Abstract
In Vibrio cholerae, the transmembrane DNA binding proteins, ToxR and TcpP, activate expression of the regulatory gene toxT in response to specific environmental signals. The resulting enhanced level of ToxT leads to a coordinated increase in the production of a subset of virulence factors, including cholera toxin (CT) and toxin-coregulated pilus (TCP). The effect of anaerobiosis on expression of the V. cholerae virulence regulatory cascade was examined. The expression of the major regulatory genes, tcpP, toxR, and toxT, in anaerobically grown V. cholerae was comparable to that in cells grown under aerobic conditions, and no significant difference in the ToxT-dependent expression of tcpA was detected when aerobic and anaerobic cultures were compared. However, in spite of the presence of functional ToxT, ctxAB expression was drastically reduced, and practically no CT was detected in cells grown under anaerobic conditions. In a V. cholerae hns mutant, however, high levels of ctxAB expression occurred even under anaerobic conditions. Also, deletion of the H-NS binding site from the ctxAB promoter eliminated anaerobic repression of ctxAB expression. These results suggest that H-NS directly represses ctxAB expression under anaerobic growth conditions. It has been reported that in the first stage of infection of infant mice by V. cholerae, tcpA is expressed but ctxAB expression is shut off (S. H. Lee, D. L. Hava, M. K. Waldor, and A. Camilli, Cell 99: 625-634, 1999). This pattern is similar to the pattern in anaerobic cultures of V. cholerae. Under all other in vitro conditions, ctxAB and tcpA are known to be coordinately expressed.
Vibrio cholerae, a gram-negative, noninvasive enteric bacterium, is the causative agent of the diarrheal disease cholera (15). For successful infection, V. cholerae must colonize the intestinal epithelium and secrete cholera toxin (CT), a potent enterotoxin that causes the severe fluid loss characteristic of the disease. A toxin-coregulated pilus (TCP), coordinately expressed with CT, is thought to be the major colonization factor of V. cholerae, and mutants deficient in TCP are greatly attenuated for virulence both in animal models and in human volunteers (13, 30). A subset of virulence factors, including CT and TCP, is coordinately regulated by the hierarchical expression of regulatory proteins comprising the ToxR regulon (7). At the top of the hierarchy are ToxR and TcpP (11), which are inner membrane proteins with cytoplasmic DNA binding domains that act synergistically to promote transcription of the toxT gene. ToxT, in turn, activates transcription of several virulence genes, including ctxAB and tcpA, which code for CT and TcpA, the major subunit of TCP, respectively (6).
A common theme in bacterial pathogenesis is a sensory transduction mechanism that allows coordinated expression of virulence factors in response to the external environment of the bacteria. Pathogenic bacteria are known to exploit physical and chemical parameters that distinguish the host from the external environment as signals for the expression of virulence determinants (23). Thus, in most pathogens, environmental conditions characteristic of the host physiological sites of infection activate central regulators of virulence determinants (9). Paradoxically, the intestinal environment may be presumed to have parameters similar to those of the nonpermissive conditions for induction of the ToxR regulon of V. cholerae. In vitro, the ToxR regulon is maximally expressed in cells grown at 30°C in media with a starting pH of 6.6 and an osmolarity equivalent to 66 mM NaCl. In the intestinal lumen, the temperature is 37°C, the pH is alkaline, and the osmolarity is thought to be equivalent to 300 mM NaCl or higher, conditions that repress expression of ToxR-activated virulence factors in vitro (reviewed in references 19 and 29). Furthermore, bile, a major constituent of the small intestine, represses expression of virulence factors (10, 27). Critical differences have been found in the requirements of virulence regulatory proteins for expression of the ctxAB and tcpA genes between growth of V. cholerae in vitro and growth of V. cholerae in the infant mice intestine (22). The mechanism of activation of the ToxR regulon and the mechanism of environmental modulation of the regulatory processes in vivo are unknown. It is likely that the concerted functioning of regulatory systems responding to different environmental conditions encountered at different stages of infection may fine-tune expression of virulence genes for successful infection. It is generally presumed that the oxygen concentration in the intestine is low, and recently transcriptome analysis of V. cholerae grown in vivo has revealed that several genes involved in anaerobic respiration are strongly induced during intraintestinal growth (2, 31). There is some evidence that enteric pathogens have adapted so that they express virulence factors in response to low oxygen concentrations; notably, this occurs in Salmonella, in which a low level of oxygen stimulates bacterial invasion from the gastrointestinal tract (21). In Escherichia coli, anaerobic growth has been shown to modulate expression of flagellar genes and lipopolysaccharide production (20). It is in this context that in the present report we describe experiments in which we investigated the effect of anaerobiosis on expression of virulence factors in V. cholerae.
MATERIALS AND METHODS
Bacterial strains and plasmids.
