Abstract
Objective
To determine swallowing, speech and quality of life (QOL) outcomes following transoral robotic surgery (TORS) for oropharyngeal squamous cell carcinoma (OPSCC).
Design
Prospective cohort study.
Setting
Tertiary care academic comprehensive cancer center.
Patients
81 patients with previously untreated OPSCC.
Intervention
Primary surgical resection via TORS and neck dissection as indicated.
Main Outcome Measures
Patients were asked to complete the Head and Neck Cancer Inventory (HNCI) pre-operatively and at 3 weeks as well as 3, 6 and 12 months post-operatively. Swallowing ability was assessed by independence from a gastrostomy tube (G-Tube). Clinicopathological and follow-up data were also collected.
Results
Mean follow-up time was 22.7 months. HNCI response rates at 3 weeks and 3, 6, and 12 months were 79%, 60%, 63%, 67% respectively. There were overall declines in speech, eating, aesthetic, social and overall QOL domains in the early post-operative periods. However, at 1 year post-TORS scores for aesthetic, social and overall QOL remained high. Radiation therapy was negatively correlated with multiple QOL domains (p<0.05), while age > 55 years correlated with lower speech and aesthetic scores (p<0.05). HPV status did not correlate with any QOL domain. G-Tube rates at 6 and 12 months were 24% and 9%, respectively. The extent of TORS (> 1 oropharyngeal site resected) and age > 55 years predicted the need for a G-Tube at any point after TORS (p<0.05).
Conclusions
Patients with OPSCC treated with TORS maintain a high QOL at 1 year after surgery. Adjuvant treatment and advanced age tend to decrease QOL.
Keywords: Transoral robotic surgery, oropharyngeal cancer, health related quality of life
INTRODUCTION
Oropharyngeal squamous cell carcinoma (OPSCC) was historically treated with primary open-surgery. Cure rates were low, complication rates were high and patient Health-Related Quality of Life (HRQOL) suffered. In an effort to at least minimize morbidity, a quest for organ preservation protocols was undertaken,1–3 and treatment paradigms shifted towards primary external beam radiation therapy (XRT) or chemo-radiation therapy (CRT). Unfortunately, these protocols failed to provide desired solutions as they were often associated with significant acute and chronic toxicities.4–6 The result was impaired upper aerodigestive tract function and suboptimal HRQOL.7, 8 As such, head and neck surgeons have regained an interest in pursuing the ultimate balance between cancer cure, functional outcomes, minimal morbidity and HRQOL.
In the 1990s transoral laser microsurgery (TLM) was pioneered by Steiner for laryngeal tumors9 and eventually was adapted to the oropharynx. Since that time, proponents of primary TLM have demonstrated favorably balanced treatment outcomes in OPSCC.10–14 In 2005 a novel minimally invasive approach to the oropharynx was born: transoral robotic surgery (TORS).5, 15 Soon after, Weinstein et al recognized the potential for TORS as an oncologically sound and function-preserving tool for treating OPSCC.16 The technique improves visualization and adds degrees of freedom to surgical movements. Complication rates are low17 and swallowing function remains high.8, 17, 18 Initial, limited HRQOL data has shown that speech, eating, social and overall QOL domains tend to decrease from baseline, but remain high at 3 months post-TORS.18 However, long term results with significant patient numbers are lacking.
The aim of this study was to explore the short and long term HRQOL as well as functional outcomes in patients with OPSCC undergoing TORS. Secondly, factors correlated with QOL outcomes and factors predicative of poor swallowing were determined.
METHODS
Institutional review board research ethics approval was granted by the Ohio State University Office of Responsible Research Practices (OSU-07061). This study was conducted at a tertiary care academic referral center and comprehensive cancer center.
Setting and Study Design
Patients were enrolled from the Head and Neck Cancer Clinic at the Ohio State University/Arthur G. James Cancer Hospital at their first new-patient referral visit. Following their consultation with a head and neck surgeon, patients met a study coordinator, who explained the study, obtained written consent and formally registered patients for the trial. At this time, baseline data was collected. All cases were formally discussed at a weekly head and neck cancer multidisciplinary tumor board prior to finalizing treatment plans. The design was a prospective cohort study with patients enrolled from April 2008 – September 2012. All patients meeting study criteria were offered TORS as a primary treatment modality.
Patient Selection
Inclusion Criteria
Biopsy proven OPSCC
Clinical T1–T3 disease
Scheduled for TORS
Exclusion Criteria
Inadequate transoral exposure to allow for TORS instrumentation
Inability to complete HNCI
Pre-operative positron emission-computed tomography (PET-CT) demonstrating distant metastases
Panendoscopy demonstrating an unresectable primary tumor or a synchronous second primary tumor
Research Questions
What are the short and long term quality of life outcomes in patients with OPSCC undergoing TORS?
What are the short and long term G-Tube dependence rates in patients with OPSCC undergoing TORS?
Are there any peri-operative variables predictive of QOL or G-Tube dependence in OPSCC patients undergoing TORS?
Treatment
Patients with head and neck cancers at the Ohio State University underwent a standard metastatic work-up including a full body PET-CT and panendoscopy.19 Those that chose to undergo TORS were booked for a single-staged procedure including panendoscopy, TORS tumor resection as well as concurrent neck dissection. TORS was performed with the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, California) after panednoscopy as per previously described protocols.16, 18, 20 Following tumor resection, frozen section biopsies were taken from all mucosal and deep margins and sent to a head and neck pathologist for immediate analysis. During margin review the robot was removed from the operative field and the patient was prepared for concurrent neck dissection. Bilateral neck dissection was performed on patients with lesions encroaching the midline. Following neck dissection, the robot was brought back into the field if margins needed to be revised based on frozen section analysis.
The extent of TORS resection was determined by the number of oropharyngeal sites significantly (tonsil, BOT, soft-palate) involved in the resection. Significant involvement included ≥ 1 cm of the ipsilateral portion of the subsite. These patients also underwent a local uvular mucosal rotational flap closure of the palate defect. All other patients were not reconstructed, but rather left to heal by secondary intention.
Adjuvant treatment including external beam radiation therapy (XRT) or concurrent chemo-radiation therapy (CRT) was delivered within 6 weeks of TORS. Post-operative XRT/CRT was offered in the presence of high-risk disease features as per National Comprehensive Cancer Network guidelines.21 The mean XRT dose was 65 Gy (60–74 Gy) divided over a 6–7 week treatment course. Chemotherapy regimens were either cisplatin (75%) or cetuximab (25%) based with 3 scheduled doses, 3 wks apart.
