Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous and often carcinogenic contaminants released into the environment during natural and anthropogenic combustion processes. Benzo[a]pyrene (B[a]P) is the prototypical carcinogenic PAH, and dibenzo[def,p]chrysene (DBC) is a less prevalent, but highly potent transplacental carcinogenic PAH. Both are metabolically activated by isoforms of the cytochrome P450 enzyme superfamily to form reactive carcinogenic and cytotoxic metabolites. Metabolism of B[a]P and DBC was studied in hepatic microsomes of male Sprague-Dawley rats, naïve and pregnant female B6129SF1/J mice, and female humans, corresponding to available pharmacokinetic data. Michaelis–Menten saturation kinetic parameters including maximum rates of metabolism (VMAX, nmol/min/mg microsomal protein), affinity constants (KM, μM), and rates of intrinsic clearance (CLINT, ml/min/kg body weight) were calculated from substrate depletion data. CLINT was also estimated from substrate depletion data using the alternative in vitro half-life method. VMAX and CLINT were higher for B[a]P than DBC, regardless of species. Clearance for both B[a]P and DBC was highest in naïve female mice and lowest in female humans. Clearance rates of B[a]P and DBC in male rat were more similar to female human than to female mice. Clearance of DBC in liver microsomes from pregnant mice was reduced compared to naïve mice, consistent with reduced active P450 protein levels and elevated tissue concentrations and residence times for DBC observed in previous in vivo pharmacokinetic studies. These findings suggest that rats are a more appropriate model organism for human PAH metabolism, and that pregnancy's effects on metabolism should be further explored.
Keywords: Benzo[a]pyrene; Dibenzo[def,p]chrysene; Michaelis–Menten; VMAX; KM; Intrinsic clearance
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are xenobiotics observed ubiquitously as complex mixtures throughout the environment. The release of PAHs into the environment results from natural and anthropogenic combustion of organic materials, including forest fires, fossil fuel powered transport, industrial manufacturing, smoking cigarettes and grilling meat (USEPA, 1988). PAHs are highly hydrophobic and non-volatile (Cerniglia, 1992) due to their structure of two or more fused benzene rings (Sims and Overcash, 1983), and are therefore persistent pollutants in the atmosphere, soil and water. As the number of fused benzene rings in the PAH increases, water solubility and volatility decrease (Wilson and Jones, 1993).
Many high molecular weight PAHs have been shown to be carcinogenic in humans and animals, including the prototypic carcinogenic PAH, benzo[a]pyrene (B[a]P), and the lesser studied but highly potent dibenzo[def,p]chrysene (DBC), also known as dibenzo[a,l]pyrene. Due to their adverse effects on human health, persistence in the environment, and tendency to be transformed into more reactive and dangerous metabolites, B[a]P and DBC have been classified by the International Agency for Research on Cancer (IARC) as Group 1, carcinogenic to humans, and Group 2A, probably carcinogenic to humans, respectively (WHO, 2010, 2012).
Major routes of PAH exposure occur via ingestion of contaminated food and water or inhalation of particulate matter in the ambient air (Miller and Ramos, 2001). Though natural forms of combustion such as forest fires, volcanic eruptions, and lightening contribute to human PAH exposure, anthropogenic sources including industrial manufacturing, petrol powered transportation, tobacco and cooking processes are principally responsible for the majority of human exposure (Guo et al., 2011). Once released into the atmosphere, PAHs immediately bind to particulate matter, which may then be inhaled or ingested to reach the metabolically active lungs and liver, and the rest of the body.
An alternate, lesser investigated, method of exposure is the exchange between mother and fetus during gestation. The placenta is a vital gestational organ that permits the exchange of blood, nutrients, and oxygen between mother and fetus (Miller and Ramos, 2001). It is also capable of transferring maternal PAHs, resulting in significant decreases in infant birth weight, length, and head and chest circumference (Hunter et al., 2010). Neonates may also be exposed during lactation, as breast milk consists of approximately 7–10% fat (Mandel et al., 2005), providing a hydrophobic environment in which PAHs may stably exist. In mice, we have shown DBC to be a potent transplacental carcinogen affecting multiple organs (Castro et al., 2008; Shorey et al., 2012; Yu et al., 2006).
The liver is an important location of metabolic activation due to its production of many xenobiotic metabolizing enzymes involved in PAH bioactivation and detoxification (Miller and Ramos, 2001). PAHs are procarcinogenic molecules, requiring enzymatic processing to form reactive metabolites capable of exerting carcinogenic effects. The cytochrome P450 superfamily is largely responsible for PAH metabolism and is highly inducible in the liver (Nebert et al., 2004).
