Abstract
The aim of this study was to investigate the role of the superficial zone on the mechanical behavior of articular cartilage. Confined compression of articular cartilage was modeled using a biphasic finite element analysis to calculate the one-dimensional deformation of the extracellular matrix (ECM) and movement of the interstitial fluid through the ECM and articular surface. The articular cartilage was modeled as an inhomogeneous, nonlinear hyperelastic biphasic material with depth and strain-dependent material properties. Two loading conditions were simulated, one where the superficial zone was loaded with a porous platen (normal test) and the other where the deep zone was loaded with the porous platen (upside down test). Compressing the intact articular cartilage with 0.2 MPa stress reduced the surface permeability by 88%. Removing the superficial zone increased the rate of change for all mechanical parameters and decreased the fluid support ratio of the tissue, resulting in increased tissue deformation. Apparent permeability linearly increased after superficial removal in the normal test, yet it did not change in the upside down test. Orientation of the specimen affected the time-dependent biomechanical behavior of the articular cartilage, but not equilibrium behavior. The two tests with different specimen orientations resulted in very different apparent permeabilities, suggesting that in an experimental study which quantifies material properties of an inhomogeneous material, the specimen orientation should be stated along with the permeability result. The current study provides new insights into the role of the superficial zone on mechanical behavior of the articular cartilage.
Keywords: Articular cartilage, Superficial zone, Biphasic, Finite element analysis, Confined Compression
1. Introduction
Articular cartilage is a connective tissue serving as a low-friction, load-bearing material in diarthrodial joints. Mature articular cartilage has a depth-dependent heterogeneous composition and is usually divided into three zones: superficial, middle and deep zones (Mow et al., 2005). Collagen fibers are oriented parallel to the cartilage surface in the superficial zone (SZ),, becoming more randomly oriented in the middle zone and perpendicular to the articular surface in the deep zone, where they are imbedded into calcified cartilage and subchondral bone (Clark, 1985; Jeffery et al., 1991). The equilibrium aggregate modulus (HA) of mature articular cartilage increases with depth (Schinagl et al., 1997) while the tensile modulus decreases with depth (Krishnan et al., 2003). The SZ represents about 20% of the total thickness and has a critical role in the normal function of the articular cartilage, including load-distribution and viscoelastic response (Flannery et al., 1999; Gannon et al., 2012; Hosseini et al., 2014; Korhonen et al., 2002; Owen and Wayne, 2006; Setton et al., 1993). Breakdown and loss of the SZ is an early sign of osteoarthritis (Heinegård and Saxne, 2011; Hollander et al., 1995). Collapse of the SZ is believed to be responsible for controlling interstitial fluid transport across the articular surface (exudation and imbibition), and more important, the overall mechanical response of the articular cartilage (Torzilli et al., 1983; Torzilli, 1984). While there is experimental evidence to support this hypothesis, computational validation of this mechanism is lacking.
The aim of this study was to use a hyperelastic biphasic finite element analysis to investigate the role of the SZ on the mechanical behavior of articular cartilage in two different confined compression creep test configurations, one in which the articular surface was loaded with a porous platen (normal test) and the other with the cartilage inverted to load the deep zone with the porous platen (upside down test). Depth and strain-dependent material properties of the articular cartilage were included. Different amounts of superficial tissue were removed in both test configurations to investigate whether the deformation (collapse) of the SZ had a significant influence on the mechanical behavior of the articular cartilage.
2. Method
Hyperelastic biphasic theory
The hyperelastic biphasic theory proposed by Holmes and Mow (Holmes and Mow, 1990) was used in the current study. The governing equations are
| (1) |
| (2) |
where p is the fluid pressure, I the identity tensor, the solid phase velocity, k the permeability, and the effective stress of the solid matrix defined as
| (3) |
where J is the volume ratio defined as J= det(F), F the Jacobian determinant of the deformation gradient, and (Bonet and Wood, 1997) the Green-Lagrangian strain tensor. The strain energy density function is defined by (Holmes and Mow, 1990)
| (4) |
where I1, I2, and I3 are the invariants of the right Cauchy-Green deformation tensor, C, defined as C=FTF, the dimensionless nonlinear stiffening coefficient β=α1+2α2, and α0, α1 and α2 positive material parameters. α0, α1, and α2 are related to β, aggregate modulus, HA, and Poisson’s ratio, ν, by
| (5) |
The deformation-dependent permeability (Lai and Mow, 1980) is defined as
| (6) |
where k0 is the initial permeability and m a material parameter.