V. cholerae O1 strains O395, O395H29 (hns), and O395F32 (fnr) were used in the present study. The strains were grown in Luria-Bertani (LB) medium containing 1% tryptone (Difco), 0.5% yeast extract (Difco), and 0.5% NaCl and were stored at −70°C in 20% (vol/vol) glycerol. The suicide vector pGP704, used for site-directed mutagenesis, was maintained in E. coli strain SM10 λ pir (24). Plasmids pGS810 and pGS814 contained the anaerobiosis-activated melR promoter and anaerobiosis-repressed promoter of the ndh gene, respectively, fused to a reporter lacZ gene in plasmid pBR322 (8). Plasmids pKDctx335 and pKDctx75 were constructed by cloning 335- and 75-bp fragments of the ctxAB promoter in plasmid pKK232.8 (Pharmacia) immediately upstream of the promoterless cat gene. Plasmids were introduced into E. coli cells by transformation and into V. cholerae by triparental conjugation with E. coli strain MM294(pRK2013) as a donor of mobilization factors. Ampicillin (100 μg ml−1) and streptomycin (100 μg ml−1) were used when appropriate. Tetracycline was used at a concentration of 15 μg ml−1 for E. coli and at a concentration of 5 μg ml−1 for V. cholerae.
Growth conditions.
V. cholerae strains from glycerol stock cultures stored at −70°C were streaked on LB agar plates and incubated overnight at 37°C. A loopful of cells from a plate was inoculated into 5 ml of LB medium and grown overnight (14 to 16 h) at 37°C with shaking. Cultures were diluted 1:200 in 5 ml of LB medium in 18-mm-diameter 15-cm-long culture tubes and were grown with vigorous shaking for aerobic growth. Anaerobic conditions were achieved either by using an anaerobic-atmosphere-generating system (Oxoid) or by using 10-mm-diameter 4-cm-long screw-cap tubes that were filled to the brim, sealed with tape, and incubated without shaking. In some experiments diluted LB medium containing 0.5% tryptone, 0.25% yeast extract, and 0.5% NaCl was used for aerobic growth. For assays for virulence factors, V. cholerae was grown in LB medium (pH 6.6) at 30°C (24) under aerobic or anaerobic conditions.
Construction of V. cholerae hns and fnr mutants.
A 180-bp fragment spanning nucleotides 60 to 240 from the 5′ end of the hns open reading frame and a 279-bp internal fragment of the fnr gene were PCR amplified by using appropriate primers. The primers were designed based on the hns and fnr gene sequences obtained from the V. cholerae genome sequence database (12). The PCR-amplified internal fragments of the hns and fnr genes were cloned at the EcoRV site of the suicide vector pGP704 (Apr) and were transformed into a λ pir lysogen of E. coli SM10 (24). Ampicillin-resistant transformants containing the recombinant plasmid were selected and conjugally transferred to V. cholerae strain O395 (Smr). Transconjugants resistant to both ampicillin and streptomycin were selected. Southern blot analysis was used to confirm that integration had occurred at an appropriate position within the chromosomal hns or fnr gene. The V. cholerae hns and fnr mutant strains containing the suicide vector pGP704 inserted into the chromosomal gene were designated O395H29 and O395F32, respectively.
RNA isolation and RT-PCR.
For isolation of RNA, V. cholerae strains O395 and O395H29 were grown to a density of about 0.6 A600 unit (∼6 × 108 CFU ml−1) in LB medium (pH 6.6) at 30°C under aerobic or anaerobic conditions. Total RNA was extracted and purified by using guanidium isothiocyanate (1). The RNA was treated with RNase-free DNase I (1 U/μg; amplification grade; Invitrogen) in the presence of an RNase inhibitor (RNasin; Gibco-BRL), and reverse transcription (RT)-PCR was performed by using a single-tube RT-PCR kit (Gibco-BRL). Two hundred nanograms of DNase-treated RNA was used in all reactions. Amplification was performed for 25 to 35 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by a 7-min extension at 72°C) for tcpP-, toxR-, toxT-, ctxAB-, and tcpA-specific primers, and 25 amplification cycles were used for 16S rRNA. Genomic DNA was used as a positive control, and RNA that had been treated with DNase but not reverse transcribed was used as a negative control. Fifteen microliters of each PCR product was electrophoresed on a 1.5% agarose gel with ethidium bromide, and the gels were analyzed by using a Gel Doc 1000 system (Bio-Rad Laboratories). PCR products were normalized according to the amount of 16S rRNA detected in the same cDNA sample. Each set of experiments was performed at least three times.
Assays for β-galactosidase and CAT activities.