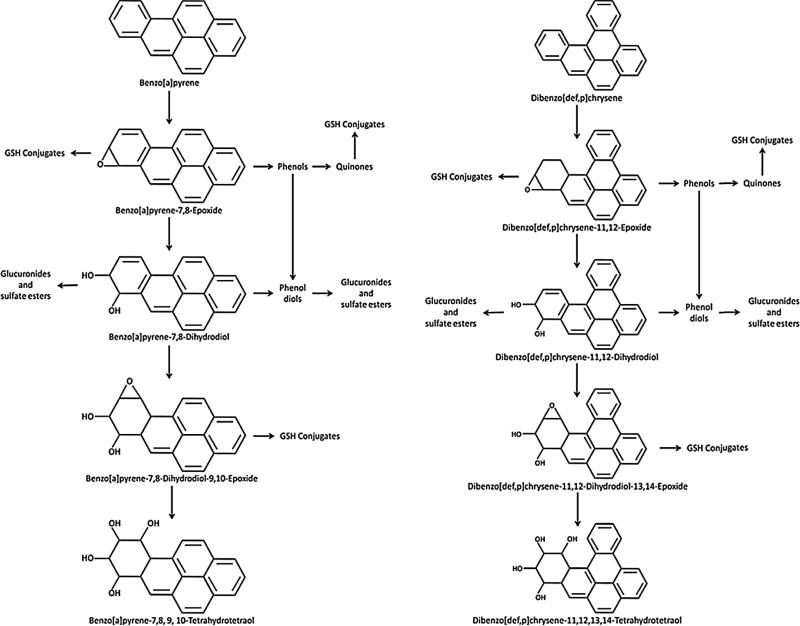
The principal CYP 450 isoforms responsible for activation of most carcinogenic PAHs are CYP 1A1 and 1B1 (Shimada and Fujii-Kuriyama, 2004), which work in conjunction with epoxide hydrolases, peroxidases and other enzymes to transform the B[a]P and DBC parent compounds to a variety of intermediates including epoxides, phenols, quinones and diol epoxides (Fig. 1). Examination of these metabolites found the epoxides and diol epoxides to be extremely reactive with DNA (Conney, 1982), readily forming adducts that resulted in mutation in growth control genes such as p53 (Pfeifer et al., 2002).
Fig. 1.
Metabolic pathway of benzo[a]pyrene (B[a]P) and dibenzo[def,p]chrysene (DBC).
The purpose of this study was to determine the in vitro Michaelis–Menten kinetic parameters involved in the initial step in the metabolism of B[a]P and DBC across multiple species using liver microsomes obtained from male Sprague-Dawley rats, naïve and pregnant female B6129SFI/J mice, and female human. These species were selected due to the volume of previous literature on B[a]P in the rat, our ongoing research into the mode of action and pregnancy-dependent pharmacokinetics of the transplacental carcinogenicity of DBC in the mouse, and the ultimate goal of predicting the disposition of PAHs in humans (Castro et al., 2008; Crowell et al., 2011; Shorey et al., 2012; Yu et al., 2006). Kinetic parameters obtained from these studies are critical for revising preliminary physiologically based pharmacokinetic (PBPK) models for each PAH (Crowell et al., 2011) to facilitate comparisons between target tissue doses from high-dose toxicity studies with pregnant and non-pregnant animals to humans under realistic exposure conditions.
2. Materials and methods
2.1. Reagents and chemicals
DBC, DBC-11,12-diol, DBC-11,12,13,14-teraols, B[a]P-7,8-diol, and B[a]P-7,8,9,10-tetraols were synthesized according to previously reported methods (Krzeminski et al., 1994; Luch et al., 1998; Sharma et al., 2004). B[a]P, sodium sulfate, sulfuric acid, acetone, methanol, ethyl acetate, phosphate buffered saline, magnesium chloride, tris acetate, potassium chloride, potassium pyrophosphate, ethylenediaminetetracetic acid (EDTA) and nicotinamide adenine dinucleotide phosphate (NADPH) were purchased from Sigma (St. Louis, MO). All solvents were of high-performance liquid chromatography (HPLC) grade.
Highly concentrated stocks of B[a]P and DBC were diluted with acetone to create working solutions with concentrations ranging from 0.01 to 2.1 mM (B[a]P) and 0.004–0.9 mM (DBC) for use as analytical and internal standards, as well as substrates in metabolism assays. Solutions were stored at −80 °C in Teflon capped amber vials wrapped with parafilm to prevent degradation or evaporation. Theoretical stock concentrations were confirmed by HPLC with fluorescence detection (FLD).
2.2. Animals
Female B6129SF1/J mice, male 129S/SvImj mice, and male Sprague-Dawley rats (Jackson Laboratory, Bar Harbor, ME, USA) were housed separately in suspended plastic cages with chipped bedding, in rooms maintained at 21 ± 2 °C and 50 ± 10% relative humidity with a 12-h light/dark cycle. Animals were given a minimum acclimation period of seven days before breeding or harvesting tissues. Lab diet certified rodent chow and water were provided ad libitum. For metabolism studies with liver microsomes from pregnant animals, naïve female B6129SF1/J mice were bred with 129S/SvImj males after the one-week acclimation period. The appearance of a vaginal plug was designated gestational day (GD) 0, as in previous studies (Castro et al., 2008; Yu et al., 2006). Livers from pregnant mice were isolated on GD17 corresponding to the exposure day used in prior pharmacokinetic and carcinogenicity studies with DBC (Castro et al., 2008; Crowell et al., 2011; Shorey et al., 2012; Yu et al., 2006). The animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). All animal protocols were approved by the Institutional Animal Care and Use Committee at Pacific Northwest National Laboratory and studies were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals (NIH, 2011).