The hyperelastic biphasic theory was implemented in COMSOL Multiphysics (Burlington, MA). Solid mechanics in the Structural Mechanics Module and Darcy’s Law in the Earth Science Module were used (Guo et al., 2013; Guo et al., 2012; Guo and Spilker, 2011; Guo and Spilker, 2014). The user defined strain energy density function in COMSOL was used to input the strain energy density function (Eqn. 4) (Guo et al., 2014b).
Confined compression of the articular cartilage
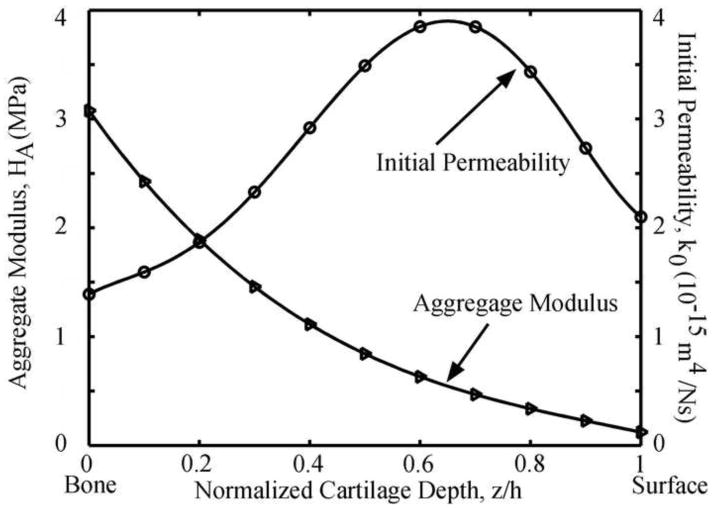
Confined compression test is widely used to measure the material properties of articular cartilage (Lu and Mow, 2008). Test is normally performed by confining a full-depth cartilage within a nonporous chamber and loading the articular surface with a rigid porous platen, resulting in one-dimensional interstitial fluid exudation through the articular surface and tissue. To evaluate the role of the SZ, two loading configurations were simulated using our hyperelastic biphasic finite element (HBFE) model: loading the articular surface (Fig. 1, normal) and inverting the cartilage to load the deep zone (Fig. 1, upside down). Four conditions were simulated for each test configuration: intact and after removal of 100, 200, and 300 μm of the superficial zone. The thickness of the intact cartilage was ho=1.5 mm. A stress of 0.2 MPa was linearly applied on the articular cartilage in 100 seconds and thereafter held constant for additional 2,900 seconds. The magnitude of the applied stress was chosen to limit the equilibrium strain to ~25%. The nonlinear depth-dependent aggregate modulus and initial permeability of the articular cartilage were obtained from published experimental measurements (Chen et al., 2001; Maroudas, 1968; Schinagl et al., 1997; Wang et al., 2001) (Fig. 2). Material parameters ν, β and m were 0.1, 0.35 and 2.2 (Wang et al., 2001), respectively.
Fig. 1.
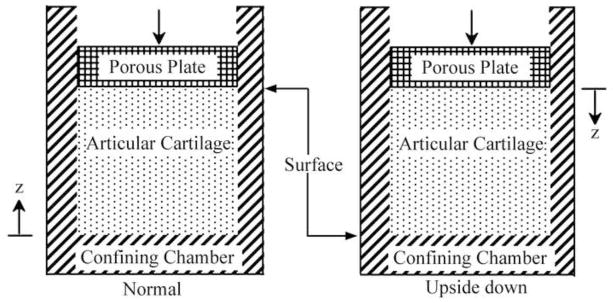
Schematic diagram of the confined compression tests modeled where the cartilage is orientated normal (left) and upside down (right).
Fig. 2.
Depth-dependent aggregate modulus (Chen et al., 2001; Schinagl et al., 1997; Wang et al., 2001) and initial permeability (Maroudas, 1968) of the articular cartilage used in the models.