Data Collection
Clicopathologic data was collected prospectively by a research coordinator as it became available. Preoperative data included: age at surgery, sex, race, tissue diagnosis, site of tumor, Charleson Comorbidity Index (CCI), and smoking status/pack year history. Post-operative data included: extent of TORS, type of neck dissection, adjuvant treatment, Human Papilloma Virus (HPV) status, protein p16INKa (p16) status, nodal status, TNM classification, AJCC staging,22 peri-operative complications, length of hospital stay, follow-up time, G-Tube dependence, and quality of life scores. The presence of HPV in tumor tissue was determined via chromogenic in situ hybridization for high-risk types of HPV. Immunohistochemical staining was performed on sections of paraffin-embedded tumor tissue for p16. All pathologic protocols were implemented and standardized by a group of Ohio State University head and neck pathologists. Time frames were referenced from the day of surgery (baseline), with assessments occurring at 3 weeks, as well as at 3, 6 and 12 months post-TORS.
Outcome Measures
Health-Related Quality of Life (HRQOL)
The Head and Neck Cancer Inventory (HNCI) was utilized to determine head and neck cancer specific HRQOL.23 This is a validated, quantitative QOL instrument with excellent inter-rater and intra-rater reliability. It employs a 30-item multi-dimensional survey that measures head and neck cancer specific outcomes in 4 domains: speech, eating, social disruption and aesthetics. For each domain, the patient’s functional (ability to perform the task) and attitudinal (satisfaction with the task performance) scores are determined. The final item includes an overall QOL of life score. Each item is scored on an ordinal scale ranging from 1 to 5, with the scores being converted to a 0–100 scale to aid in interpretation.23 Previous studies have stratified mean domain scores into 3 groups: high (70–100), intermediate (31–69) and low (0–30) HRQOL.7, 24 Patients were asked to complete the HNCI unaided, in the absence of a health care professional after their routinely scheduled follow-up visits. The questionnaires were collected by a research coordinator and the results were input into a database.
Swallowing Function
G-Tube dependence was used as a surrogate for swallowing ability. This is a well accepted standard measure of swallowing function and was defined as having to utilize tube feeds to maintain daily caloric needs.25 Patients using G-Tube feeding for any caloric needs were coded as G-Tube dependent. The rate of G-Tube dependence (# of patients using a G-Tube/# of patients assessed at time of follow-up) was determined at baseline as well as 6 and 12 months post-TORS.
Statistical Analysis
Statistical analysis was conducted using the SPSS 17.0 software package (SPSS Inc., Chicago, IL). Continuous variables were compared using a Mann-Whitney U test, and categorical variables with a Chi-squared test. The Mann-Whitney U test was also used to compare ordinal values across time periods. Peri-operative variables potentially predictive of G-Tube dependence after TORS were identified in a logistic regression analysis. Peri-operative patient and tumor variables correlated with HRQOL domains were identified using correlation analysis based on Spearman’s Rho coefficient. All comparisons were two-tailed and statistical significance was set as p < 0.05. Estimated 2 and 4 year survival rates were calculated with Kaplan-Meier analysis.
RESULTS
98 patients with OPSCC were evaluated at the Head and Neck Cancer Clinic at the Ohio State University/Arthur G. James Cancer Hospital and offered TORS. 11 patients refused surgical treatment and opted for XRT/CRT. Thus, 87 patients with OPSCC were enrolled in the study protocol. 6 were excluded: 3 dropped out, 1 cancelled their operation, 1 could not be adequately exposed to allow for TORS and 1 was found to have distant metastases on pre-operative imaging. Thus, 81 patients with OPSCC undergoing TORS were included in the analysis.
The mean age at TORS was 58.3 years (range: 39.0–80.6 years). 16 patients were female (20%) and 65 were male (80%). 1 patient was African American (1%), while the remainder were Caucasian. The mean CCI score was 6.6 (range:2–17). 62 patients (77%) were smokers with a mean pack year history of 31.7 years (range: 1–120 years). All patients had SCC with 65 (80%) lesions occurring in a palatine tonsil and 16 (20%) in the base of tongue (BOT). 8 (9.9%) resection margins were positive as per widely accepted guidelines.26 Table 1 demonstrates tumor and staging data.
Table 1.
Pathologic Information
| Characteristic | n (%) |
|---|---|
|
| |
| pT (n=81) | |
| T1 | 34 (42) |
| T2 | 39 (48) |
| T3 | 6 (7) |
| T4 | 2 (3) |
| pN (n=79) | |
| N0 | 9 (11) |
| N1 | 9 (11) |
| N2a | 21 (27) |
| N2b | 31 (39) |
| N2c | 3 (4) |
| N3 | 6 (8) |
| pOverall Stage (n=79) | |
| I | 7 (9) |
| III | 9 (11) |
| IV | 63 (80) |
| HPV status (n=71) | |
| HPV+ | 51 (72) |
| HPV− | 20 (28) |
| p16 Status (n=71) | |
| p16+ | 60 (85) |
| p16− | 11 (15) |
Abbreviations: n, number; pT, pathological Tumor-classification; pN, pathologic Node-classification; HPV, Human Papilloma Virus; protein p16INKa.
Table 2 presents treatment details. 2 patients withdrew consent for neck dissection at the time of surgery and chose to have neck XRT instead. 2 (2.5%) patients had received CRT preoperatively, but had persistent disease. As such, pathologic staging information could not be obtained and they were classified as Nx. The mean number of positive lymph nodes on final pathologic review was 2.2 (range: 0–18) and total nodes was 30.7 (range: 5–77). 3 (4%) patients had neck hematomas requiring operative evacuation within 8 hours of surgery. No sequalae resulted from these cases. 2 intraoperative fistulae from the pharynx to the submandibular space were detected. One was repaired with alloderm and the other by transposing the submandibular gland and re-enforcing it with a digastric muscle flap. In both cases no orocutaneous fistulae developed post-operatively. No other perioperative complications requiring operative intervention were encountered. There were no incidences of hypoglossal or lingual nerve injury. The mean length of hospital stay was 3.7 days (range: 1–9). The mean follow-up time was 22.7 months (range: 2.5–51.2 months). At the time of the current study 8 patients had passed away. The 2 and 4 year disease specific survival were 92% and 89%, respectively. There were no cases of 30 day mortality.
Table 2.
Treatment Details
| Treatment variable | n (%) |
|---|---|
|
| |
| Extent of TORS | |
| 1 oropharyngeal site | 52 (64) |
| > 1 oropharyngeal site | 26 (32) |
| Neck dissection | |
| None | 2 (2) |
| Unilateral | 79 (98) |
| Bilateral | 9 (11) |
| Adjuvant therapy | |
| XRT | 69 (87) |
| CRT | 49 (62) |
Abbreviations: n, number; TORS, transoral robotic surgery; XRT, external beam radiation therapy; CRT, chemo-radiation therapy.
