by FACS. An anti-murine dectin-1 MAb, MAb 4B2, was prepared. This antibody was selected from hybridoma clones because of its blocking effect on the binding of SPG-biotin to the soluble form of dectin-1 on an ELISA plate. Cytochemical staining of dectin-1 mutants with 4B2 was verified by FACS. As shown in Fig. 6, 4B2 staining was prevented by replacing Trp221 with Ala. I222A and H223A also resulted in slightly reduced staining with 4B2. However, other single-amino-acid mutants, including the V220A and G224A mutants, did not exhibit reduced staining. The W221A-I222A and W221A-H223A double mutations resulted in no reactivity with 4B2, as expected. In addition to the reactivities of the double mutants which included the W221A residue, the reactivity of an I222A-H223A mutant with 4B2 was also diminished (Fig. 6). These results support the hypothesis that Trp221 and surrounding amino acid residues are critical for the formation of β-glucan binding sites.
FIG. 6.
Anti-mouse dectin-1 antibody 4B2 recognizes the glucan binding site of dectin 1. The levels of expression of various dectin-1 mutants were monitored by using anti-FLAG MAb. The cells were also assessed to determine their reactivities to dectin-1 MAb 4B2. Dectin-1-transduced cells were incubated with MAb 4B2 for 30 min on ice. After washing, anti-rat IgG-biotin for 4B2 staining and streptavidin-Alexa 488 were added and incubated for 30 min on ice. The cells were analyzed by flow cytometry after antibody staining. The shaded histograms show the results for dectin-1 mutants. The open histograms with the black, blue, and green lines show the results for wild-type dectin-1-transduced cells plus 4B2, control vector-transduced cells plus 4B2, and wild-type dectin-1-transduced cells plus isotype control antibody, respectively.
W221A-H223A mutation failed to activate the NF-κB nuclear factor in response to zymosan.
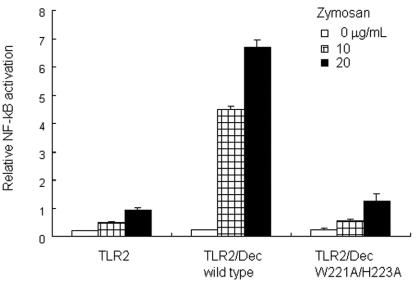
It has been reported that coligation of dectin 1 and TLR2 by a yeast cell wall preparation, zymosan, enhances TLR2-mediated NF-κB activation. The concurrent stimulation by zymosan through dectin 1 and TLR2 was reduced by pretreatment with antagonistic soluble (1→3)-β-d-glucans, and this implied that the dectin-1-mediated recognition of the (1→3)-β-d-glucan moiety in zymosan is important. To examine the effect of the W221A-H223A mutation in dectin 1 on the TLR2-mediated NF-κB activation by zymosan, HEK293 cells were transduced with a reporter plasmid for NF-κB binding motif-conjugated luciferase cDNA and with expression vectors containing cDNA encoding murine TLR2 and the dectin-1 mutant. As shown in Fig. 7, wild-type dectin-1- and TLR2-transduced cells showed significantly higher NF-κB activation than TLR2-transduced cells during stimulation with zymosan. However, transduction of the dectin-1 mutant in TLR2-transduced cells did not increase the NF-κB activation in response to zymosan. These results suggest that the W221A-H223A mutation functionally affects the cellular activation by zymosan.
FIG. 7.
Dectin-1 mutant did not augment TLR2-mediated NF-κB activation in response to zymosan. HEK293 cells were transfected with TLR2- and dectin-1 (Dec)-expressing plasmids and an ELAM-1 promoter-conjugated luciferase reporter plasmid and were stimulated with zymosan, a yeast cell wall preparation, containing 1,3-β-glucan. NF-kB activation was monitored by the luciferase reporter assay as described in Materials and Methods. Mutation of Trp221 and His223 in the CRD of dectin 1 that resulted in replacement of both residues by alanine resulted in decreased NF-κB activation in response to zymosan.
Characteristics of negative dectin-1 mutants.