Overall strain was defined as the tissue deformation (displacement of the porous platen) divided by the initial tissue thickness, while the apparent aggregate modulus and initial permeability were extracted by curve-fitting the HBFE model to the overall strain. The local peak strain, permeability and maximum principal shear stress, compressive stress and compressive strain were calculated at z=1.2 mm for all test conditions. Fluid support ratio, defined as ratio of average fluid pressure to average total normal stress, was also computed.
3. Results
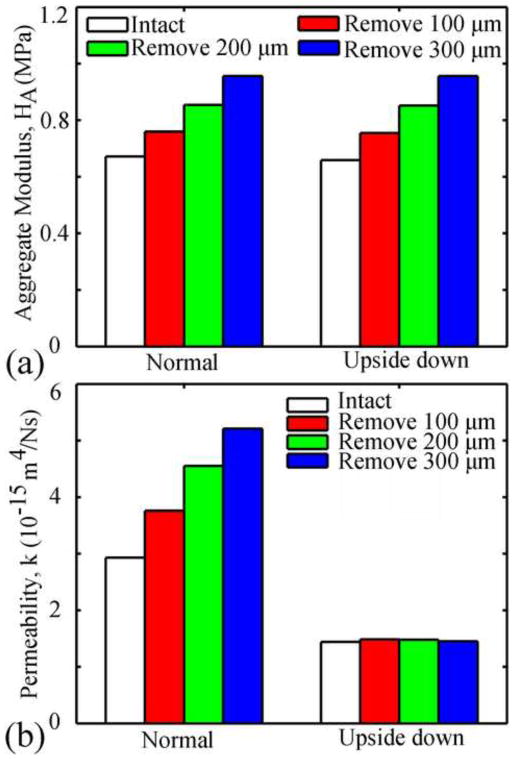
For both types of confined compression tests (normal and upside-down orientations), removal of the SZ resulted in greater overall strain than the intact tissue at early times (<500s) and smaller overall strain at larger times (Fig. 3a). The overall strain at equilibrium (3,000s) decreased with increasing superficial removal. For the normal test, peak strain occurred at the surface of the SZ and remained constant once the applied force was constant (Fig. 3b). In the upside-down test, the peak strain initially occurred at the bone surface of the deep zone (<100s), then shifted to the surface of the SZ (100–150s), and increased thereafter until equilibrium was reached. Removal of the SZ increased the apparent aggregate modulus of the tissue in both types of tests (Fig. 4a) and the apparent permeability in the normal test, however, SZ removal did not affect the apparent permeability in the upside-down test (Fig. 4b). In both tests the magnitudes of the apparent aggregate modulus were similar; however those for the apparent permeability were different (Fig. 4).
Fig. 3.
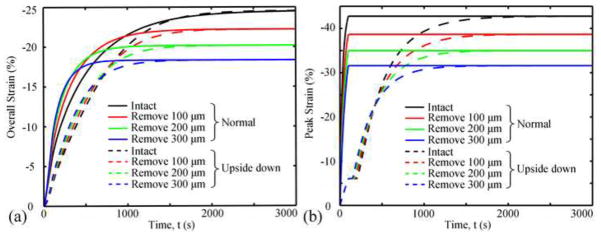
Overall strain (a) and peak strain (b) as functions of time in different cases.
Fig. 4.
Apparent aggregate modulus (a) and permeability (b) for different cases.
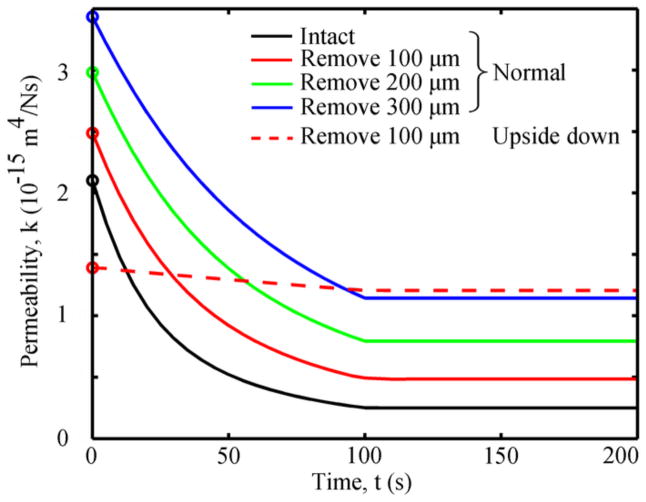
In the normal test, the permeability at the superficial surface of the articular cartilage (z=1.5 mm) decreased by 88% during the ramp loading phase and remained constant thereafter (Fig. 5), while the tissue permeability at other depths gradually reached equilibrium (Supplementary Fig. S1). Compressing cartilage after SZ removal of 100, 200 and 300 μm resulted in an 80%, 73% and 67% decrease in the surface permeability, respectively (max decrease = 2.1×10−15 m4/Ns). In all cases for the upside-down test, the permeability at the bone surface decreased by 13% up to 100s and then remained constants thereafter (Fig. 5).