β-Galactosidase activity in permeabilized cells was assayed by measuring the hydrolysis of o-nitrophenyl galactopyranoside. Chloramphenicol acetyltransferase (CAT) activity in sonicated cell lysates was measured by using a Quan-T-CAT kit (Amersham) as recommended by the manufacturer. The results presented below are averages of at least three independent experiments.
GM1-ganglioside-dependent enzyme-linked immunosorbent assay for CT.
CT production in culture supernatants or sonicated cell pellets was determined by performing GM1-dependent enzyme-linked immunosorbent assays with polyclonal rabbit serum directed against purified CT. Anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Jackson Laboratories) was used as the secondary antibody. Dilutions of CT of known concentrations (Sigma) were used to estimate the amounts of CT in the samples. The amount of CT was expressed as the amount of toxin produced per milliliter of culture at a cell density corresponding to 0.6 A600 unit.
Ligated rabbit ileal loop model.
Expression from anaerobiosis-regulated promoters during in vivo growth of V. cholerae was assayed by using the ligated rabbit ileal loop model (5). The fluid that accumulated in each loop was separately collected and measured, after which the loops were slit open and scraped. The fluid and scrapings were centrifuged (8,000 × g, 5 min) to collect the bacteria, washed twice with normal saline, and finally resuspended in saline. The bacterial count in each suspension was determined by plating the suspension on thiosulfate-citrate-bile salt-sucrose agar plates containing appropriate antibiotics when necessary, and the β-galactosidase activity in a measured amount of cells grown in vivo was determined. At least two loops in the same animal were inoculated with each bacterial strain, and each strain was tested in at least three individual animals.
RESULTS
Growth of V. cholerae under anaerobic conditions.
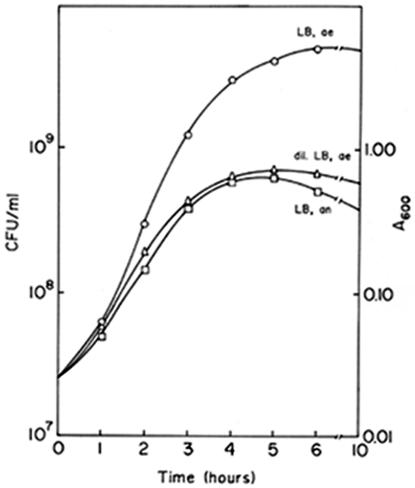
V. cholerae strain O395 was grown in LB medium under aerobic or anaerobic conditions, and at regular intervals the optical density of the culture and the number of CFU were measured. Under anaerobic conditions the culture attained a maximum density of about 0.6 A600 unit, corresponding to about 6× 108 CFU ml−1, in 4 to 5 h before the stationary phase was reached (Fig. 1). When the strain was grown in the same medium under aerobic conditions, the cell density reached about 6 × 108 CFU ml−1 in 2.5 h, and the maximum density, about 5 × 109 to 6 × 109 CFU ml−1, was reached in 6 to 7 h. Thus, the growth rate of V. cholerae in LB medium under aerobic conditions was much higher than the growth rate under anaerobic conditions. To obtain comparable growth rates for aerobic and anaerobic cultures, the growth of V. cholerae in dilute LB medium was monitored. When V. cholerae was grown in 1:1 diluted LB medium under aerobic conditions, the growth rate was comparable to the growth rate in LB medium under anaerobic conditions (Fig. 1).
FIG. 1.
Growth of V. cholerae O395 under aerobic conditions in LB medium (○) and dilute LB medium (▵) and under anaerobic conditions in LB medium (□). dil., dilute; ae, aerobic; an, anaerobic.
CT production is reduced in V. cholerae grown with a low oxygen concentration.
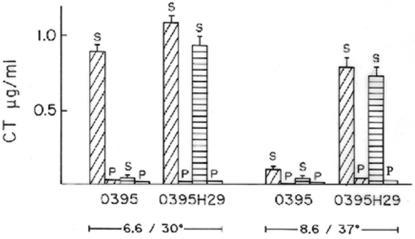
To examine if the oxygen concentration has any effect on the virulence of V. cholerae, production of CT, the major virulence factor of the organism, was estimated by using cells grown aerobically or anaerobically to an optical density of 0.6 in LB medium (pH 6.6) at 30°C. Although under aerobic conditions V. cholerae produced 0.8 to 1 μg of CT ml−1 in culture supernatants, very little CT (<0.1 μg ml−1) was detected in culture supernatants of cells grown under anaerobic conditions (Fig. 2). To examine if the decrease in CT production was due to a lower growth rate of V. cholerae in LB medium under anaerobic conditions (Fig. 1) or to anaerobiosis per se, cells were grown to an optical density of 0.6 in (i) LB medium with aeration for 2 to 2.5 h (Fig. 1), (ii) dilute LB medium with aeration for 4.5 to 5 h (Fig. 1), or (iii) LB medium under anaerobic conditions for 4.5 to 5 h (Fig. 1). Thus, comparisons could be made between cultures grown aerobically and anaerobically to similar cell densities (i) at the same growth rate (dilute LB medium under aerobic conditions for 4.5 to 5 h versus LB medium under anaerobic conditions for 4.5 to 5 h) or (ii) at different growth rates (LB medium under aerobic conditions for 2 to 2.5 h versus LB medium under anaerobic conditions for 4.5 to 5 h). When V. cholerae was grown under aerobic conditions in LB medium and in dilute LB medium to an optical density of 0.6, the cultures produced similar amounts of CT (0.8 to 1 μg of CT ml−1), but practically no CT was detected in V. cholerae cultures grown in LB medium under anaerobic conditions. These results indicate that anaerobiosis, and not a decrease in the growth rate, drastically reduces CT production in V. cholerae cells. Since there was no difference in CT production between cultures grown to an optical density of 0.6 in LB medium and cultures grown to an optical density of 0.6 in dilute LB medium, in subsequent experiments aerobic cultures were grown in LB medium for 2 to 2.5 h.