All patients were discharged home on a full oral diet, without any patient requiring nasogastric feeding during their hospital stay. No patients were re-admitted for dysphagia prior to starting XRT. 17 (21%) patients required G-Tube insertion at some point after TORS due to dysphagia and inability to maintain daily caloric needs. In 8 (47%) of these patients the G-Tube was inserted temporarily during XRT/CRT and was removed before 1 year post-TORS. 4 (24%) patients had G-Tubes placed for palliative reasons at 12 months or later after TORS. The remaining 5 (29%) G-Tubes were placed during XRT/CRT; however, these patients could not regain sufficient swallowing function to become G-Tube independent. The mean time to G-Tube insertion was 5.5 months (range: 1.0–30.8 months) post-TORS. 1 patient had a G-Tube pre-operatively, secondary to previous XRT, and did not regain swallowing function after TORS. The specific perioperative G-Tube rates are shown in Table 3. Only 1 (1%) patient required a tracheostomy tube, which was removed prior to leaving the hospital.
Table 3.
Perioperative G-Tube Dependence Rates
| Time Frame | G-Tube Dependence, n (%) |
|---|---|
|
| |
| Pre-TORS (n=81) | 1 (1) |
| 6 months post-TORS (n=77) | 18 (24) |
| 12 months post-TORS (n=66) | 6 (9) |
Abbreviations: G-Tube, gastrostomy tube; n, number; TORS, transoral robotic surgery.
The results of logistic regression analysis of factors predictive of G-Tube dependence are shown in Table 4. The analysis was carried out to identify patients at risk of needing a G-Tube at some point during treatment and those who would retain the G-Tube without maintenance of adequate oral nutrition. Age ≥ 55 years and the extent of TORS predicted the need for a G-Tube, while advanced pT-classification (pT3/pT4) predicted patients who would not be able to rid their G-Tube once it was inserted. A similar analysis of factors potentially predictive of G-Tube dependence at 12 months post-TORS was also carried out and all of the same factors were found not to be statistically significant predictors (p>0.05).
Table 4.
Logistic Regression Analysis to Predict G-Tube Dependence
| G-Tube after TORS | Permanent G-Tube after TORS | |||||
|---|---|---|---|---|---|---|
| p-Value | OR | 95% CI | p-Value | OR | 95% CI | |
| Sex | 0.28 | 2.4 | 0.5–11.6 | 0.99 | 1.0 | 0.1–9.8 |
| Age ≥ 55 y | 0.047 a | 4.8 | 1.0–23.0 | 0.70 | 0.7 | 0.1–4.4 |
| CCI > 6 | 0.13 | 0.4 | 0.1–1.3 | 0.67 | 0.7 | 0.1–4.2 |
| Smoking | 0.09 | 0.4 | 0.1–1.1 | 0.94 | 0.9 | 0.1–5.9 |
| HPV | 0.68 | 0.8 | 0.2–2.6 | 0.99 | NA | NA |
| Tumor Site | 0.97 | 0.8 | 0.2–3.4 | 0.90 | 0.9 | 0.09–8.3 |
| pT | 0.46 | 2.3 | 0.7–7.5 | 0.002a | 27.0 | 3.5–210.4 |
| pN | 0.91 | NA | NA | 0.99 | NA | NA |
| Stage | 0.80 | NA | NA | 0.99 | NA | NA |
| XRT | 0.32 | 2.9 | 0.3–25.0 | 0.99 | NA | NA |
| CRT | 0.65 | 1.3 | 0.4–3.9 | 0.99 | NA | NA |
| Extent of TORS | 0.01a | 5.6 | 1.5–21.4 | 0.12 | 5.8 | 0.6–54.5 |
Abbreviations: G-Tube, gastrostomy tube; TORS, transoral robotic surgery; OR, odds ratio; CI, confidence interval; y, years; CCI, Charelson Comorbidty Index; HPV, Human Papilloma Virus; pT, pathological Tumor-classification; pN, pathologic Node-classification; NA, not analyzable due to low numbers; XRT, post-operative external beam radiation therapy; CRT, post-operative chemo-radiation therapy.
Denotes statistical significance
Table 6 provides HRQOL outcome values and comparisons. Figure 1 illustrates the QOL outcomes by time frame and domain. Long term outcomes are represented at 12 months post-TORS with differences and comparisons being calculated from the baseline. 76 (94%) patients completed the questionnaire at baseline, 64 (79%) at 3 weeks, 49 (60%) at 3 months, 47 (63%) at 6 months and 42 (67%) at 12 months post-TORS. All patients were able to complete the questionnaire on their own without the assistance of a hospital staff, research team or family member. There were no statistically significant differences in patient age (p=0.61), sex (p=0.28), CCI (p=0.10), smoking status (p=0.36), tumor site (p=0.08), T-classification (p=0.51), N-classification (p=0.24), overall stage (p=0.41), HPV status (p=0.62), extent of surgery (p=0.58), adjuvant therapy (p=0.49), G-Tube dependence (p=0.16), or complication status (p=0.67) between patients who completed and did not complete the questionnaires at 12 months. Table 5 illustrates clinicopathologic factors and their associations with HRQOL domains on the HNCI.
Table 6.
Perioperative HNCI HRQOL scores by QOL domain.
| Time | HNCI HRQOL Domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Speech function | Speech attitude | Eating function | Eating attitude | Aesthetic attitude | Social function | Social attitude | Overall function | Overall attitude | Overall QOL | |
| Baseline | 89.5 (16.6) | 84.0 (18.4) | 86.3 (17.5) | 85.5 (19.2) | 90.1 (20.4) | 85.1 (22.7) | 86.9 (13.7) | 87.0 (16.1) | 86.9 (13.7) | 76.3 (21.7) |
| 3 wks | 81.7 (20.5) | 80.3 (21.5) | 64.4 (21.7) | 75.5 (20.1) | 81.2 (25.5) | 60.4 (21.5) | 80.2 (18.9) | 69.8 (18.3) | 79.4 (16.2) | 71.1 (20.5) |
| 3 mos | 72.0 (26.6) | 72.9 (26.0) | 58.1 (23.9) | 50.3 (24.1) | 83.9 (22.5) | 61.2 (29.8) | 76.0 (26.6) | 65.9 (19.3) | 70.6 (18.8) | 61.2 (27.0) |
| 6 mos | 78.4 (20.6) | 79.0 (20.9) | 57.8 (24.8) | 49.8 (23.3) | 83.1 (22.6) | 72.0 (27.8) | 80.2 (20.3) | 67.5 (20.3) | 73.5 (18.0) | 66.0 (25.8) |
| 12 mos | 80.3 (20.5) | 81.4 (21.8) | 58.5 (27.5) | 57.9 (30.1) | 84.2 (24.2) | 78.7 (27.7) | 84.2 (21.4) | 70.5 (21.7) | 78.0 (19.4) | 76.8 (20.5) |
|
| ||||||||||
| 12 mos – baseline | −9.2 | −2.6 | −27.8 | −27.6 | −7.0 | −6.4 | −2.7 | −19.5 | −8.9 | +0.5 |
| p-value | 0.002a | 0.77 | <0.001 a | <0.001 a | 0.07 | 0.28 | 0.37 | <0.001 a | 0.01 a | 0.98 |
Abbreviations: HNCI, Head and Neck Cancer Inventory; HRQOL, Health Related Quality of Life; QOL, Quality of Life; wks, weeks; mos, months.