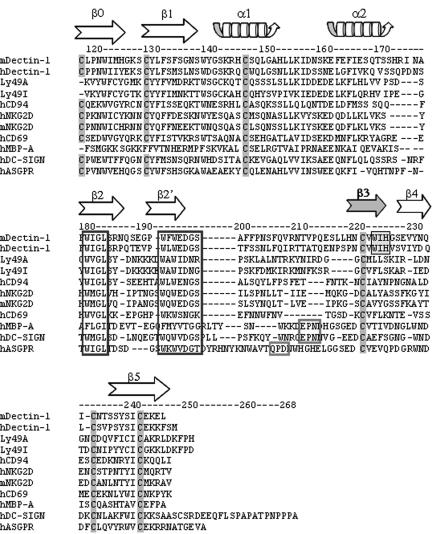
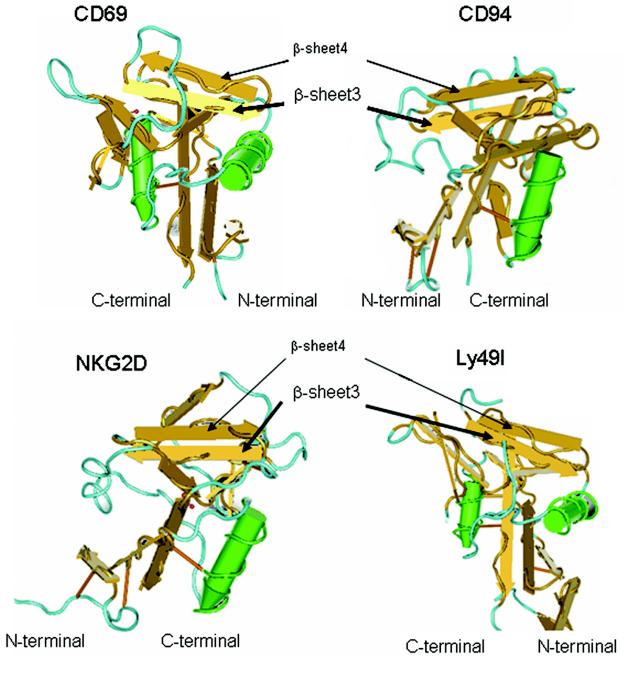
The results described above indicate that the peptide sequence around Trp221 is important for ligand binding. This portion of the molecule is located between the fourth and fifth cysteine residues of the CRD. The amino acid sequence of human dectin 1 is significantly similar to that of mouse dectin 1 (54% identity and 64% similarity). Sequential amino acid residues, including the fourth cysteine at positions 219 and 220 of humans and rhesus monkeys (human Cys219 and rhesus monkey Cys220), human Val220 and rhesus monkey Val221, human Trp221 and rhesus monkey Trp222, human Ile222 and rhesus monkey Ile223, and human His223 and rhesus monkey His224, are conserved in both mice and humans or rhesus monkeys (12). Six cysteine residues are highly conserved in the CRD of C-type lectin (14). It has been demonstrated that three pairs of cysteine residues (the first and second residues, the third and sixth residues, and the fourth and fifth residues) form disulfide bonds (14). In the case of C-type lectins belonging to the NK receptor gene family, such as CD94, NKG2D, and CD69, the CRD has a series of secondary structures consisting of β-sheet 0, β-sheet 1, α-helix 1, α-helix 2, β-sheet 2, β-sheet 3, β-sheet 4, and β-sheet 5 (39). Two of these secondary structures, β-sheets 3 and 4, are between the fourth and fifth cysteine residues (Fig. 8) (14). β-Sheets 3 and 4 create double-stranded antiparallel β-sheet structures and form an interface for major histocompatibility complex (MHC) class I molecules (39). By superimposing the amino acid residues of dectin 1 in the sequence of the C-type lectin NK receptor family, Trp221 should be localized on β-sheet 3 (Fig. 9). Although X-ray crystallography of dectin 1 has not been performed yet, the putative β-sheet 3 in the CRD may form a critical ultrastructure for interacting with β-glucans.