Fig. 5.
Permeabilities at the cartilage surface close to the porous plate in different cases. The permeabilities were constant after 100s, and only the results for 0 to 200s are shown. For the upside–down orientation, the permeability was similar for all cases (intact and SZ removed), and thus only one case was plotted. Initial permeabilities are represented by open circles.
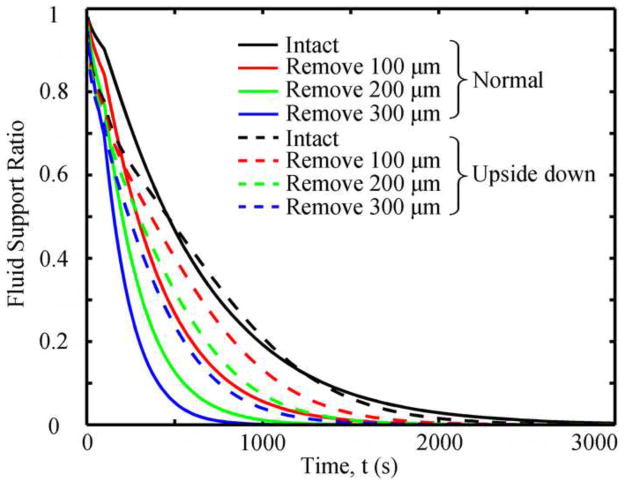
For both tests, SZ removal resulted in increased rates of change for tissue permeability, compressive strain, compressive stress, and maximum principal shear stress for up to 3,000s, where they converged to the same equilibrium values (Fig. 6). The normal test had greater rates of change than the upside-down tests. Removal of the SZ decreased the fluid support ratio in both types of tests (Fig. 7). The more SZ removed, the faster the fluid support ratio decreased. The fluid support ratio of the intact specimens was greater within the first 500s in the normal test than in the upside-down test, becoming similar after 500s. After superficial removal, the fluid support ratio in both tests were similar in the early phase of creep (<300 s), while after 300s the fluid support ratio in the normal test was smaller than that in the upside-down test.
Fig. 6.
Permeability (a), compressive strain (b), compressive stress (c), and maximum principal shear stress (d) at z=1.2 mm of the articular cartilage in different cases.
Fig. 7.
Fluid support ratios as functions of time in different cases.
4. Discussion
The objective of the current study was to use a biphasic finite element analysis to investigate the role of the superficial zone on the mechanical behavior of articular cartilage. Our computational model found that when intact articular cartilage was loaded in confined compression, the superficial zone collapses and the permeability at the articular surface decreased by 88%, which effectively trapped and pressurized the interstitial fluid within the middle and deep zones of the ECM. The dramatic decrease of the permeability in the surface was caused by the large deformation or strain in the SZ. Removing the SZ decreased the fluid support ratio of the articular cartilage, lowering the fluid pressure and increasing strain. Removing the SZ also increased rates of change in the overall strain and apparent permeability of the tissue, which are consistent with previous experimental studies (Setton et al., 1993; Torzilli, 1984). Our results provided direct computational evidence supporting the widely accepted yet computationally untested biphasic mechanism of cartilage mechanics that the SZ of the articular cartilage will collapse under compressive loading, decreasing the surface porosity and permeability to pressurize the interstitial fluid within the ECM (Setton et al., 1993; Torzilli et al., 1983). This is somewhat intuitive for several reasons. First, the aggregate modulus of the SZ of the articular cartilage is much lower (~25-fold) than the deeper zones (Hosoda et al., 2008; Laasanen et al., 2003; Schinagl et al., 1997). Second, the collagen fibrils within the SZ are closely packed and parallel to the articular surface, resulting in the surface having a lower permeability than the deeper regions (Maroudas, 1968). Third, once compressed the SZ of the articular cartilage will undergo a large deformation (Gratz et al., 2009) and compact the collagen fibrils within the SZ, further lowering the permeability and restricting fluid exudation (Torzilli et al., 1983; Torzilli, 1984).