Data are given as: mean (standard deviation). p-Values demonstrate a comparison between baseline and 12-months post-TORS values.
Denotes statistical significance
Figure 1.
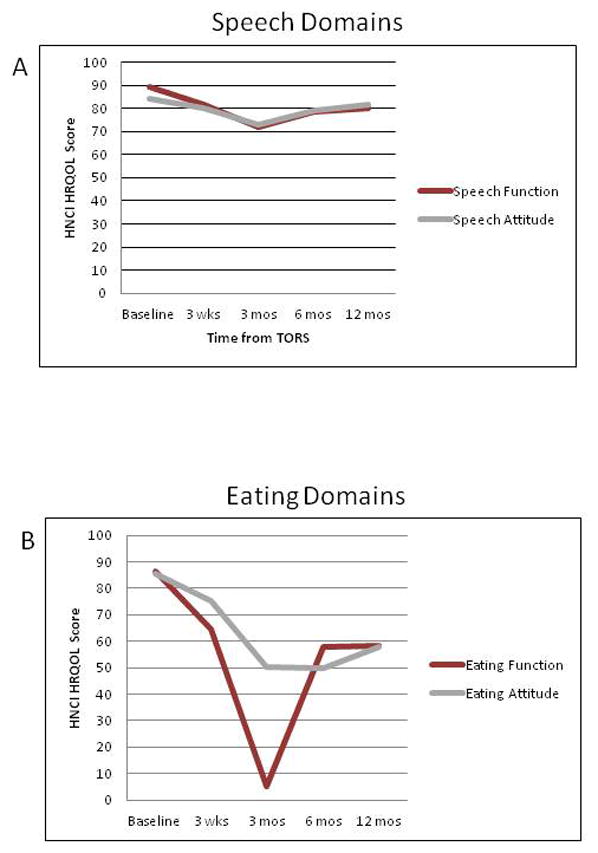

Perioperative HNCI HRQOL scores by QOL domain.
Abbreviations: HNCI, Head and Neck Cancer Inventory; HRQOL, Health Related Quality of Life; QOL, Quality of Life; wks, weeks; mos, months.
Table 5.
Patient-Related Factors and HRQOL Outcomes 12 Months Post-TORS
| HNCI HRQOL Domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Variable | n | Speech function |
Speech attitude |
Eating function |
Eating attitude |
Aesthetic attitude |
Social function |
Social attitude |
Overall function |
Overall attitude |
Overall QOL |
| Sex | 0.30 | 0.60 | 0.06 | 0.13 | 0.61 | 0.14 | 0.64 | 0.07 | 0.27 | 0.21 | |
| Male | 35 | 78 (21) | 81 (23) | 55 (27) | 55 (30) | 84 (25) | 76 (29) | 83 (23) | 68 (22) | 77 (20) | 75 (20) |
| Female | 7 | 88 (13) | 86 (17) | 77 (26) | 74 (24) | 88 (24) | 93 (12) | 90 (12) | 84 (17) | 85 (15) | 86 (20) |
|
| |||||||||||
| Age | 0.25 | 0.03a | 0.43 | 0.30 | 0.04a | 0.23 | 0.44 | 0.38 | 0.17 | 0.11 | |
| ≥ 55 y | 26 | 84 (15) | 88 (14) | 61 (26) | 62 (28) | 91 (15) | 83 (24) | 86 (21) | 74 (17) | 82 (14) | 81 (19) |
| < 55 y | 16 | 72 (27) | 71 (27) | 54 (30) | 51 (33) | 73 (21) | 71 (32) | 81 (23) | 65 (27) | 70 (25) | 70 (21) |
|
| |||||||||||
| Smoking | 0.95 | 0.89 | 0.71 | 0.58 | 0.98 | 0.88 | 0.17 | 0.99 | 0.34 | 0.98 | |
| Smoker | 28 | 78 (21) | 80 (24) | 54 (28) | 55 (29) | 79 (27) | 76 (31) | 81 (24) | 67 (22) | 75 (21) | 78 (20) |
| Non-Smoker | 14 | 84 (19) | 85 (17) | 68 (25) | 63 (33) | 94 (15) | 84 (19) | 91 (12) | 77 (20) | 83 (14) | 75 (22) |
|
| |||||||||||
| HPV | 0.43 | 0.47 | 0.70 | 0.96 | 0.97 | 0.81 | 0.57 | 0.99 | 0.56 | 0.72 | |
| HPV + | 31 | 79 (19) | 80 (19) | 60 (26) | 58 (29) | 84 (21) | 78 (27) | 83 (21) | 71 (21) | 77 (17) | 75 (22) |
| HPV − | 8 | 81 (28) | 81 (33) | 54 (37) | 56 (36) | 78 (36) | 75 (32) | 83 (27) | 67 (29) | 76 (30) | 78 (16) |
|
| |||||||||||
| Site | 0.86 | 0.56 | 0.80 | 0.83 | 0.91 | 0.72 | 0.99 | 0.88 | 0.73 | 0.46 | |
| Tonsil | 35 | 80 (20) | 81 (22) | 58 (27) | 58 (29) | 83 (25) | 79 (29) | 84 (23) | 70 (21) | 78 (20) | 78 (20) |
| BOT | 7 | 78 (23) | 84 (22) | 62 (32) | 60 (36) | 88 (19) | 77 (24) | 87 (14) | 71 (26) | 80 (18) | 71 (22) |
|
| |||||||||||
| pT | 0.75 | 0.86 | 0.35 | 0.70 | 0.95 | 0.50 | 0.53 | 0.45 | 0.73 | 0.86 | |
| T1/T2 | 38 | 81 (21) | 82 (22) | 59 (29) | 58 (31) | 84 (24) | 77 (28) | 84 (22) | 71 (23) | 78 (20) | 77 (22) |
| T3/T4 | 4 | 70 (16) | 75 (21) | 53 (5) | 53 (5) | 88 (25) | 90 (21) | 88 (15) | 65 (9) | 77 (17) | 75 (1) |
|
| |||||||||||
| pN | 0.88 | 0.87 | 0.77 | 0.93 | 0.40 | 0.48 | 0.25 | 0.80 | 0.60 | 0.48 | |
| N0 | 7 | 76 (29) | 79 (35) | 66 (37) | 68 (40) | 79 (39) | 75 (35) | 79 (27) | 71 (33) | 77 (33) | 79 (22) |
| N+ | 34 | 80 (19) | 82 (19) | 57 (26) | 55 (28) | 85 (21) | 79 (27) | 85 (20) | 70 (19) | 78 (16) | 76 (20) |
|
| |||||||||||
| Overall Stage | 0.66 | 0.70 | 0.90 | 0.77 | 0.88 | 0.25 | 0.12 | 0.95 | 0.62 | 0.41 | |
| I/II | 5 | 73 (33) | 76 (42) | 61 (41) | 61 (46) | 70 (45) | 67 (39) | 70 (28) | 66 (37) | 70 (38) | 75 (25) |
| III/IV | 36 | 81 (19) | 82 (18) | 58 (26) | 57 (28) | 86 (21) | 80 (26) | 86 (20) | 71 (20) | 79 (16) | 76 (20) |
|
| |||||||||||
| Extent of TORS | 0.43 | 0.13 | 0.80 | 0.80 | 0.70 | 0.40 | 0.86 | 0.49 | 0.93 | 0.33 | |
| 1 Site | 22 | 78 (22) | 77 (25) | 57 (26) | 56 (31) | 80 (29) | 77 (31) | 82 (25) | 69 (23) | 75 (22) | 80 (21) |
| > 1 Site | 19 | 83 (18) | 86 (17) | 61 (30) | 60 (30) | 89 (17) | 80 (24) | 87 (17) | 72 (21) | 82 (15) | 74 (19) |
|
| |||||||||||
| XRT | 0.007a | 0.01a | 0.003a | 0.005a | 0.06 | 0.09 | 0.10 | 0.002a | 0.003a | 0.13 | |
| No XRT | 5 | 98 (3) | 98 (4) | 91 (14) | 90 (11) | 100 (1) | 97 (7) | 96 (8) | 94 (7) | 96 (2) | 90 (14) |
| XRT | 37 | 78 (21) | 79 (22) | 54 (26) | 53 (29) | 82 (25) | 76 (29) | 82 (22) | 67 (21) | 75 (19) | 75 (20) |
|
| |||||||||||
| CRT | 0.10 | 0.05 | 0.04a | 0.04a | 0.20 | 0.27 | 0.26 | 0.006a | 0.04a | 0.31 | |
| No CRT | 5 | 87 (24) | 91 (20) | 79 (24) | 78 (22) | 95 (11) | 88 (18) | 93 (10) | 84 (22) | 89 (15) | 85 (22) |
| CRT | 25 | 78 (19) | 80 (24) | 53 (23) | 53 (29) | 80 (27) | 75 (26) | 82 (21) | 66 (19) | 75 (20) | 75 (20) |
|
| |||||||||||
| Adjuvant Treatment | 0.76 | 0.72 | 0.74 | 0.95 | 0.41 | 0.37 | 0.55 | 0.42 | 0.91 | 0.99 | |
| XRT | 12 | 76 (25) | 78 (20) | 56 (32) | 53 (31) | 86 (22) | 78 (35) | 84 (27) | 70 (26) | 76 (19) | 75 (22) |
| CRT | 25 | 78 (19) | 80 (24) | 53 (23) | 80 (27) | 75 (26) | 82 (20) | 82 (20) | 66 (19) | 75 (20) | 75 (20) |
|
| |||||||||||
| G-Tube | 0.99 | 0.42 | <0.001a | <0.001a | 0.92 | 0.98 | 0.98 | 0.20 | 0.27 | 0.93 | |
| No G-Tube | 40 | 80 (20) | 82 (22) | 60 (27) | 60 (30) | 84 (25) | 79 (28) | 84 (21) | 71 (22) | 79 (20) | 77 (20) |
| G-Tube | 2 | 78 (32) | 73 (25) | 29 (15) | 25 (12) | 88 (18) | 79 (29) | 84 (22) | 54 (7) | 68 (15) | 75 (35) |
Abbreviations: HNCI, Head and Neck Cancer Inventory; HRQOL, Health Related Quality of Life; n, number; y, years; HPV, Human Papilloma Virus; BOT, Base of Tongue; pT, pathologic Tumor-classification; pN, pathologic Node-classification; N+, pathologically positive neck; TORS, transoral robotic surgery; XRT, post-operative external beam radiation therapy; CRT, post-operative chemo-radiation therapy; G-Tube, gastrostomy tube.
Data are given as: p-value; mean (standard deviation)
Denotes statistical significance
COMMENT