FIG. 2.
CT production in aerobic and anaerobic cultures. V. cholerae strains O395 and O395H29 (hns) were grown to an optical density of 0.6 in LB medium (pH 6.6) at 30°C or in LB medium (pH 8.6) at 37°C under aerobic conditions (diagonally cross-hatched bars) or under anaerobic conditions (horizontally cross-hatched bars), and the CT concentrations in culture supernatants (S) or sonicated cell pellets (P) were estimated.
In order to examine if anaerobic growth affects secretion of CT, the amounts of CT in sonicated cell pellets from cultures grown under aerobic and anaerobic conditions were also estimated, and in both cases almost no CT was detected in the cell lysates (Fig. 2). Since the pH of the growth medium is known to affect CT production (24), we confirmed that the anaerobic growth conditions did not have any effect on the pH of the culture.
Expression of ctxAB but not expression of tcpA is reduced during anaerobic growth.
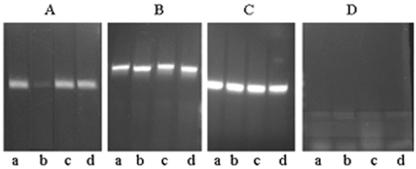
To examine if the inhibition of CT production in anaerobically grown cells is at the level of transcription, RNA was isolated from cultures grown to an optical density of 0.6 in LB medium (pH 6.6) at 30°C under aerobic and anaerobic conditions, and the amount of ctxAB-specific mRNA in each sample was estimated by RT-PCR. 16S rRNA production was used as an internal control (Fig. 3C). Analysis of the results obtained indicated that although high levels of ctxAB-specific mRNA were produced in cells grown with aeration (Fig. 3A, lane a), the amount of ctxAB mRNA was drastically reduced in anaerobic cultures (Fig. 3A, lane b).
FIG. 3.
Effect of anaerobiosis on ctxAB and tcpA gene expression. RT-PCR was performed with RNA isolated from V. cholerae strain O395 (lanes a and b) or strain O395H29 (lanes c and d) grown under aerobic conditions (lanes a and c) or anaerobic conditions (lanes b and d) to an optical density of 0.6 in order to estimate levels of ctxAB (A), tcpA (B), or 16S rRNA (C). DNase-treated RNA samples that had not been reverse transcribed were used as negative controls (D).
Environmental modulation of the tcpA gene is thought to follow the same pattern as environmental modulation of ctxAB, with both genes under the coordinated regulation of ToxT (30). In view of the fact that ctxAB gene expression was significantly reduced in anaerobic cultures of V. cholerae, tcpA expression was examined in the cells and compared to the tcpA expression in cells grown with aeration. RT-PCR analysis indicated that there was not a significant difference in the amounts of tcpA-specific transcript in cells grown under aerobic conditions and cells grown under anaerobic conditions (Fig. 3B, lanes a and b). Thus, contrary to expectations, although ctxAB gene expression was drastically reduced in anaerobic cultures of V. cholerae, the expression of the tcpA gene was similar to that in cells grown with aeration.
Expression of virulence regulatory genes.
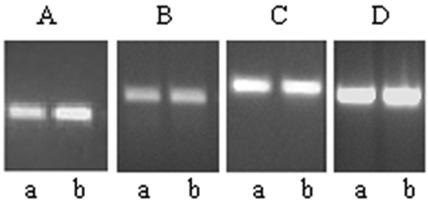
Expression of the ctxAB and tcpA genes is positively regulated by ToxT, and toxT gene expression is controlled by the synergistically acting ToxR and TcpP proteins (6, 7, 11). RT-PCR analysis indicated that the levels of expression of the toxR and tcpP genes in cells grown in LB medium to an optical density of 0.6 under aerobic and anaerobic conditions were comparable (Fig. 4B and C), although an increase in toxT expression was detected in the anaerobic cultures (Fig. 4A). The increased expression of toxT in anaerobic cultures may have been due to the anaerobiosis-activated transcriptional regulator ArcA that has recently been shown to activate toxT expression (28).