FIG. 8.
Alignment of the dectin-1 CRD sequence with related C-type lectin CRD sequences. Highly conserved cysteine residues in CRD and β-glucan binding sites (WIH) in dectin 1 are shaded. Homologous amino acid residues involved in mannose and galactose binding and conserved amino acid sequences are enclosed in boxes. The arrows show the β-sheet strand structure of C-type lectin NK-receptors. m, mouse; h, human; MBP-A, mannose binding protein A.
FIG. 9.
Typical ultrastructure of the C-type lectin NK-receptor family. The ultrastructures of C-type lectin NK receptors CD69 (Protein Data Bank identification number, 1FM5), CD94 (Protein Data Bank identification number, 1B6E), NKG2D (Protein Data Bank identification number, 1MPU), and Ly49I (Protein Data Bank identification number, 1JA3) were visualized by using the Cn3D software, version 4.1, on the Entrez structure web server. Antiparallel β-sheets 3 and 4, which are highly conserved in other C-type lectin NK receptors, are located in the apical region of each molecule (39). Trp221 and His223 in the CRD of dectin 1 were assumed to be on β-sheet 3.
DISCUSSION
In this study we demonstrated that dectin 1 can bind soluble (1→3)-β-d-glucan, and this binding is influenced by replacement of Trp-Ile-His residues around β-sheet 3 following the fourth cysteine residue in the CRD. Since (1→3)-β-d-glucan is a major cell wall component of fungi (22, 32, 34), it has been thought that the host defense system may be equipped with specific proteins to recognize (1→3)-β-d-glucans (27). Some (1→3)-β-d-glucan recognition proteins in plants (21, 36), insects (35, 41), and conchostracans (49, 50, 51, 56, 59, 60) have been identified. β-Glucan recognition proteins from silkworms (41), earthworms (6), and horseshoe crabs (50) commonly contain a β-glucanase-like domain. However, the recognition moieties of the proteins from silkworms and horseshoe crabs are composed of about 100 to 130 amino acid residues and are distinct from the β-glucanase-like domain (41, 50). Furthermore, it has not been determined whether the recognition moieties have a characteristic amino acid sequence. Thus, the recognition sites of these proteins have not been fully elucidated.
Recently, a β-glucan recognition protein, dectin 1, was found in vertebrates (8, 29, 58). The specificity of dectin 1 for (1→3)-β-d-glucans is shown by the inhibitory effect of soluble (1→3)-β-d-glucans on binding to the yeast form of C. albicans and zymosan from the cell wall of Saccharomyces cerevisiae (8, 9). The results of this study suggest that the binding of a gel-forming immunostimulatory β-d-glucan, SPG, to dectin 1 is specific for the (1→3)-β-d-glucosyl linkage (Fig. 2B). SPG possesses a 1,6-monoglucosyl branch on a (1→3)-β-glucosyl main chain, which forms a triple-helix conformation in a physiological solution. The conformation is formed by hydrogen bonding of C-2 hydroxyl groups of main-chain glucosyl residues. Alkaline treatment of triple-helix (1→3)-β-d-glucans results in a conformational change in the random coil structure, and subsequent neutralization results in a single-helix conformation or a partially opened triple-helix conformation (62). Therefore, the significant differences between the triple- and single-helix or partially opened conformations include the accessibility to C-2 hydroxyl groups of the main-chain strand. The SPG-OH used in this study was prepared by alkaline treatment and neutralization to form a single-helix conformation. The inhibitory effect of SPG-OH on the binding of SPG-biotin to dectin-1-transduced cells was almost identical to that of SPG (Fig. 2B). This result implies that dectin 1 may not recognize the C-2 hydroxyl group of the (1→3)-β-d-glucosyl linkage. Dectin 1 may contact other reactive groups, such as C-4 and C-6 hydroxyl groups or glucosyl linkages, on the (1→3)-β-glucosyl main chain.