The goals of head and neck cancer treatment are continually redefined. In recent years head and neck oncologists have focused on maximizing survival while optimizing QOL during that survival. Many studies have implemented self-assessment QOL tools to determine if these goals are met. As TORS is a rather novel treatment tool for OPSCC, there is a paucity of HRQOL information. To our knowledge, this is the largest study to evaluate long term QOL post-TORS in a single-center, prospective manner.
The patients studied represent a population similar to those described in previous reports.18, 20, 27, 28 Most patients have early T-classification disease with significant nodal burden. Thus, most patients exhibit stage IV disease. Furthermore, consistent with the current viral-induced cancer epidemic17 > 70% of patients are HPV+ or p16+; yet, smoking remains prominent in more than ¾ of these patients. Patients underwent similar treatment protocols described previously with comparable survival rates.46
Temporal changes for HRQOL scores in this study followed expected trends. All HRQOL scores declined at 3 weeks after TORS. Speech, eating, social and overall scores continued to drop and bottomed out at 3 months post-TORS. This time frame coincides with XRT/CRT treatment, during which patients face many challenges with the acute toxicity of adjuvant treatment.4, 29, 30 HRQOL and functional outcomes tend to be lowest at this point; the magnitude of dysfunction often determines how patients recover.18, 29 Haughey et al have found parallel patterns in TLM for OPSCC.11, 13, 14 Fortunately, most XRT/CRT disturbances tend to recover by 12 months and scores return to intermediate – high levels (Figure 1). Speech attitude, aesthetic, social and overall scores demonstrated the greatest recovery and were statistically indifferent from baseline (p>0.05). Speech function, and aesthetic attitude showed partial recovery, but remained significantly below baseline (p<0.05). Speech function, eating function and eating attitude scores dropped the most with minimal recovery by 12 months (p<0.05). While statistical differences helped identify HNCI domains affected most by treatment, these values need to be correlated with clinical meaning. Funk et al. determined Clinically Important Differences (CIDs) for the HNCI domains to fall into three categories: small, medium and high.24
Speech function showed a statistically significant and small CID from baseline (Figure 1A). Previous data supports that patients with OPSCC treated with primary surgery can maintain significant speech function as long as the majority of critical speech structures are maintained.20, 31, 32 TORS OPSCC resections are largely limited to the tonsillar fossa/lateral pharyngeal wall with preservation of most of the soft palate and BOT; thus, it is expected that speech should be preserved. Similar to this study, Leonhardt et al. also found that speech function is only moderately affected by TORS.29 However, adjuvant XRT was found to be significantly correlated with lower speech function (p=0.007) and speech attitude scores (p=0.01) at 12 months post-TORS. XRT is known to cause irreversible long term fibrosis and impaired mobility of the upper aerodigestive tract,33 which can result in poor long term functional recovery.29 Age < 55 years was also found to correlate with lower speech attitude scores (p=0.03). Because younger patients tend to have a higher baseline functional status, it is postulated that their attitude towards lowered HRQOL physical domains declines; thus, producing lower scores.18, 34, 35
Aesthetic attitude showed a small CID without statistical significance (p>0.05) (Table 6). Lower scores were correlated with age < 55 years (p=0.04), which is often observed in head and neck cancer patients.36, 37 This is likely due to a dynamic self-perception of facial aesthetics38 and less importance placed on this domain in determining HRQOL with aging.37 Social function and attitude also showed non-statistically significant small CIDs over time. No perioperative factors correlated with lower scores in these domains. These results compare favorably with previous studies and suggest that social domains are maintained in the long term after TORS and seem to be less affected than in open surgical approaches.39,40
Eating function and attitude were the most affected HRQOL domains at 12 months after TORS (Figure 1B). Both domains suffered statistically significant and large CIDs from baseline (Table 6). Earlier results on a smaller group of patients showed similar differences, but lacked statistical significance18. Smaller studies, using a different QOL scale, found smaller declines in patient-perceived swallowing function after TORS.28, 29 However, poor recovery at 6 and 12 months was also observed in similar patient populations.28, 29 Patients who undergo adjuvant XRT or CRT have the lowest eating HRQOL domain scores (p<0.05) with differences of nearly 40 (XRT) or 30 (CRT) points compared to their counterparts who avoided XRT or CRT. This finding is not unique; XRT and CRT are known to cause significant deterioration in perceived swallowing function.7, 18, 28, 29, 41 However, when scores for patients who underwent adjuvant CRT versus XRT-only were compared, there were no statistically significant correlations (p>0.05) or CIDs within eating domains (Table 5). It is postulated that it is adjuvant XRT after TORS, and not the chemotherapy, which influences long term eating function the most. Previous studies have emphasized the importance of CRT on long term HRQOL, but have not compared XRT directly to CRT in TORS patients.29,28, 42 Patients who avoided any adjuvant treatment showed superior HRQOL outcomes, as supported by other data.18, 28, 29