Removing the SZ increased the rates of change for the permeability, compressive stress and strain, and maximum principal shear stress within the ECM. The rapid change in these mechanical parameters, especially the shear stress, may damage the collagen fiber network within the articular cartilage. The tangentially oriented fibers in the SZ help the articular cartilage withstand tensile and shear forces (Roth and Mow, 1980; Zhu et al., 1993), while the middle and deep zones have inferior tensile and shear mechanical properties (Krishnan et al., 2003). Removing the SZ will expose the middle and deep zones to higher magnitudes of stress and strain. Biphasic multiscale finite element studies (Guo et al., 2014a) are necessary in the further to investigate how the changes at tissue level affect the microenvironment of a cell within the articular cartilage. While only static creep loading was investigated in the current study, it would be of interest in future studies to investigate the effect of the SZ on the dynamic mechanical behavior of the articular cartilage, as similar findings have been previously reported for cyclic creep loading (Torzilli, 1984).
Finally, changing the orientation of the specimen (normal to upside down) affected the time-dependent biomechanical behavior of the articular cartilage, but not the equilibrium behavior, which is consistent with a previous computational study (Wang et al., 2001). The normal tests had greater rates of change than the upside-down tests. The two tests with different orientations had very different apparent permeabilities, suggesting that when measuring the permeability of an inhomogeneous material, the specimen orientation is very important, and should be reported along with the permeability results. One unique finding of the current study was that the apparent permeability progressively increased with increasing superficial removal in the normal test, yet it did not change in the upside-down test. This phenomenon is due to the fact that the modulus of the deep zone is over 25 times that of the superficial zone and therefore deep zone does not collapse in a similar manner to the SZ.
In summary, the current study provided for the first time computational evidence that the SZ will collapse under compressive loading and aid in the pressurization of the interstitial fluid within the articular cartilage. Removing the SZ decreased the fluid support ratio of the articular cartilage, resulting in increased tissue deformation. Specimen orientation has a significant effect on the time-dependent behavior of the articular cartilage, but as expected, not on the equilibrium behavior.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR057343. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonet J, Wood RD. Nonlinear continuum mechanics for finite element analysis. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- Chen A, Bae W, Schinagl R, Sah R. Depth-and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. Journal of Biomechanics. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Clark JM. The organization of collagen in cryofractured rabbit articular cartilage: a scanning electron microscopic study. Journal of Orthopaedic Research. 1985;3:17–29. doi: 10.1002/jor.1100030102. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochemical and Biophysical Research Communications. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- Gannon AR, Nagel T, Kelly DJ. The role of the superficial region in determining the dynamic properties of articular cartilage. Osteoarthritis and Cartilage. 2012;20:1417–1425. doi: 10.1016/j.joca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Gratz KR, Wong BL, Bae WC, Sah RL. The effects of focal articular defects on cartilage contact mechanics. Journal of Orthopaedic Research. 2009;27:584–592. doi: 10.1002/jor.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Maher SA, Spilker RL. Biphasic finite element contact analysis of the knee joint using an augmented Lagrangian method. Medical Engineering and Physics. 2013;35:1313–120. doi: 10.1016/j.medengphy.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Maher SA, Torzilli PA. A biphasic multiscale study of the mechanical microenvironment of chondrocytes within articular cartilage under unconfined compression. Journal of Biomechanics. 2014a;47:2721–2729. doi: 10.1016/j.jbiomech.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Nickel JC, Iwasaki LR, Spilker RL. An augmented Lagrangian method for sliding contact of soft tissue. Journal of Biomechanical Engineering. 2012;134:084503. doi: 10.1115/1.4007177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Shah M, Spilker RL. A finite element implementation for biphasic contact of hydrated porous media under finite deformation and sliding. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2014b;228:225–236. doi: 10.1177/0954411914522782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Spilker RL. Biphasic finite element modeling of hydrated soft tissue contact using an augmented Lagrangian Method. Journal of Biomechanical Engineering. 2011;133:111001. doi: 10.1115/1.4005378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Spilker RL. An augmented Lagrangian finite element formulation for 3D contact of biphasic tissues. Computer Methods in Biomechanics and Biomedical Engineering. 2014;17:1206–1216. doi: 10.1080/10255842.2012.739166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nature Reviews Rheumatology. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- Hollander A, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. Journal of Clinical Investigation. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MH, Mow VC. The nonlinear characteristics of soft gels and hydrated connective tissues in ultrafiltration. Journal of Biomechanics. 1990;23:1145–1156. doi: 10.1016/0021-9290(90)90007-p. [DOI] [PubMed] [Google Scholar]
- Hosoda N, Sakai N, Sawae Y, Murakami T. Depth-dependence and time-dependence in mechanical behaviors of articular cartilage in unconfined compression test under constant total deformation. Journal of Biomechanical Science and Engineering. 2008;3:209–220. [Google Scholar]
- Hosseini SM, Wu Y, Ito K, van Donkelaar CC. The importance of superficial collagen fibrils for the function of articular cartilage. Biomechanics and Modeling in Mechanobiology. 2014;13:41–51. doi: 10.1007/s10237-013-0485-0. [DOI] [PubMed] [Google Scholar]
- Jeffery A, Blunn G, Archer C, Bentley G. Three-dimensional collagen architecture in bovine articular cartilage. Journal of Bone & Joint Surgery, British Volume. 1991;73:795–801. doi: 10.1302/0301-620X.73B5.1894669. [DOI] [PubMed] [Google Scholar]
- Korhonen R, Wong M, Arokoski J, Lindgren R, Helminen H, Hunziker E, Jurvelin J. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Medical Engineering and Physics. 2002;24:99–108. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Eckstein F, Ateshian GA, Park S. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. Journal of Biomechanical Engineering. 2003;125:569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laasanen M, Töyräs J, Korhonen R, Rieppo J, Saarakkala S, Nieminen M, Hirvonen J, Jurvelin J. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133–140. [PubMed] [Google Scholar]
- Lai WM, Mow VC. Drag-induced compression of articular cartilage during a permeation experiment. Biorheology. 1980;17:111–123. doi: 10.3233/bir-1980-171-213. [DOI] [PubMed] [Google Scholar]
- Lu X, Mow V. Biomechanics of articular cartilage and determination of material properties. Medicine Science in Sports and Exercise. 2008;40:193–199. doi: 10.1249/mss.0b013e31815cb1fc. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Permeability of Articular Cartilage. Nature. 1968;219:1260–1261. doi: 10.1038/2191260a0. [DOI] [PubMed] [Google Scholar]
- Mow VC, Gu WY, Chen FH. Structure and function of articular cartilage and meniscus. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-biology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 181–258. [Google Scholar]
- Owen J, Wayne J. Influence of a superficial tangential zone over repairing cartilage defects: implications for tissue engineering. Biomechanics and Modeling in Mechanobiology. 2006;5:102–110. doi: 10.1007/s10237-006-0022-5. [DOI] [PubMed] [Google Scholar]
- Roth V, Mow V. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. The Journal of Bone and Joint Surgery. 1980;62:1102–1117. [PubMed] [Google Scholar]
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. Journal of Orthopaedic Research. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- Setton LA, Zhu W, Mow VC. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. Journal of Biomechanics. 1993;26:581–592. doi: 10.1016/0021-9290(93)90019-b. [DOI] [PubMed] [Google Scholar]
- Torzilli P, Dethmers D, Rose D, Schryuer H. Movement of interstitial water through loaded articular cartilage. Journal of Biomechanics. 1983;16:169–179. doi: 10.1016/0021-9290(83)90124-0. [DOI] [PubMed] [Google Scholar]
- Torzilli PA. Mechanical response of articular cartilage to an oscillating load. Mechanics Research Communications. 1984;11:75–82. [Google Scholar]
- Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. Journal of Biomechanics. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. Journal of Orthopaedic Research. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.