Many C-type lectins have been identified on DC, and these lectins are represented by DC-SIGN, dectin 2, langerin, BCDA-2, DCIR, DLEC, CLEC-1, CLEC-2, and DC-ASGPR (25). In the CRD of most C-type lectins, the highly conserved EPN and QPD sequences are essential for recognizing mannose- and galactose-containing structures with Ca2+ dependence, respectively (13). On the other hand, murine dectin 1 has no Ca2+ dependence for ligand binding and no EPN or QPD sequences (5). The results obtained in this study suggest that recognition of β-d-glucan involves an amino acid sequence that is distinct from the amino acid sequences of other C-type lectins. NK cells also possess C-type lectins as NK receptors for recognizing MHC class I proteins, which are represented by Ly49A, Ly49I, NKG2D, CD69, and CD94 (39). The ultrastructure of the CRD of NK receptors has been well characterized by crystallography (Fig. 9) (39). Most of the CRD of NK receptors exits as disulfide-linked homo- or heterodimers at the cell surface (7, 33). The CRD subunit associates through the first β-strand, β0, creating one extended six-strand anti-parallel β-sheet (Fig. 8) (39). It has been deduced that β-sheet strands β3 and β4 are located on top of the CRD subunits with which MHC class I molecules are recognized (Fig. 9) (39).
Gene investigation of human dectin-1 molecules has suggested that there are at least nine splicing variants in humans (61). Two variants, isoforms A and B, are similar to mouse dectin 1A and 1B, which lack the stalk region of isoform A (5). These variants contain an intact CRD that acts as a β-glucan recognition protein (58). However, isoform C lacking the exon 5 region of isoforms A and B is not able to bind to β-glucans (58). This finding implies that the exon 5 or 6 region of dectin 1A and 1B may result in a functional polypeptide for recognizing β-glucans. Therefore, in our study we intensively focused on mutating these two exons, especially exon 6. An Ala mutation at the Trp221 and His223 residues affected the interaction with SPG (Fig. 4). These amino acid residues may be located on β-sheet 3, which is encoded in exon 6 of the CRD (Fig. 8). If the ultrastructure of the CRD in dectin 1 is similar to that of other C-type lectins, the β-glucan recognition site is presumably on the top surface of the CRD molecule (Fig. 9).
MAb 4B2 was prepared in this study by screening its inhibitory effect on SPG binding to dectin 1. The 4B2 staining of dectin-1 mutants was also examined by FACS. The reactivity of MAb 4B2 with dectin-1 mutants with single amino acid replacements was reduced by mutation of Trp221 (Fig. 6). However, another inactive mutant for SPG binding, the H223A mutant, remained antigenic for 4B2. The I222A mutant also showed significant reactivity for 4B2 staining. An additional mutant in which Ile222 of the H223A mutant was replaced by Ala exhibited significantly decreased reactivity with 4B2. These results suggest that the amino acid side chains of Ile222 and His223 may not directly contact a complementary determining region of 4B2 immunoglobulin but may affect the conformation of the region, including Trp221 of dectin 1.
The binding of a particulate β-glucan preparation resulted in production of reactive oxygen species by macrophages (2, 23). It has been reported that dectin-1 ligation with zymosan results in tyrosine phosphorylation of the receptor's ITAM-like signaling motif, generating intracellular signals that mediate phagocytosis and activation of NADPH oxidase (12), which contributes to microbial death. Furthermore, the concurrent engagement of dectin 1 enhances TLR2-mediated cell activation in combination with lipopeptide and a β-glucan-enriched zymosan derivative (24). As shown in Fig. 7, we also examined whether mutation of the CRD, especially at W221A-H223A, affected the cellular activation by zymosan. Mutation of both Trp221 and His223 of the dectin-1 molecule resulted in a significant loss of the enhancing effect on TLR2-mediated NF-κB activation, suggesting that these amino acid residues contribute to formation of the functional dectin-1 molecule.