All patients were able to tolerate a full oral diet by the time of hospital discharge. The TORS literature quotes return to swallowing times of approximately 0–14 days.18, 43–45 However, it is known that objective swallowing ability will deteriorate with adjuvant treatment. 7, 8, 11, 13, 28, 29, 46, 47 A fifth of patients required a G-Tube at some point after TORS with 24% still using their G-Tube at 6 months. The most common indication for tube feeding was dysphagia during XRT/CRT. Approximately half of these patients were able to regain swallowing ability by 12 months post-TORS. It was found that patients with G-Tubes had significantly worse HRQOL eating scores, as would be predicted by landmark head and neck cancer QOL literature.7, 48
To better counsel patients, it is worth knowing variables predictive of needing a G-Tube. In the current analysis, it was found that older patients (≥ 55 years) were nearly 5 times as likely to need a G-Tube after TORS compared to their younger counterparts. This is potentially due to a lower baseline functional status and less of a capacity for aggressive swallowing therapy in the elderly. Secondly if TORS resection included > 1 oropharyngeal subsite, patients had a 5.6 time increased risk of needing a G-Tube. This is a novel piece of information in the TORS literature, but is supported by previous findings that as more swallowing structures are violated by surgery/XRT, swallowing function deteriorates and recovery is poor.25, 49, 50 One factor predicted the need for a permanent G-Tube after TORS: pT-classification. Patients with pT3 or pT4 tumors were 27 times as likely to not be weaned off of G-Tube feeds. Previous TORS studies have also shown advanced T-classification to be predictive of poor swallowing function and retained G-Tubes.8, 44
Although most authors were using peri-operative tracheostomy tubes with the introduction of TORS, this seems to be a passing trend. Only 1 patient received a perioperative tracheostomy in this series. The current literature reports tracheostomy rates of 0–31%, with most authors demonstrating the safety of the technique without a surgical airway.47
Overall HRQOL scores provide a summary of all patient perceived outcomes (Figure 1D). Overall function showed similar trends to eating domains with initial drop-offs and incomplete recovery. The difference from baseline was a significant medium CID (<0.001). Overall attitude also demonstrated a significant change from baseline, but this represented only a small CID with good 12 month recovery. Lastly, overall 12 month QOL demonstrated no significant change from baseline (p>0.05). This is despite significant deterioration in eating and speech domains. Previous studies have found similar results with high overall quality of life, despite major disruptions in other areas of HRQOL.7, 18 Possible explanation for this paradox stems from the definition of QOL: the perceived discrepancy between reality and what a person expected this reality to be.51 It is conceivable that with appropriate pre-operative counseling, patients are able to set appropriate expectations; therefore, maintaining their pre-TORS overall QOL.
Many studies evaluating HRQOL in OPSCC after surgery or XRT/CRT exist with a wide range of outcomes. Recent literature continues to show that XRT/CRT have a negative impact on QOL and swallowing function52 with xerostomia-related complications being the most prominent obstacles for patients to overcome.53 Minimizing and focusing XRT, while avoiding chemotherapy lead to less treatment toxicity and improved outcomes.53–55 Patients with early stage disease treated with surgery alone, demonstrate superior outcomes by avoiding the toxicity of XRT.27,54,56 Although this data is still in its infancy, TORS is showing promise as an optimal treatment strategy in early stage disease.
Despite the abundance of QOL data available, direct comparisons between treatment modalities remains a challenge. The crux of the matter is a lack of standardized outcome measures.25, 57 One study with similar patients, who were treated with primary CRT or surgery and XRT (SRT), using the same measures as this study (HNCI) was identified.7 All HRQOL domains in the work published by El-Deiry et al demonstrated lower scores compared to this TORS cohort. The most striking domains differences were in the mean eating: CRT: 37.8, SRT: 40.8, TORS: 58.2 and speech: CRT: 65.1, SRT: 56.0, TORS: 80.9. Overall QOL also differed: CRT: 55.0, SRT: 64.0, TORS: 76.8. Overall there appears to be a pattern of increased HRQOL scores favoring TORS. This could very well be to the minimally invasive nature of the technique and lowered XRT dose used in the post-operative setting.
Limitations of this study are acknowledged. Although it is the largest cohort of its kind, 12 month follow-up data was not available for all patients, leaving the data open to selection bias. There was also a lack of a comparison arm, which ideally would be addressed with a randomized trial with primary CRT. Due to the geographic nature of the treatment facility, many patients did not have adjuvant treatment at The Ohio State University Wexner Medical Center. Thus, there was a lack of standardization of type and dose of XRT/CRT. It would be difficult to convince all patients to travel to a standardized location for adjuvant treatment; thus, eliminating this confounding variable would be unrealistic.