2.3. Microsome preparation
Pooled female human liver microsomes generated from 25 human females, 31–86 years of age, were obtained from Celsis In Vitro Technologies (Baltimore, MD, USA). Rodent hepatic microsomes were prepared according to the methods of Guengerich (Guengerich, 1994). Briefly, naïve and GD 17 female B6129SF1/J mice and male Sprague-Dawley rats were euthanized by CO2 asphyxiation, exsanguinated, and liver tissue excised. Tissue was immediately placed in ice-cold 0.1 M PBS solution (pH 7.4). Livers were subsequently weighed and placed in 2 mL per gram tissue of homogenization buffer (1 M tris acetate, pH 7.4; 1 M KCl; 100 mM EDTA; d-H2O), and homogenized by 6–8 passes of a drill press. Homogenate was centrifuged in a Beckman ultracentrifuge at 4 °C, 10,000 × g, for 30 min. Supernatant (S9 fraction) was decanted, and centrifuged for an additional 60 min at 4 °C and 105,000 × g. Supernatant (cytosolic fraction) was decanted and stored at −80 °C. Wash buffer (100 mM potassium pyrophosphate; 100 mM EDTA; d-H2O) was added in excess to the ultracentrifuge tube to dislodge the pellets (microsomal fraction), which were then pooled and centrifuged a final time for 45 min at 4 °C and 105,000 × g. Supernatant was discarded and the pellets resuspended with 0.5 mL per gram original tissue of resuspension buffer (1 M tris acetate; 100 mM EDTA; d-H2O). Resuspended pellets were homogenized with 5–6 passes of the drill press. Microsomes were alliquoted by 0.5 mL volumes and stored at −80 °C. Microsomal protein (MSP) concentration was determined by bicinchoninic acid spectrophotometric assay (BCA), using bovine serum albumin as a standard of known concentration; CO difference spectra assays were performed to determine the microsomal CYP concentration.
2.4. In vitro metabolism studies
Reactions were conducted in 500 μL total volumes, consisting of 0.1 M phosphate buffer (pH 7.4), MSP (0.2–1.0 mg/ml), MgCl2 (3 mM), and excess NADPH (1.5 mM). Reactions were initiated by the addition of the chemical of interest (DBC or B[a]P) dissolved in an acetone vehicle (total organic in reaction less than 1%, v/v) to a final reaction concentration of 0.05–35 μM (B[a]P) or 0.05–9.0 μM (DBC) and were incubated at 37 °C for 0–30 min. Control experiments were performed to verify that reaction kinetics were linear with regard to time and MSP content, and without key substrates to establish baseline chromatography (without PAH) and substrate stability over time (without NADPH). Reactions were quenched with the addition of 500 μL 0.9 M H2SO4, followed by the addition of a 5 μL internal standard (B[a]P for DBC kinetic experiments; DBC for B[a]P kinetic experiments). Samples were then vortexed and placed on ice until extraction.
Liquid–liquid extractions were performed as follows: 250 mg Na2SO4 was added to each sample, followed by 500 μL ethyl acetate. Samples were vortexed, then centrifuged for 10 min at 4 °C and 1600 × g. The supernatant was drawn off using a glass Pasteur pipette to a new tube, and then the extraction repeated. The combined supernatants were blown to dryness under a gentle stream of nitrogen. Samples were reconstituted in 500 μL methanol and analyzed as described below.
2.5. Sample analysis
DBC, B[a]P, and their hydroxylated metabolites were quantified by reverse-phase HPLC using an Agilent 1100 HPLC system equipped with a fluorescence detector (Santa Clara, CA, USA). Twenty μL of reconstituted sample were injected onto an Ascentis 25 cm × 4.6 mm, 5 μm C18 column (Sigma–Aldrich, St. Louis, MO, USA). A water:acetonitrile gradient from 45:55 to 0:100 was employed from 0 to 10 min, and then held at 100% acetonitrile until 22 min, at a constant flow rate of 0.95 mL/min. Excitation and emission wavelengths were 245 and 430 for tetraol metabolites, 360 and 430 for diol metabolites, and 235 and 430 for B[a]P and DBC. Elution times were 20.9 and 18.1 min for DBC and B[a]P, 10.7 and 8.5 min for DBC-diol and B[a]P-diol, 5.4, 6.0, and 6.6 min for DBC tetraols, and 3.3, 3.9, and 4.6 min for B[a]P tetraols. The limits of reliable quantitation (LOQ) for B[a]P and DBC were 0.0.04 and 0.02 μM, respectively.
2.6. Data analysis
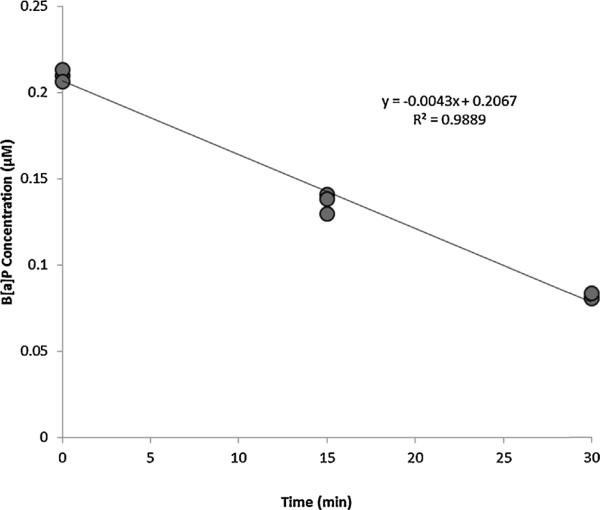
Initial enzyme velocity (Vs, nmol/mL/min) was obtained by linear regression of parent PAH concentration (μM) versus time (min) data from individual kinetic experiments; a representative time course appears in Fig. 2. Regressions included at least six data points and were only considered valid if R2 was equal to or greater than 0.7. To determine kinetic parameters, nonlinear regressions of protein-normalized initial velocities versus initial reaction substrate concentrations ([S], μM) were performed using R: A Language and Environment for Statistical Computing Version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria) according to the Michaelis–Menten model:
| (1) |
where VMAX (nmol/min/mg MSP) is the maximum enzyme velocity and KM (μM) is the Michaelis constant, defined as the initial reaction concentration required to reach half the VMAX.