In addition to the inflammatory response, dectin 1 binds to T lymphocytes and augments their mitogenic response by cross-linking T-cell receptors (5). The binding domain on dectin-1 molecules for T-cell activation has not been clarified, although it has been confirmed that β-glucan binding does not interfere with T-cell interactions (58). Recognition of β-glucan and immunogenic substances from a pathogen by antigen-presenting cells such as DC and macrophages may augment pathogen-specific T-cell activation. Ligation of dectin 1 may therefore modulate various immunological responses for infectious diseases, not only by activation of innate immunity but also by antigen-specific acquired immunity. Our results suggest that the β-glucan binding domain is located around putative β-sheet 3 in the CRD. Although the crystal structure of the dectin-1 protein is still unclear, the information obtained in this study may point the way for exploration of a specific site for the T-cell interaction, which is a tantalizing problem.
Acknowledgments
We thank Naoki Hara, Yuya Kato, and Naoki Matsutani for their technical assistance.
This work was supported by a grant for private universities from the Ministry of Education, Culture, Sports, Science, and Technology and by the Japan Private School Promotion Foundation.
Editor: T. R. Kozel
REFERENCES
- 1.Adachi, Y., N. Ohno, M. Ohsawa, K. Sato, S. Oikawa, and T. Yadomae. 1989. Physiochemical properties and antitumor activities of chemically modified derivatives of antitumor glucan “grifolan LE” from Grifola frondosa. Chem. Pharm. Bull. (Tokyo) 37:1838-1843. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, Y., N. Ohno, and T. Yadomae. 1993. Inhibitory effect of β-glucans on zymosan-mediated hydrogen peroxide production by murine peritoneal macrophages in vitro. Biol. Pharm. Bull. 16:462-467. [DOI] [PubMed] [Google Scholar]
- 3.Adachi, Y., N. Ohno, and T. Yadomae. 1994. Preparation and antigen specificity of an anti-(1→3)-β-d-glucan antibody. Biol. Pharm. Bull. 17:1508-1512. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14:S43-S53. [DOI] [PubMed] [Google Scholar]
- 5.Ariizumi, K., G. L. Shen, S. Shikano, S. Xu, R. Ritter III, T. Kumamoto, D. Edelbaum, A. Morita, P. R. Bergstresser, and A. Takashima. 2000. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275:20157-20167. [DOI] [PubMed] [Google Scholar]
- 6.Beschin, A., M. Bilej, F. Hanssens, J. Raymakers, E. Van Dyck, H. Revets, L. Brys, J. Gomez, P. Timmermans, and M. De Baetselier. 1998. Identification and cloning of a glucan- and lipopolysaccharide-binding protein from Eisenia foetida earthworm involved in the activation of prophenoloxidase cascade. J. Biol. Chem. 273:24948-24954. [DOI] [PubMed] [Google Scholar]
- 7.Borrego, F., J. Kabat, D. K. Kim, L. Lieto, K. Maasho, J. Pena, R. Solana, and J. E. Coligan. 2002. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol. Immunol. 38:637-660. [DOI] [PubMed] [Google Scholar]
- 8.Brown, G. D., and S. Gordon. 2001. Immune recognition. A new receptor for β-glucans. Nature 413:36-37. [DOI] [PubMed] [Google Scholar]
- 9.Brown, G. D., P. R. Taylor, D. M. Reid, J. A. Willment, D. L. Williams, L. Martinez-Pomares, S. Y. Wong, and S. Gordon. 2002. Dectin 1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cambi, A., and C. G. Figdor. 2003. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell Biol. 15:539-546. [DOI] [PubMed] [Google Scholar]
- 11.Childs, R. A., T. Feizi, C. T. Yuen, K. Drickamer, and M. S. Quesenberry. 1990. Differential recognition of core and terminal portions of oligosaccharide ligands by carbohydrate-recognition domains of two mannose-binding proteins. J. Biol. Chem. 265:20770-20777. [PubMed] [Google Scholar]