FIG. 4.
Expression of toxT, toxR, and tcpP in aerobic and anaerobic cultures of V. cholerae. RT-PCR was performed with RNA isolated from strain O395 grown under aerobic conditions (lanes a) or anaerobic conditions (lanes b) in order to estimate levels of toxT (A), toxR (B), tcpP (C), or 16S rRNA (D).
FNR has no role in anaerobic repression of ctxAB expression.
The global anaerobiosis response regulator FNR activates expression of a set of genes required for anaerobic respiration and represses genes involved in aerobic respiration (8) To examine if FNR has any role in the anaerobic repression of ctxAB expression, a V. cholerae O395 fnr mutant strain, strain O395F32, was constructed. Appropriate expression from the FNR-activated promoter in the reporter plasmid pGS810 and the FNR-repressed promoter in pGS814 (8) in strain O395F32 confirmed the loss of functional FNR in this strain (Table 1). CT production was next measured in the fnr mutant strain O395F2 and the parent strain O395. Under aerobic conditions both strains produced 0.8 to 1 μg of CT per ml at an optical density of 0.6 (data not shown). Similar to wild-type strain O395, fnr mutant strain O395F32 did not produce detectable CT in culture supernatants or sonicated cell pellets under anaerobic conditions, indicating that FNR has no role in the anaerobic repression of CT production in V. cholerae.
TABLE 1.
β-Galactosidase activity in V. cholerae O395 carrying plasmid pGS810 or pGS814
| V. cholerae strain | β-Galactosidase activity in strains growna:
|
||
|---|---|---|---|
| In vitro
|
In vivo | ||
| Aerobic conditions | Anaerobic conditions | ||
| O395 | 8 | 5 | 5.6 |
| O395/pGS814 | 1,993 ± 135 | 809 ± 37 | 440 ± 31 |
| O395/pGS810 | 271 ± 9 | 1,667 ± 143 | 1,080 ± 206 |
| O395F32/pGS814 | 1,603 ± 102 | 1,756 ± 127 | NDb |
V. cholerae strains were grown aerobically or anaerobically in LB medium at 37°C (in vitro) or in ligated rabbit ileal loops (in vivo), bacterial counts were determined, and the β- galactosidase activity per 5 × 108 CFU was measured. The results (means ± standard deviations for three independent experiments) are expressed in Miller units.
ND, not done.
Role of H-NS in anaerobic repression of ctxAB expression.
It has been reported that the histone-like protein H-NS can silence ctxAB expression (25, 32). Indeed, the V. cholerae O395 hns mutant strain O395H29 constructed in this study showed high levels of ctxAB expression even under normally repressive conditions (LB medium, pH 8.6, 37°C) (Fig. 2). Furthermore, strain O395H29 grown anaerobically to an optical density of 0.6 in LB medium (pH 6.6) at 30°C produced about 0.8 μg of CT ml−1 in culture supernatants, although practically no CT was detected in anaerobic cultures of wild-type strain O395 under identical conditions (Fig. 2). RT-PCR analysis indicated that there was a statistically significant (P = 0.001) difference in the relative intensities of PCR products corresponding to ctxAB mRNA obtained from anaerobic cultures of strains O395 and O395H29. After normalization according to the amount of 16S rRNA present in each RNA population, the amount of ctxAB mRNA in anaerobic cultures of strain O395H29 was at least 10-fold higher than the amount in anaerobic cultures of parental strain O395 (Fig. 3A, lanes b and d). The ctxAB expression in anaerobic cultures of strain O395H29 was comparable to that in aerobically grown strain O395 cultures (Fig. 3A, lanes a and d). Thus, repression of CT production under anaerobic conditions was completely eliminated in the V. cholerae hns mutant.
A region on the ctxAB promoter between approximately position −400 and position −70 with respect to the transcription start site was demonstrated to contain H-NS binding sites by Yu and DiRita (32), who used plasmids carrying various amounts of the ctxAB upstream region fused to a reporter gene. To determine if anaerobic repression of ctxAB expression is due to a direct effect of H-NS on ctxAB, the H-NS binding region was deleted from the ctxAB promoter, and expression from the promoter was assayed under anaerobic conditions. Reporter plasmids carrying the ctxAB promoter either up to position −335 (pKDctx335) or up to position −75 (pKDctx75), fused to a promoterless cat gene in plasmid pKK232.8, were constructed. Thus, H-NS binding sites were present in the ctxAB promoter in plasmid pKDctx335 but absent from the ctxAB promoter in plasmid pKDctx75. Both plasmids were conjugally transferred to V. cholerae O395, and the CAT activities in transconjugants grown under anaerobic conditions were assayed. Very little CAT activity was detected in anaerobic cultures of O395 carrying plasmid pKDctx335, although an approximately fivefold increase in CAT activity was detected in cells carrying plasmid pKDctx75 (Table 2). Cells carrying pKDctx335 and cells carrying pKDctx75 produced comparable amounts of CAT under aerobic conditions. Thus, deletion of H-NS binding sites from the ctxAB promoter caused derepression of ctxAB under anaerobic conditions.