Fig. 2.
Representative B[a]P substrate depletion over 30 min reaction period in the female human at an initial velocity (V0) of 0.0043 nmol/mL/min.
From VMAX and KM, intrinsic clearance rates (CLINT) were predicted by applying the methods of Obach et al. (1997). CLINT was scaled up from microsomal (mL/min/mg MSP) to tissue (mL/min/g liver) to organismal (mL/min/kg body weight) levels utilizing liver weights of 55, 41.8, 36.6 and 25.7 g per kg body weight for naïve and pregnant (GD 17) mice, rats and humans, respectively (Brown et al., 1997; Oflaherty et al., 1992), as described in Eq. (2).
| (2) |
Though the organism level CLINT is more useful in development of physiologically based pharmacokinetic (PBPK) models, tissue level CLINT involves fewer extrapolations from the microsomal level at which experiments were conducted, and was therefore also included for comparison. Alternatively, intrinsic clearances were also determined directly using the in vitro half-life method (Obach, 1999) for comparison to values determined from classic Michaelis–Menten regressions of substrate depletion data. Scaled in vitro metabolic parameters such as these can be used in physiologically based pharmacokinetic models alongside parameters describing physiology and biochemistry governing chemical disposition within an organism, as described previously (Crowell et al., 2013).
3. Results
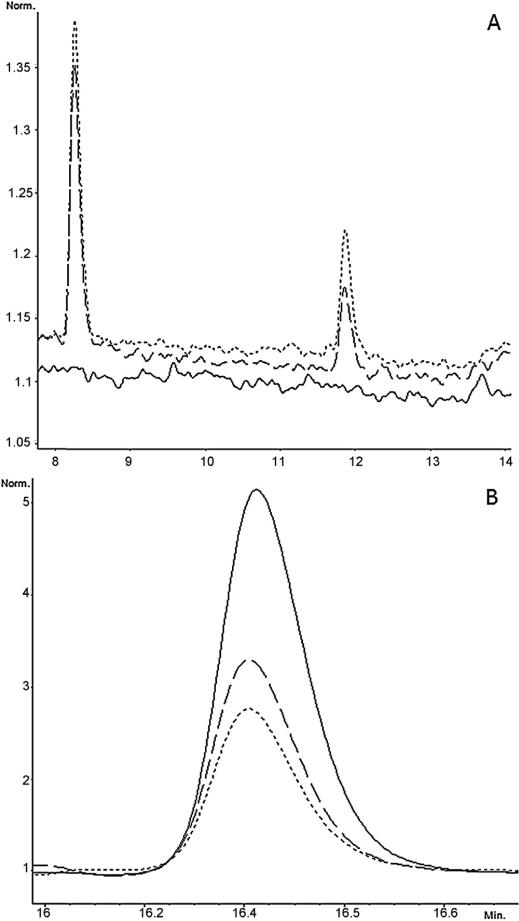
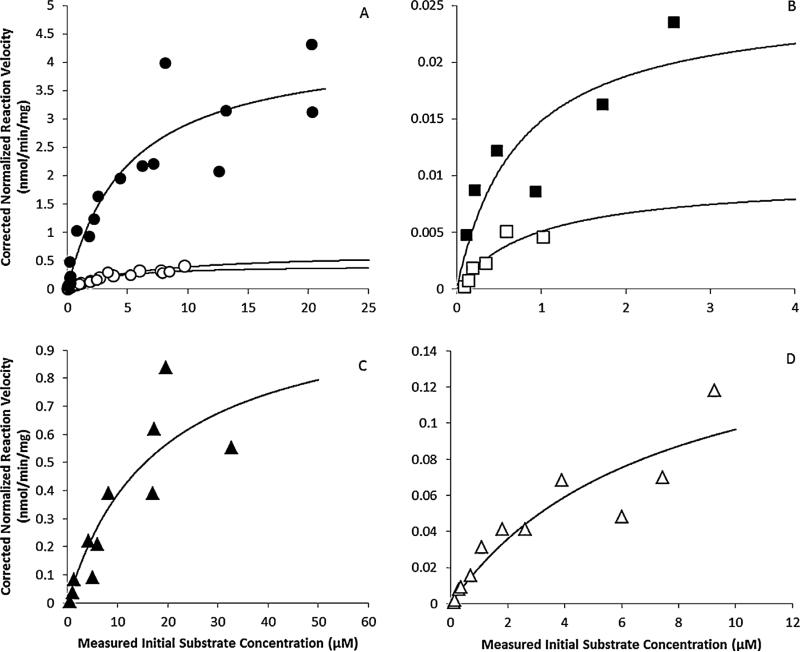
PAH metabolism was observed regardless of sex or species of organism. This was demonstrated by decreasing parent compound and increasing metabolite peak area count over time (Fig. 3). Michaelis–Menten regressions appear alongside measured data points in Fig. 4.