- 12.Choi, Y. K., B. A. Fallert, M. A. Murphey-Corb, and T. A. Reinhart. 2003. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101:1684-1691. [DOI] [PubMed] [Google Scholar]
- 13.Drickamer, K. 1992. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 360:183-186. [DOI] [PubMed] [Google Scholar]
- 14.Drickamer, K., and R. B. Dodd. 1999. C-type lectin-like domains in Caenorhabditis elegans: predictions from the complete genome sequence. Glycobiology 9:1357-1369. [DOI] [PubMed] [Google Scholar]
- 15.Duvic, B., and K. Soderhall. 1992. Purification and partial characterization of a β-1,3-glucan-binding-protein membrane receptor from blood cells of the crayfish Pacifastacus leniusculus. Eur. J. Biochem. 207:223-228. [DOI] [PubMed] [Google Scholar]
- 16.Duvic, B., and K. Soderhall. 1990. Purification and characterization of a β-1,3-glucan binding protein from plasma of the crayfish Pacifastacus leniusculus. J. Biol. Chem. 265:9327-9332. [PubMed] [Google Scholar]
- 17.Engering, A., T. B. Geijtenbeek, S. J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C. G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118-2126. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163-2166. [DOI] [PubMed] [Google Scholar]
- 19.Figdor, C. G., Y. van Kooyk, and G. J. Adema. 2002. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 20.Fisherman, J. A. 1992. Pneumocystis carinii pneumonia, p. 263-286. In A. P. Fisherman (ed.), Update: pulmonary diseases and disorders. McGraw-Hill, New York, N.Y.
- 21.Fliegmann, J., A. Mithofer, G. Wanner, and J. Ebel. 2004. An ancient enzyme domain hidden in the putative β-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 279:1132-1140. [DOI] [PubMed] [Google Scholar]
- 22.Fontaine, T., I. Mouyna, R. P. Hartland, S. Paris, and J. P. Latge. 1996. From the surface to the inner layer of the fungal cell wall. Biochem. Soc. Trans. 25:194-199. [DOI] [PubMed] [Google Scholar]
- 23.Gallin, E. K., S. W. Green, and M. L. Patchen. 1992. Comparative effects of particulate and soluble glucan on macrophages of C3H/HeN and C3H/HeJ mice. Int. J. Immunopharmacol. 14:173-183. [DOI] [PubMed] [Google Scholar]
- 24.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 26.Glinel, K., J. P. Sauvage, H. Oulyadi, and J. Huguet. 2000. Determination of substituents distribution in carboxymethylpullulans by NMR spectroscopy. Carbohydr. Res. 328:343-354. [DOI] [PubMed] [Google Scholar]
- 27.Gordon, S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927-930. [DOI] [PubMed] [Google Scholar]
- 28.Harada, T., A. Misaki, and H. Saito. 1968. Curdlan: a bacterial gel-forming β-1,3-glucan. Arch. Biochem. Biophys. 124:292-298. [DOI] [PubMed] [Google Scholar]
- 29.Hermanz-Falcon, P., I. Arce, P. Roda-Navarro, and E. Fernandez-Ruiz. 2001. Cloning of human DECTIN-1, a novel C-type lectin-like receptor gene expressed on dendritic cells. Immunogenetics 53:288-295. [DOI] [PubMed] [Google Scholar]
- 30.Iobst, S. T., M. R. Wormald, W. I. Weis, R. A. Dwek, and K. Drickamer. 1994. Binding of sugar ligands to Ca2+-dependent animal lectins. I. Analysis of mannose binding by site-directed mutagenesis and NMR. J. Biol. Chem. 269:15505-15511. [PubMed] [Google Scholar]
- 31.Ishibashi, K., N. N. Miura, Y. Adachi, N. Ohno, and T. Yadomae. 2001. Relationship between solubility of grifolan, a fungal 1,3-β-d-glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. 65:1993-2000. [DOI] [PubMed] [Google Scholar]