TABLE 2.
CAT activities in aerobic and anaerobic cultures of V. cholerae O395 containing ctxAB promoters fused to the reporter cat gene
| Plasmid | CAT activity in V. cholerae O395a
|
|
|---|---|---|
| Aerobic conditions | Anaerobic conditions | |
| pKK32.8 | 11 | 9 |
| pKDctx335 | 365 ± 31 | 52 ± 3 |
| pKDctx75 | 273 ± 57 | 246 ± 33 |
V. cholerae strains with plasmids were grown in LB medium (pH 6.6) at 30°C under aerobic or anaerobic conditions to an optical density of 0.6, and the CAT activity in sonicated cell pelets was measured. The results (means ± standard deviations for three independent experiments) are expressed in milliunits per A600 unit.
Although significant expression of tcpA occurred in anaerobic cultures of V. cholerae O395, there was a small but consistent increase in tcpA expression in hns mutant strain O395H9 (Fig. 3B, lanes b and d), suggesting that H-NS also represses tcpA expression in anaerobic cultures; however, the extent of repression was very small compared to the extent of H-NS-dependent repression of ctxAB expression.
Expression from anaerobiosis-regulated promoters in V. cholerae in vitro and in vivo.
Plasmids pGS810 and pGS814 contain the anaerobiosis-activated melR promoter and the anaerobiosis-repressed promoter of the ndh gene, respectively, fused to a reporter lacZ gene (8). Expression of β-galactosidase from the melR promoter in plasmid pGS810 has been reported to increase about four- to fivefold under anaerobic conditions in E. coli compared to the expression in cells grown under aerobic conditions. On the other hand, β-galactosidase expression from the ndh promoter in plasmid pGS814 decreases about fourfold in anaerobically grown E. coli cells (8). Plasmids pGS810 and pGS814 were conjugally transferred to V. cholerae O395, and the transconjugants were grown in LB medium under aerobic and anaerobic conditions. Under both anaerobic growth conditions employed in this study (see Materials and Methods), the β-galactosidase expression in cells carrying plasmid pGS810 was about sixfold higher than that in cells grown under aerobic conditions (Table 1). Furthermore, the β-galactosidase expression in pGS814-carrying cells under anaerobic conditions was about 2.5-fold lower than that in cells grown under aerobic conditions (Table 1). Thus, the growth conditions used in this study are sufficient for appropriate and full induction of anaerobiosis-responsive genes.
To determine if the intraintestinal environment is anaerobic, V. cholerae carrying plasmid pGS810 or pGS814 was also grown in rabbit intestines, and β-galactosidase expression was examined in cells ex vivo. The β-galactosidase expression from the anaerobiosis-repressed promoter in plasmid pGS814 in cells grown in the rabbit intestines was more than fourfold lower than the expression in cells grown in vitro under aerobic conditions (Table 1). Also, the approximately twofold-lower level of expression of β-galactosidase in cells carrying plasmid pGS814 than in cells carrying plasmid pGS810 was comparable to the results obtained with cells grown in vitro under anaerobic conditions (Table 1). These results indicate that the oxygen concentration in the intestinal lumen is low enough to activate expression of anaerobically induced genes. The relevance of this observation to the pathophysiology of infection is discussed below.
DISCUSSION
Expression of the major virulence factors of V. cholerae is controlled by a cascade of regulatory proteins. The transmembrane, DNA binding proteins ToxR and TcpP act synergistically to enhance transcription of the toxT gene. ToxT, the second regulator in the hierarchy, is believed to coordinately activate transcription of the genes coding for CT, the colonization factor TcpA, and other virulence determinants (7, 16). The virulence regulatory cascade in V. cholerae is influenced by several environmental signals, which exert their effects at different levels of the cascade. Under nonpermissive conditions of temperature and pH, transcription of the tcpP gene is repressed, which leads to downregulation of the entire virulence regulon (18). Bile affects the cascade at a later stage by modulating the transcriptional activity of ToxT, which leads to a decrease in the expression of both ctxAB and tcpA (10, 27). We examined the effect of growth in the presence of a low oxygen concentration on V. cholerae virulence and found an additional level of complexity governing the expression of virulence factors. Under anaerobic conditions, the transcriptional regulators ToxR, TcpP, and ToxT are produced and TcpA is synthesized, but expression of ctxAB is repressed. This pattern of expression of the virulence regulon is different from the pattern of expression under all other in vitro conditions in which tcpA and ctxAB are coordinately expressed (16, 19, 29).