Fig. 3.
(A) Chromatograph overlay of B[a]P metabolites produced at times 0 min (-----), 15 min (– – –), and 30 min (- - -). B[a]P tetraol (retention time 8.3 min) and diol (retention time 12 min) metabolite peak areas increase over reaction time as parent B[a]P compound is consumed. (B) Chromatograph overlay of B[a]P parent compound at times 0 min (-----), 15 min (– – –), and 30 min (- - -). B[a]P concentration decreases over reaction time due to formation of metabolites.
Fig. 4.
Michaelis–Menten kinetic analyses of substrate depletion for (A) B[a]P in the
naïve mouse (●) and DBC in naïve ( ) and
pregnant (
) and
pregnant ( ) mouse; (B) B[a]P (■) and DBC (□) in
female human; (C) B[a]P (▲) in male rat and (D) DBC (△) in male rat.
) mouse; (B) B[a]P (■) and DBC (□) in
female human; (C) B[a]P (▲) in male rat and (D) DBC (△) in male rat.
In all species, VMAX was higher for B[a]P than DBC, by a margin of 2.8 fold in humans, 6.5 fold in rats, and 6.7 fold in mice (Table 1 Table 1). Estimates of CLINT at both the tissue and organism level, regardless of method of calculation, were also universally higher for B[a]P than DBC. For those calculated from Michaelis–Menten regressions, CLINT for B[a]P exceeded that of DBC by a margin of 2.6 fold in male rats, 9.0 fold in female mice, and 3.3 fold in female humans; for those calculated by the in vitro half-life method, B[a]P CLINT exceeded DBC CLINT by a margin of 1.4 fold in rats, 10.6 fold in mice, and 13.3 fold in humans (Table 1). Michaelis constants did not have a similar chemical specific trend: KMs for DBC were marginally higher than for B[a]P in the female mouse and female human, while the reverse was true for male rat (Table 1).
Table 1.
Kinetic parameters of measured initial substrate concentration ranges ([S]0, μM) of B[a]P and DBC metabolism in the naïve and pregnant B6129SF1/J mouse, male Sprague-Dawley rat and female human. Maximum enzyme velocity (Vmax). Michaelis constant (Km). Michaelis-Menten and In vitro half-life intrinsic clearance rates (CLINT) scaled to the hepatic tissue and organismal level.
| Chemical | Organism | Measured [S] range (μM) | Vmax (nmol/min/mg MSP) | Km (μM) | Tissue level CLINT
(ml/min/g tissue) |
Organism level
CLINT (ml/min/kg body weight) |
||
|---|---|---|---|---|---|---|---|---|
| MM | t 1/2 | MM | t 1/2 | |||||
| B[a]P | Naïve mouse | 0.143-20.4 | 4.35 ± 0.628 | 4.97 ± 1.96 | 26.28 ± 14.14 | 48.53 ± 32.79 | 1445.2 ± 778.0 | 2699.0 ± 1803.4 |
| Male rat | 0.161-90.9 | 1.08 ± 0.401 | 17.97 ± 12.87 | 1.8 ± 1.96 | 1.40 ± 0.61 | 65.90 ± 71.68 | 51.34 ± 22.19 | |
| Female human | 0.119-2.56 | 0.025 ± 0.007 | 0.725 ± 0.581 | 1.06 ± 1.17 | 2.13 | 27.16 ± 29.98 | 54.69 | |
| DBC | Naïve mouse | 0.049-9.72 | 0.646 ± 0.055 | 6.63 ± 1.01 | 2.92 ± 0.71 | 4.59 ± 0.53 | 160.71 ± 38.94 | 252.18 ± 29.26 |
| Pregnant mouse | 0.024-8.5 | 0.417 ± 0.075 | 3.28 ± 1.38 | 3.81 ± 2.28 | 3.31 ± 1.75 | 159.43 ± 95.51 | 138.50 ± 72.96 | |
| Male rat | 0.107-9.27 | 0.167 ± 0.064 | 7.28 ± 4.99 | 0.69 ± 0.73 | 1.01 ± 0.30 | 25.14 ± 26.85 | 37.05 ± 10.96 | |
| Female human | 0.087-1.02 | 0.009 ± 0.005 | 0.915 ± 0.823 | 0.32 ± 0.46 | 0.16 | 8.19 ± 11.73 | 4.23 | |
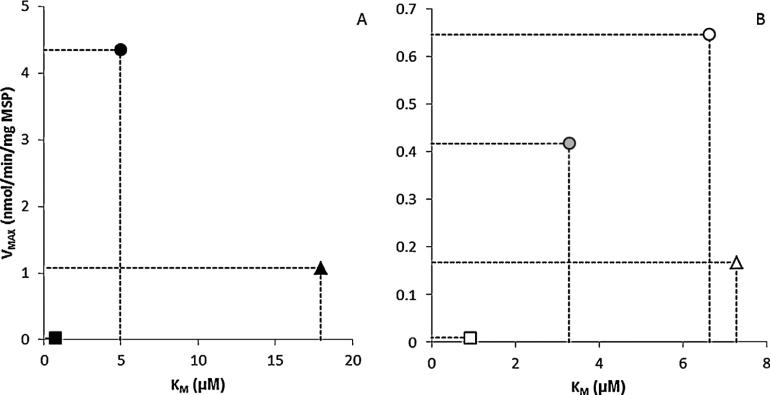
Maximum velocities of PAH depletion were lowest in female humans and highest in female mice, regardless of parent compound (Table 1, Fig. 5). For DBC, VMAX ranged from 0.009 ± 0.005 nmol/min/mg MSP in the female human to 0.646 ± 0.055 nmol/min/mg MSP in the naïve female mouse; in B[a]P from 0.025 ± 0.007 nmol/min/mg MSP in the female human and 4.35 ± 0.628 nmol/min/mg MSP in the naïve female mouse. For both compounds, VMAX in rodents far exceeded that in humans (Table 1).