- 32.Kapteyn, J. C., R. C. Montijn, G. J. Dijkgraaf, H. Van den Ende, and F. M. Klis. 1995. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J. Bacteriol. 177:3788-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanier, L. L., C. Chang, and J. H. Phillips. 1994. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J. Immunol. 153:2417-2428. [PubMed] [Google Scholar]
- 34.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, C., and M. R. Kanost. 2000. A β1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J. Biol. Chem. 275:7505-7514. [DOI] [PubMed] [Google Scholar]
- 36.Mithofer, A., J. Fliegmann, G. Neuhaus-Url, H. Schwarz, and J. Ebel. 2000. The hepta-β-glucoside elicitor-binding proteins from legumes represent a putative receptor family. Biol. Chem. 381:705-713. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki, K., H. Mizutani, H. Katabuchi, K. Fukuma, S. Fujisaki, and H. Okamura. 1995. Activated (HLA-DR+) T-lymphocyte subsets in cervical carcinoma and effects of radiotherapy and immunotherapy with sizofiran on cell-mediated immunity and survival. Gynecol. Oncol. 56:412-420. [DOI] [PubMed] [Google Scholar]
- 38.Mullin, N. P., P. G. Hitchen, and M. E. Taylor. 1997. Mechanism of Ca2+ and monosaccharide binding to a C-type carbohydrate-recognition domain of the macrophage mannose receptor. J. Biol. Chem. 272:5668-5681. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan, K., N. Dimasi, J. Wang, R. A. Mariuzza, and D. H. Margulies. 2002. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 20:853-885. [DOI] [PubMed] [Google Scholar]
- 40.Norisuye, T., T. Yanaki, and H. Fujita. 1980. Triple-helix of a Schizophyllum commune polysaccharide in aqueous solution. J. Polym. Sci. 18:547-558. [Google Scholar]
- 41.Ochiai, M., and M. Ashida. 2000. A pattern-recognition protein for β-1,3-glucan. The binding domain and the cDNA cloning of β-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem. 275:4995-5002. [DOI] [PubMed] [Google Scholar]
- 42.Ochiai, M., and M. Ashida. 1988. Purification of a β-1,3-glucan recognition protein in the prophenoloxidase activating system from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 263:12056-12062. [PubMed] [Google Scholar]
- 43.Ohno, N., T. Hashimoto, Y. Adachi, and T. Yadomae. 1996. Conformation dependency of nitric oxide synthesis of murine peritoneal macrophages by β-glucans in vitro. Immunol. Lett. 52:1-7. [DOI] [PubMed] [Google Scholar]
- 44.Ohno, N., M. Uchiyama, A. Tsuzuki, K. Tokunaka, N. N. Miura, Y. Adachi, M. W. Aizawa, H. Tamura, S. Tanaka, and T. Yadomae. 1999. Solubilization of yeast cell-wall β-(1→3)-d-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 316:161-172. [DOI] [PubMed] [Google Scholar]
- 45.Ohno, N., and T. Yadomae. 1987. Two different conformations of the antitumor β-d-glucan produced by Sclerotinia sclerotiorum IFO 9395. Carbohydr. Res. 159:293-302. [DOI] [PubMed] [Google Scholar]
- 46.Read, S. M., G. Currie, and A. Bacic. 1996. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 281:187-201. [DOI] [PubMed] [Google Scholar]
- 47.Seymour, F. R., R. D. Knapp, and S. H. Bishop. 1976. Determination of the structure of dextran by 13C-nuclear magnetic resonance spectroscopy. Carbohydr. Res. 51:179-194. [DOI] [PubMed] [Google Scholar]
- 48.Sobanov, Y., A. Bernreiter, S. Derdak, D. Mechtcheriakova, B. Schweighofer, M. Duchler, F. Kalthoff, and E. Hofer. 2001. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur. J. Immunol. 31:3493-3503. [DOI] [PubMed] [Google Scholar]