Despite the growing literature on TORS a critical question remains: how can treatment be customized to strike an optimal balance between survival, function and HRQOL? This study further continues to demonstrate that TORS is an important treatment tool in OPSCC. However, a multi-institutional, standardized protocol comparing surgery to XRT/CRT is necessary to answer this question.
CONCLUSION
This study is the largest prospective, longitudinal single-center study evaluating HRQOL and functional outcomes in OPSCC patients undergoing TORS. The results show TORS to be safe with excellent overall QOL and functional outcomes. Patients who undergo XRT tend to demonstrate worse HRQOL scores, but by 12 months post-TORS overall QOL returns to baseline values. G-Tube rates are low. However, patients with advanced age, extensive resections and advanced pT-classification are at increased risk of needing or retaining a G-Tube. These results advocate TORS as a viable alternative to primary CRT in OPSCC treatment.
Footnotes
Conflicts of Interest: Dr. Enver Ozer is a surgical proctor for Intuitive Surgical Inc.
Ethics Approval: Prior to commencement of this study, institutional research board ethics approval was obtained.
Manuscript Presentation: This study was presented as an oral presentation at the Combined Otolaryngology Spring Meeting, American Head and Neck Society; Orlando, FL; April 10, 2013.
Financial Support/Disclosure: None.
References
- 1.Soo KC, Tan EH, Wee J, et al. Surgery and adjuvant radiotherapy vs concurrent chemoradiotherapy in stage III/IV nonmetastatic squamous cell head and neck cancer: a randomised comparison. Br J Cancer. 2005 Aug 8;93(3):279–286. doi: 10.1038/sj.bjc.6602696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcial VA, Pajak TF, Rotman M, Brady LW, Amato D. “Compensated” split-course versus continuous radiation therapy of carcinoma of the tonsillar fossa. Final results of a prospective randomized clinical trial of the Radiation Therapy Oncology Group. Am J Clin Oncol. 1993 Oct;16(5):389–396. doi: 10.1097/00000421-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Morris CG, Amdur RJ, et al. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol. 2006 Jun;29(3):290–297. doi: 10.1097/01.coc.0000209510.19360.f9. [DOI] [PubMed] [Google Scholar]
- 4.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008 Feb;30(2):148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein GS, O’Malley BW, Jr, Hockstein NG. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope. 2005 Jul;115(7):1315–1319. doi: 10.1097/01.MLG.0000170848.76045.47. [DOI] [PubMed] [Google Scholar]
- 6.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009 Dec;135(12):1209–1217. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 7.El-Deiry M, Funk GF, Nalwa S, et al. Long-term quality of life for surgical and nonsurgical treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005 Oct;131(10):879–885. doi: 10.1001/archotol.131.10.879. [DOI] [PubMed] [Google Scholar]
- 8.More YI, Tsue TT, Girod DA, Harbison J, Sykes KJ, Williams C, Shnayder Y. Functional swallowing outcomes following transoral robotic surgery vs primary chemoradiotherapy in patients with advanced-stage oropharynx and supraglottis cancers. Arch Otolaryngol Head Neck Surg. 2013 Jan;139(1):43–48. doi: 10.1001/jamaoto.2013.1074. [DOI] [PubMed] [Google Scholar]
- 9.Iro H, Waldfahrer F, Altendorf-Hofmann A, Weidenbecher M, Sauer R, Steiner W. Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Arch Otolaryngol Head Neck Surg. 1998 Nov;124(11):1245–1250. doi: 10.1001/archotol.124.11.1245. [DOI] [PubMed] [Google Scholar]
- 10.Canis M, Martin A, Kron M, et al. Results of transoral laser microsurgery in 102 patients with squamous cell carcinoma of the tonsil. Eur Arch Otorhinolaryngol. Dec 29; doi: 10.1007/s00405-012-2335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. Dec;33(12):1683–1694. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 12.Grant DG, Hinni ML, Salassa JR, Perry WC, Hayden RE, Casler JD. Oropharyngeal cancer: a case for single modality treatment with transoral laser microsurgery. Arch Otolaryngol Head Neck Surg. 2009 Dec;135(12):1225–1230. doi: 10.1001/archoto.2009.185. [DOI] [PubMed] [Google Scholar]
- 13.Rich JT, Liu J, Haughey BH. Swallowing function after transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced-stage oropharyngeal cancer. Laryngoscope. Nov;121(11):2381–2390. doi: 10.1002/lary.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigby MH, Taylor SM. Review of transoral laser microsurgery for cancer of the upper aerodigestive tract. J Otolaryngol Head Neck Surg. Apr;40(2):113–121. [PubMed] [Google Scholar]
- 15.McLeod IK, Melder PC. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J. 2005 Mar;84(3):170–172. [PubMed] [Google Scholar]
- 16.Weinstein GS, O’Malley BW, Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007 Dec;133(12):1220–1226. doi: 10.1001/archotol.133.12.1220. [DOI] [PubMed] [Google Scholar]
- 17.Genden EM. The role for surgical management of HPV-related oropharyngeal carcinoma. Head Neck Pathol. Jul;6(Suppl 1):S98–103. doi: 10.1007/s12105-012-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurtuk AM, Marcinow A, Agrawal A, Old M, Teknos TN, Ozer E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg. Jan;146(1):68–73. doi: 10.1177/0194599811421298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurien G, Hu J, Harris J, Seikaly H. Cost-effectiveness of positron emission tomography/computed tomography in the management of advanced head and neck cancer. J Otolaryngol Head Neck Surg. Dec;40(6):468–472. [PubMed] [Google Scholar]
- 20.Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg. Aug;145(2):248–253. doi: 10.1177/0194599811402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfister DGAK, Brizel D, et al. [Date accessed: February 1, 2013.];NCCN Clinical Practice Guidelines in Oncology. 2010 :82. http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf.