Fig. 5.
Comparison of maximum velocities (VMAX) and
Michaelis–Menten constants (KM) calculated from (A)
B[a]P depletion data in naive female mouse (●), male SD rat (▲) and female
human (■), and (B) DBC depletion data in naïve ( ), pregnant
female mouse (
), pregnant
female mouse ( ), male SD rat (△) and female human
(□).
), male SD rat (△) and female human
(□).
Michaelis constants for DBC metabolism were generally comparable between rodents, ranging from 6.63 μM in female mice to 7.28 μM in male rats, while the KM for DBC metabolism in female humans was markedly lower (0.92 μM). For B[a]P, KM in humans was also the lowest by a large margin, at 0.73 μM, but KM in male rats exceeded that in female mice by 3.6 fold (Table 1, Fig. 5).
The effect of pregnancy on DBC metabolism kinetics was evaluated in female mice. Both VMAX and KM were reduced in the pregnant animal, by 35% and 51% respectively. Calculated organismal level CLINTs were comparable between pregnant and naïve mice, despite a 30% higher tissue level CLINT in the pregnant animal using the Michaelis–Menten regression method (Table 1). For CLINT estimated via the in vitro half-life method, the tissue level CLINT is 28% lower in the pregnant animal, and scaling up to organism level CLINT results in a 45% lower clearance rate in the pregnant animal (Table 1).
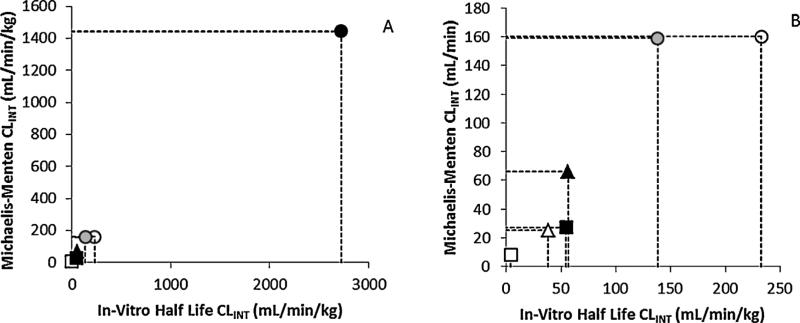
Finally, estimates of CLINT by the in vitro half-life method were generally within twofold of those calculated by the Michaelis–Menten method for both PAHs and all species. However, for both PAHs, tissue and organism level CLINTs estimated by the Michaelis–Menten method were comparable for female humans and male rats, while female mice were markedly higher; this effect was greater for B[a]P than for DBC. A similar trend was apparent for CLINT calculated by the in vitro half-life method for B[a]P metabolism, while CLINT calculated using this method for DBC metabolism were dissimilar in all organisms (Table 1, Fig. 6).
Fig. 6.
Comparison of organismal CLINT values obtained via
Michaelis–Menten and in vitro half-life methods of B[a]P in naive
female mouse (●), male SD rat (▲) and female human (■), and DBC in
naïve ( ), pregnant female mouse (
), pregnant female mouse ( ), male SD
rat (△) and female human (□). (A) All organisms and (B) B[a]P in female
mouse removed to show detail.
), male SD
rat (△) and female human (□). (A) All organisms and (B) B[a]P in female
mouse removed to show detail.
4. Discussion
Elevated maximum enzyme velocities (VMAX) and CLINTs were observed for B[a]P compared to DBC (Table 1). Composed of six fused benzene rings, DBC is larger and bulkier than five ringed B[a]P. This additional benzene ring creates steric hindrance during biotransformation (Nelson and Cox, 2008), possibly explaining the slower rates of conversion of DBC substrate to metabolite product.
The fourfold higher KM observed for B[a]P in the male rat relative to naïve mouse indicates that the CYP system of the mouse has a higher affinity for B[a]P relative to the rat, and when coupled with the higher observed mouse VMAX, may explain the two order of magnitude disparity of B[a]P organism level CLINT between mouse and rat (Fig. 6). The high error of the estimate of KM here (as well as for other kinetic parameters) may also stem from the hydrophobic nature of B[a]P and DBC in an essentially aqueous experimental system.