FNR is a transcriptional regulator that controls the expression of a large number of anoxia-responsive genes. To investigate whether FNR has a role in the anaerobic repression of ctxAB, a V. cholerae fnr mutant was constructed. ctxAB expression in the fnr mutant was repressed under anaerobic conditions like it was in the wild-type strain, suggesting that FNR has no role in the anaerobic repression of ctxAB expression. We next considered the possibility that the histone-like nucleoid-associated protein H-NS, which has been implicated in the silencing of a large number of diverse bacterial genes (14), may be involved in the repression of ctxAB under anaerobic conditions. It has previously been demonstrated that H-NS can bind to and repress expression from the ctxAB promoter (32). Even in the absence of the activator protein ToxT, high levels of CT were synthesized in a V. cholerae hns mutant under nonpermissive conditions of temperature and pH (25). To investigate whether H-NS is also involved in the repression of ctxAB under ananerobic conditions, a V. cholerae hns mutant was constructed, and ctxAB expression was examined in anaerobic cultures of this mutant. The anaerobic repression of ctxAB expression was completely eliminated in the V. cholerae hns mutant (Fig. 2 and 3), suggesting that H-NS has a role in anaerobic silencing of ctxAB. To examine if H-NS directly represses ctxAB expression, H-NS binding sites on the ctxAB promoter (32) were deleted, and expression from the promoter was assayed under anaerobic conditions (Table 2). Anaerobic repression was not observed when H-NS binding sites were deleted from the ctxAB promoter, suggesting that H-NS has a direct repressive effect on ctxAB under anaerobic conditions. It is well documented that H-NS binding to DNA is topology dependent, and the repressive nucleoprotein complexes formed by oligomerization of H-NS on DNA can be disrupted only by local supercoiling changes acting in combination with positively acting protein factors (17, 20, 21). Since anaerobiosis is known to alter DNA topology (4, 26), it may be postulated that during anaerobic growth the topology of the AT-rich ctxAB promoter region may be favorable for the assembly of a repressive H-NS oligomeric complex, resulting in repression of ctxAB expression.
Numerous studies have demonstrated that ToxT coordinately controls ctxAB and tcpA expression in response to the temperature, osmolarity, and pH of the growth medium (19, 24, 29, 30). The coordinated expression of ctxAB and tcpA appears to be lost in cells grown under anaerobic conditions since in these cells, although expression of ctxAB is drastically reduced, the tcpA gene is optimally expressed. This effect may be due to the subtle but important differences in the requirements for expression from the tcpA and ctxAB promoters (32). First, although H-NS represses expression from ctxAB and tcpA promoters, a stronger repressing effect of H-NS on ctxAB than on tcpA has been reported (32). Second, ToxT is required to achieve maximal levels of activation of the tcpA promoter even in the absence of H-NS, while ToxT is not required for ctxAB expression in the absence of H-NS (32). It has recently been shown that the global anaerobiosis response regulator ArcAB activates toxT expression under anaerobic conditions (28). It may be postulated that the combined effects of the lower affinity of H-NS for the tcpA promoter and the increased level of ToxT, which competes with H-NS for binding to the tcpA promoter, may account for tcpA expression in anaerobic cultures of V. cholerae.
An elegant study on the temporal control of virulence gene expression in vivo demonstrated that in the early stages of infection of infant mice by V. cholerae, although expression of toxT was normal and tcpA was expressed, no ctxAB expression was detected (22). Thus, the pattern of virulence gene expression in the early stages of infection was similar to that observed during in vitro growth of V. cholerae under anaerobic conditions. Under all other in vitro environmental conditions that have been examined, ToxT production is accompanied by coordinated expression of ctxAB and tcpA (16, 19). It was demonstrated in this study, as well as in recent transcriptome analyses of V. cholerae grown in human and rabbit intestines, that anaerobiosis is a major stress condition experienced by V. cholerae in the gastrointestinal tract (2, 31). In this context, it is attractive to hypothesize that the anaerobic environment in the intestine allows tcpA to be expressed while it selectively represses expression of ctxAB in the early stage of infection. The mechanism by which ctxAB repression is counteracted at later stages of infection is still obscure. However, the observation that ctxAB is expressed in bacteria that have colonized the intestine and the observation that early induction of tcpA is required for colonization and ctxAB expression (22) suggest that a signal(s) received subsequent to colonization may be important for induction of ctxAB expression. Also, contact with the intestinal epithelium might be important for activation of ctxAB expression under intestinal conditions. The relevance of the temporal control of ctxAB and tcpA expression to the pathophysiology of infection remains to be elucidated.