- 22.Edge SB. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 23.Funk GF, Karnell LH, Christensen AJ, Moran PJ, Ricks J. Comprehensive head and neck oncology health status assessment. Head Neck. 2003 Jul;25(7):561–575. doi: 10.1002/hed.10245. [DOI] [PubMed] [Google Scholar]
- 24.Funk GF, Karnell LH, Smith RB, Christensen AJ. Clinical significance of health status assessment measures in head and neck cancer: what do quality-of-life scores mean? Arch Otolaryngol Head Neck Surg. 2004 Jul;130(7):825–829. doi: 10.1001/archotol.130.7.825. [DOI] [PubMed] [Google Scholar]
- 25.Rieger JM, Tang JA, Harris J, et al. Survey of current functional outcomes assessment practices in patients with head and neck cancer: initial project of the head and neck research network. J Otolaryngol Head Neck Surg. Oct;39(5):523–531. [PubMed] [Google Scholar]
- 26.Weinstein GS, O’Malley BW, Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. Aug;122(8):1701–1707. doi: 10.1002/lary.23294. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein GS, Quon H, Newman HJ, et al. Transoral robotic surgery alone for oropharyngeal cancer: an analysis of local control. Arch Otolaryngol Head Neck Surg. Jul;138(7):628–634. doi: 10.1001/archoto.2012.1166. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. Nov;137(11):1112–1116. doi: 10.1001/archoto.2011.172. [DOI] [PubMed] [Google Scholar]
- 29.Leonhardt FD, Quon H, Abrahao M, O’Malley BW, Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. Feb;34(2):146–154. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007 Mar 1;67(3):660–669. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rieger JM, Zalmanowitz JG, Li SY, et al. Functional outcomes after surgical reconstruction of the base of tongue using the radial forearm free flap in patients with oropharyngeal carcinoma. Head Neck. 2007 Nov;29(11):1024–1032. doi: 10.1002/hed.20623. [DOI] [PubMed] [Google Scholar]
- 32.Rieger J, Dickson N, Lemire R, et al. Social perception of speech in individuals with oropharyngeal reconstruction. J Psychosoc Oncol. 2006;24(4):33–51. doi: 10.1300/J077v24n04_03. [DOI] [PubMed] [Google Scholar]
- 33.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007 Oct;85(1):64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Hammerlid E, Bjordal K, Ahlner-Elmqvist M, et al. A prospective study of quality of life in head and neck cancer patients. Part I: at diagnosis. Laryngoscope. 2001 Apr;111(4 Pt 1):669–680. doi: 10.1097/00005537-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, et al. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001 Aug;111(8):1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Funk GF, Karnell LH, Christensen AJ. Long-term health-related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. Feb;138(2):123–133. doi: 10.1001/archoto.2011.234. [DOI] [PubMed] [Google Scholar]
- 37.Flexen J, Ghazali N, Lowe D, Rogers SN. Identifying appearance-related concerns in routine follow-up clinics following treatment for oral and oropharyngeal cancer. Br J Oral Maxillofac Surg. Jun;50(4):314–320. doi: 10.1016/j.bjoms.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Sezgin B, Findikcioglu K, Kaya B, Sibar S, Yavuzer R. Mirror on the wall: a study of women’s perception of facial features as they age. Aesthet Surg J. May;32(4):421–425. doi: 10.1177/1090820X12442083. [DOI] [PubMed] [Google Scholar]
- 39.Oskam IM, Verdonck-de Leeuw IM, Aaronson NK, et al. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. Jan 11; doi: 10.1016/j.oraloncology.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Pourel N, Peiffert D, Lartigau E, Desandes E, Luporsi E, Conroy T. Quality of life in long-term survivors of oropharynx carcinoma. Int J Radiat Oncol Biol Phys. 2002 Nov 1;54(3):742–751. doi: 10.1016/s0360-3016(02)02959-0. [DOI] [PubMed] [Google Scholar]
- 41.Tschudi D, Stoeckli S, Schmid S. Quality of life after different treatment modalities for carcinoma of the oropharynx. Laryngoscope. 2003 Nov;113(11):1949–1954. doi: 10.1097/00005537-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Moore EJ, Henstrom DK, Olsen KD, Kasperbauer JL, McGree ME. Transoral resection of tonsillar squamous cell carcinoma. Laryngoscope. 2009 Mar;119(3):508–515. doi: 10.1002/lary.20124. [DOI] [PubMed] [Google Scholar]
- 43.Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009 Mar;31(3):283–289. doi: 10.1002/hed.20972. [DOI] [PubMed] [Google Scholar]
- 44.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2009 Aug;141(2):166–171. doi: 10.1016/j.otohns.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009 Nov;119(11):2156–2164. doi: 10.1002/lary.20647. [DOI] [PubMed] [Google Scholar]
- 46.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. Mar;87(3):219–225. doi: 10.1016/j.mayocp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genden EM, O’Malley BW, Jr, Weinstein GS, et al. Transoral robotic surgery: role in the management of upper aerodigestive tract tumors. Head Neck. Jun;34(6):886–893. doi: 10.1002/hed.21752. [DOI] [PubMed] [Google Scholar]
- 48.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004 Apr;130(4):401–408. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 49.O’Connell DA, Rieger J, Harris JR, et al. Swallowing function in patients with base of tongue cancers treated with primary surgery and reconstructed with a modified radial forearm free flap. Arch Otolaryngol Head Neck Surg. 2008 Aug;134(8):857–864. doi: 10.1001/archotol.134.8.857. [DOI] [PubMed] [Google Scholar]
- 50.Dziegielewski PT, Ho ML, Rieger J, et al. Total glossectomy with laryngeal preservation and free flap reconstruction: objective functional outcomes and systematic review of the literature. Laryngoscope. Jan;123(1):140–145. doi: 10.1002/lary.23505. [DOI] [PubMed] [Google Scholar]
- 51.Morton RP. Studies in the quality of life of head and neck cancer patients: results of a two-year longitudinal study and a comparative cross-sectional cross-cultural survey. Laryngoscope. 2003 Jul;113(7):1091–1103. doi: 10.1097/00005537-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Tulunay-Ugur OE, McClinton C, Young Z, Penagaricano JA, Maddox AM, Vural E. Functional outcomes of chemoradiation in patients with head and neck cancer. Otolaryngol Head Neck Surg. Jan;148(1):64–68. doi: 10.1177/0194599812459325. [DOI] [PubMed] [Google Scholar]
- 54.Al-Mamgani A, Rooij PV, Tans L, Verduijn GM, Sewnaik A, Jong RJ. A prospective evaluation of patient-reported quality-of-life after (chemo)radiation for oropharyngeal cancer: Which patients are at risk of significant quality-of-life deterioration? Radiother Oncol. Feb 7; doi: 10.1016/j.radonc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Broglie MA, Soltermann A, Haile SR, et al. Quality of life of oropharyngeal cancer patients with respect to treatment strategy and p16-positivity. Laryngoscope. Jan;123(1):164–170. doi: 10.1002/lary.23622. [DOI] [PubMed] [Google Scholar]
- 56.Myers C, Kerr P, Cooke A, Bammeke F, Butler J, Lambert P. Functional outcomes after treatment of advanced oropharyngeal carcinoma with radiation or chemoradiation. J Otolaryngol Head Neck Surg. Apr;41(2):108–118. [PubMed] [Google Scholar]
- 57.Mlynarek AM, Rieger JM, Harris JR, et al. Methods of functional outcomes assessment following treatment of oral and oropharyngeal cancer: review of the literature. J Otolaryngol Head Neck Surg. 2008 Feb;37(1):2–10. [PubMed] [Google Scholar]