While clearance values obtained from the in vitro half-life method were generally similar to those calculated from Michaelis–Menten analyses, there are limitations to this method that should be considered. Estimates of clearance are made using much less data than for Michaelis–Menten regressions, and therefore may not accurately reflect clearance values or their variability. The in vitro half-life method also required that reaction concentrations fall well below KM, information which may not be well established for a given compound. Relatedly, the clearance values cannot be used to determine VMAX and KM, and therefore are only reflective of clearance in the first-order range of substrate concentrations (i.e., clearance of low exposures). The in vitro half-life method is certainly useful as an initial, rapid, and low cost method of investigation of metabolism kinetics. Additionally, in situations where substrate solubility may hinder accurate Michaelis–Menten analyses, as may be the case for B[a]P in male rat, the availability of an alternate method is invaluable. In the pregnant mouse, VMAX and KM of DBC depletion were reduced compared to the non-pregnant mouse (Table 1, Fig. 5). It has been shown that pregnant rats in mid to late gestation (GD13 and GD19) have significantly decreased hepatic expression of CYP1A1 (He et al., 2005)–one of the primary isoforms involved in PAH metabolism (Shimada and Fujii-Kuriyama, 2004). While comprehensive information on enzyme expression and activity during pregnancy in the mouse is not available, we have recently used a novel functional proteomic approach, activity based protein profiling, to assess the relative abundances of active enzyme protein levels in pregnant and naïve mice, and found a 2–10 fold reduction in over 30 active P450 enzyme isoforms in the livers of pregnant mice (Crowell et al., 2013). These findings support the observed reduction in overall microsomal VMAX levels but say little about PAH-specific KMs. If the depletion of parent PAH were dependent on a single enzyme, we would not expect to see a change in enzyme affinity with a purported reduction in enzyme expression or abundance. However, because multiple CYP isoforms contribute to PAH metabolism, the observed reduction in overall apparent KM may be attributable to an uneven reduction in several isoforms that contribute to DBC metabolism.
Regardless, overall microsomal apparent KMs estimated from studies with higher molecular weight PAHs are subject to greater error than those determined for lower molecular weight PAHs. This is due to a corresponding decreased water solubility, even with closely related PAHs like B[a]P and DBC, that hinders the ability to conduct studies over a sufficiently broad concentration range to adequately characterize the shift between first-order and zero-order reaction rates. This dose-dependent shift in overall tissue kinetics is further complicated by potential changes in isoform ratios as a result of pregnancy. As a result, overall tissue and organismal level CLINT values determined using the in vitro half-life method that does not depend upon accurate estimates of KM are more likely relevant for comparing across species and pregnancy status as long as exposures are well below those that cause metabolic saturation.
When using rodent data to estimate human risk it is vital to understand interspecies variability in both expression and catalytic activity of relevant CYP isoforms. It was previously believed that CYP1A1 was predominantly responsible for PAH detoxification and activation (Conney, 1982). Studies showed that PAH exposure induced increased expression of CYP1A1; however, CYP1B1 has been shown to transform PAH parent compounds into reactive and carcinogenic metabolites at a higher rate than CYP1A1 (Murray et al., 2001). Both isoforms are predominantly expressed in organs other than the liver (Shimada, 2006) and are highly inducible by PAHs (Murray et al., 2001; Uno et al., 2004). Strong regulatory conservation of CYP1A isoforms has been observed between male rat and humans (Mugford and Kedderis, 1998), corroborating our finding that human metabolism kinetics were more similar to male rat than to female mouse. Clearance values for B[a]P and DBC in female mice were generally far in excess of those in male rat or female human (Table 1, Fig. 6), supporting the previous reports that rats may provide a more accurate model for human risk assessment.
In order to establish more accurate human dosimetry, in vitro studies using human tissues must be continued. As the CYP iso-forms involved in the metabolism of B[a]P and DBC are highly inducible, future studies should focus on how pre-exposure to PAHs affects kinetic parameters. PAHs occur environmentally as complex mixtures of varying composition, making accurate risk assessment and exposure estimates in humans difficult to evaluate (Wenzl et al., 2006). To produce more accurate human models, studies should be performed applying PAHs as simple as well as environmentally relevant mixtures. Though the liver is considered an important target of PAH parent compounds due to its production of many of the enzymes involved in bioactivation and detoxification (Miller and Ramos, 2001), lung and other tissues are also metabolically active target sites and should be investigated. To better quantify rates of production and depletion of carcinogenic metabolites, studies of downstream metabolites as the initial substrate should be performed. Focus should be placed on the hydroxylated diol and tetraol metabolites as the carcinogenic epoxide metabolites are too unstable for in vitro study.
The findings reported here provide an important basis for further development of quantitative models of PAH disposition, such as those previously described (Crowell et al., 2011, 2013), and facilitate more robust extrapolations and estimations of human risk.
Supplementary Material
HIGHLIGHTS.
PAH clearance was highest in naïve female mice and lowest in female humans.
PAH clearance rates in male rat were more similar to female human than female mice.
Metabolism of B[a]P was generally more rapid than DBC, regardless of organism.
DBC metabolism kinetics were reduced in the pregnant mouse relative to naïve mice.