- 49.Sritunyalucksana, K., S. Y. Lee, and K. Soderhall. 2002. A β-1,3-glucan binding protein from the black tiger shrimp, Penaeus monodon. Dev. Comp. Immunol. 26:237-245. [DOI] [PubMed] [Google Scholar]
- 50.Takaki, Y., N. Seki, S. Kawabata, S. Iwanaga, and T. Muta. 2002. Duplicated binding sites for (1→3)-β-d-glucan in the horseshoe crab coagulation factor G: implications for a molecular basis of the pattern recognition in innate immunity. J. Biol. Chem. 277:14281-14287. [DOI] [PubMed] [Google Scholar]
- 51.Tamura, H., S. Tanaka, T. Oda, Y. Uemura, J. Aketagawa, and Y. Hashimoto. 1996. Purification and characterization of a (1→3)-β-d-glucan-binding protein from horseshoe crab (Tachypleus tridentatus) amoebocytes. Carbohydr. Res. 295:103-116. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka, S., J. Aketagawa, S. Takahashi, Y. Shibata, Y. Tsumuraya, and Y. Hashimoto. 1993. Inhibition of high-molecular-weight-(1→3)-β-d-glucan-dependent activation of a limulus coagulation factor G by laminaran oligosaccharides and curdlan degradation products. Carbohydr. Res. 244:115-127. [DOI] [PubMed] [Google Scholar]
- 53.Taylor, P. R., G. D. Brown, D. M. Reid, J. A. Willment, L. Martinez-Pomares, S. Gordon, and S. Y. Wong. 2002. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169:3876-3882. [DOI] [PubMed] [Google Scholar]
- 54.Tsuchiya, Y., M. Igarashi, M. Inoue, and K. Kumagai. 1989. Cytokine-related immunomodulating activities of an anti-tumor glucan, Sizofiran (SPG). J. Pharmacobio-Dyn. 12:616-625. [DOI] [PubMed] [Google Scholar]
- 55.Valladeau, J., V. Duvert-Frances, J. J. Pin, M. J. Kleijmeer, S. Ait-Yahia, O. Ravel, C. Vincent, F. Vega, Jr., A. Helms, D. Gorman, S. M. Zurawski, G. Zurawski, J. Ford, and S. Saeland. 2001. Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J. Immunol. 167:5767-5774. [DOI] [PubMed] [Google Scholar]
- 56.Vargas-Albores, F., F. Jimenez-Vega, and G. M. Yepiz-Plascencia. 1997. Purification and comparison of β-1,3-glucan binding protein from white shrimp (Penaeus vannamei). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 116:453-458. [DOI] [PubMed] [Google Scholar]
- 57.Walsh, T. J., C. Gonzalez, E. Roilides, B. U. Mueller, N. Ali, L. L. Lewis, T. O. Whitcomb, D. J. Marshall, and P. A. Pizzo. 1995. Fungemia in children infected with the human immunodeficiency virus: new epidemiologic patterns, emerging pathogens, and improved outcome with antifungal therapy. Clin. Infect. Dis. 20:900-906. [DOI] [PubMed] [Google Scholar]
- 58.Willment, J. A., S. Gordon, and G. D. Brown. 2001. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276:43818-43823. [DOI] [PubMed] [Google Scholar]
- 59.Yepiz-Plascencia, G., T. G. Galvan, F. Vargas-Albores, and M. Garcia-Banuelos. 2000. Synthesis of hemolymph high-density lipoprotein β-glucan binding protein by Penaeus vannamei shrimp hepatopancreas. Mar. Biotechnol. (New York) 2:485-492. [DOI] [PubMed] [Google Scholar]
- 60.Yepiz-Plascencia, G., F. Vargas-Albores, F. Jimenez-Vega, L. M. Ruiz-Verdugo, and G. Romo-Figueroa. 1998. Shrimp plasma HDL and β-glucan binding protein (BGBP): comparison of biochemical characteristics. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 121:309-314. [DOI] [PubMed] [Google Scholar]
- 61.Yokota, K., A. Takashima, P. R. Bergstresser, and K. Ariizumi. 2001. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene 272:51-60. [DOI] [PubMed] [Google Scholar]
- 62.Young, S. H., W. J. Dong, and R. R. Jacobs. 2000. Observation of a partially opened triple-helix conformation in 1→3-β-glucan by fluorescence resonance energy transfer spectroscopy. J. Biol. Chem. 275:11874-11879. [DOI] [PubMed] [Google Scholar]