Examination of the virulence regulatory process in V. cholerae has revealed that temperature, pH, and osmolarity conditions presumed to resemble intestinal conditions closely are nonpermissive for expression of the virulence regulon in vitro (24). There is evidence that the model proposed for heat shock-mediated regulation of virulence based on in vitro studies could not be extrapolated to the in vivo situation (3). Nevertheless, in spite of recent developments, methodological limitations still require that detailed genetic analysis of the virulence regulatory process be carried out in vitro, emphasizing the necessity of identifying environmental conditions for these studies that are physiologically relevant and provide authentic information similar to that obtained from in vivo studies. We demonstrate that virulence regulation during anaerobic growth of V. cholerae resembles events that occur in vivo, at least in the early stages of infection, more closely than regulation under the other in vitro conditions studied resembles the in vivo situation.
Acknowledgments
We thank all members of the Biophysics Division for their cooperation, encouragement, and helpful discussions during this study and I. Guha Thakurta and P. Majumdar for excellent technical support.
H.H.K. and A.G. are grateful to the Council of Scientific and Industrial Research, Government of India, for research fellowships.
Editor: J. T. Barbieri
REFERENCES
- 1.Ausbel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 2.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci USA 100:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty, S., N. Sengupta, and R. Chowdhury. 1999. Role of DnaK in in vitro and in vivo expression of virulence factors of Vibrio cholerae. Infect. Immun. 67:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortassa, S., and M. A. Aon. 1993. Altered topoisomerase activities may be involved in the regulation of DNA supercoiling in aerobic-anaerobic transitions in Escherichia coli. Mol. Cell. Biochem. 126:115-124. [DOI] [PubMed] [Google Scholar]
- 5.De, S. N., and S. N. Chatterjee. 1953. An experimental study of the mechanisms of action of Vibrio cholerae on intestinal mucosal membrane. J. Pathol. Bacteriol. 46:559-562. [DOI] [PubMed] [Google Scholar]
- 6.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiRita, V. J. 1992. Co-ordinate regulation of virulence genes by ToxR in Vibrio cholerae. Mol. Microbiol. 6:451-458. [DOI] [PubMed] [Google Scholar]
- 8.Green, J., B. Bennet, P. Jordan, E. T. Ralph, A. J. Thomson, and J. R. Guest. 1996. Reconstitution of the [4Fe-4S] cluster in FNR and demonstration of the aerobic-anaerobic transcription switch in vitro. Biochem. J. 316:887-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guiney, D. G. 1997. Regulation of virulence gene expression by the host environment. J. Clin. Investig. 99:565-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta, S., and R. Chowdhury. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect. Immun. 65:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hase, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, O. H. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin co-regulated pili and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid protein H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 15.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klose, K. E. 2001. Regulation of virulence in Vibrio cholerae. Int. J. Med. Microbiol. 291:81-88. [DOI] [PubMed] [Google Scholar]
- 17.Krispin, O., and R. Allmansberger. 1995. Changes in DNA supertwist as a response of Bacillus subtilis towards different kinds of stress. FEMS Microbiol. Lett. 134:129-135. [DOI] [PubMed] [Google Scholar]
- 18.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krokonis, E. S., and V. J. DiRita. 2003. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Curr. Opin. Microbiol. 6:186-190. [DOI] [PubMed] [Google Scholar]
- 20.Landini, P., and A. J. Zehnder. 2002. The global regulatory hns gene negatively affects adhesion to solid surfaces by anaerobically grown Escherichia coli by modulating expression of flagellar genes and lipopolysaccharide production. J. Bacteriol. 184:1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc, G. J., C. Tartera, and E. S. Metcalf. 1998. Environmental regulation of Salmonella typhi invasion-defective mutants. Infect. Immun. 66:682-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 23.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in the construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295-4303. [DOI] [PMC free article] [PubMed]
- 26.O'Byrne, C. P., N. Ni Bhriain, and C. J. Dorman. 1992. The DNA supercoiling sensitive expression of the S. typhimurium his operon requires the his attenuator and is modulated by anaerobiosis and by osmolarity. Mol. Microbiol. 6:2467-2476. [DOI] [PubMed] [Google Scholar]
- 27.Schuhmacher, D. A., and K. K. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in V. cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta, N., K. Paul, and R. Chowdhury. 2003. The global regulator ArcA modulates expression of virulence factors in Vibrio cholerae. Infect. Immun. 71:5583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skorupski, K., and R. K. Taylor. 1997. Control of ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu, Q., M. Dziejman, and J. J. Mekalanos. 2003. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc. Natl. Acad. Sci. USA 100:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]