Acknowledgments
Funding
This research was supported by Award Number P42ES016465 from the National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Castro DJ, Baird WM, Pereira CB, Giovanini J, Loehr CV, Fischer KA, Yu Z, Gonzalez FJ, Krueger SK, Williams DE. Fetal mouse Cyp1b1 and transplacental carcinogenesis from maternal exposure to dibenzo(a,l)pyrene. Cancer Prev. Res. 2008;1:128–134. doi: 10.1158/1940-6207.CAPR-07-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- Conney AH. Induction of microsomal-enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic-hydrocarbons – Clowes G.H.A. memorial lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA. Preliminary physiologically based pharmacokinetic models for benzo a pyrene and dibenzo[def,p]chrysene in rodents. Toxicol. Appl. Pharmacol. 2011;257:365–376. doi: 10.1016/j.taap.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell SR, Sharma AK, Amin S, Soelberg JJ, Sadler NC, Wright AT, Baird WM, Williams DE, Corley RA. Impact of pregnancy on the pharmacokinetics of dibenzo[def,p]chrysene in mice. Toxicol. Sci. 2013;135:48–62. doi: 10.1093/toxsci/kft124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. Analysis and Characterization of Enzymes and Nucleic Acids. Raven Press; New York: 1994. [Google Scholar]
- Guo Y, Wu K, Huo X, Xu X. Sources, distribution, and toxicity of polycyclic aromatic hydrocarbons. J. Environ. Health. 2011;73:22–25. [PubMed] [Google Scholar]
- He XJ, Ejiri N, Nakayama H, Doi K. Effects of pregnancy on CYPs protein expression in rat liver. Exp. Mol. Pathol. 2005;78:64–70. doi: 10.1016/j.yexmp.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hunter S, Myers S, Radmacher P, Eno C. Detection of polycyclic aromatic hydrocarbons (PAHs) in human breast milk. Polycyclic Aromat. Compd. 2010;30:153–164. [Google Scholar]
- Krzeminski J, Lin JM, Amin S, Hecht SS. Synthesis of Fjord region diol expoxides as potential ultimate carcinogens of dibenzo[a,l]pyrene. Chem. Res. Toxicol. 1994;7:125–129. doi: 10.1021/tx00038a002. [DOI] [PubMed] [Google Scholar]
- Luch A, Platt KL, Seidel A. Synthesis of Fjord region tetraols and their use in hepatic biotransformation studies of dihydrodiols of benzo[c]chrysene, benzo[g]chrysene and dibenzo[a,l]pyrene. Carcinogenesis. 1998;19:639–648. doi: 10.1093/carcin/19.4.639. [DOI] [PubMed] [Google Scholar]
- Mandel D, Lubetzky R, Dollberg S, Barak S, Mimouni FB. Fat and energy contents of expressed human breast milk in prolonged lactation. Pediatrics. 2005;116:e432–e435. doi: 10.1542/peds.2005-0313. [DOI] [PubMed] [Google Scholar]
- Miller KP, Ramos KS. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 2001;33:1–35. doi: 10.1081/dmr-100000138. [DOI] [PubMed] [Google Scholar]
- Mugford CA, Kedderis GL. Sex-dependent metabolism of xenobiotics. Drug Metab. Rev. 1998;30:441–498. doi: 10.3109/03602539808996322. [DOI] [PubMed] [Google Scholar]
- Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu. Rev. Pharmacol. Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. WH Freeman and Company; New York: 2008. [Google Scholar]
- NIH . Guide for the Care and Use of Laboratory Animals. National Research Council; Washington, DC.: 2011. [Google Scholar]
- Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
- Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. JPET. 1997;283:46–58. [PubMed] [Google Scholar]
- Oflaherty EJ, Scott W, Schreiner C, Beliles RP. A physiologically based kinetic-model of rat and mouse gestation – disposition of a weak acid. Toxicol. Appl. Pharmacol. 1992;112:245–256. doi: 10.1016/0041-008x(92)90194-w. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Kumar S, Amin S. A highly abbreviated synthesis of dibenzo[def,p]chrysene and its 12-methoxy derivative, a key precursor for the synthesis of the proximate and ultimate carcinogens of dibenzo[def,p]chrysene. J. Org. Chem. 2004;69:3979–3982. doi: 10.1021/jo0303822. [DOI] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab. Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey LE, Castro DJ, Baird WM, Siddens LK, Leohr CV, Matzke MM, Waters KM, Corley RA, Williams DE. Transplacental carcinogenesis with dibenzo[def,p]chrysene (DBC): timing of maternal exposures determines target tissue response in offspring. Cancer Lett. 2012;317:49–55. doi: 10.1016/j.canlet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RC, Overcash MR. Fate of polynuclear aromatic-compounds (PNAS) in soil–plant systems. Residue Rev. 1983;88:1–68. [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- USEPA . Methods for the Determination of Organic Compounds in Drinking Water. USEPA; 1988. [Google Scholar]
- Wenzl T, Simon R, Kleiner J, Anklam E. Analytical methods for poly-cyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trac-Trends Analyt. Chem. 2006;25:716–725. [Google Scholar]
- WHO Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer. 2010;92 [PMC free article] [PubMed] [Google Scholar]
- WHO . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 100. World Health Organization, International Agency for Research on Cancer; 2012. Chemical agents and related occupations. [PMC free article] [PubMed] [Google Scholar]
- Wilson SC, Jones KC. Bioremediation of soil contaminated with polynuclear aromatic-hydrocarbons (PAHS) – a review. Environ. Pollut. 1993;81:229–249. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]
- Yu Z, Loehr CV, Fischer KA, Louderback MA, Krueger SK, Dashwood RH, Kerkvliet NI, Pereira CB, Jennings-Gee JE, Dance ST, Miller MS, Bailey GS, Williams DE. In utero exposure of mice to dibenzo[a,l]pyrene produces lymphoma in the offspring: Role of the aryl hydrocarbon receptor. Cancer Res. 2006;66:755–762. doi: 10.1158/0008-5472.CAN-05-3390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.