Abstract
The mesolimbic dopamine system, originating in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc), has been heavily implicated in the reinforcing effects of ethanol. Recent slice voltammetry studies have shown that ethanol inhibits dopamine release selectively during highfrequency activity that elicits phasic dopamine release shown to be important for learning and reinforcement. Presently, we examined ethanol inhibition of electrically evoked NAc dopamine in two mouse strains with divergent dopamine responses to ethanol, C57BL/6 (C57) and DBA/2J (DBA) mice. Previous electrophysiology and microdialysis studies have demonstrated greater ethanol induced VTA dopaminergic firing and NAc dopamine elevations in DBA compared to C57 mice. Additionally, DBA mice have greater ethanol responses in dopamine-related behaviors, including hyperlocomotion and conditioned place preference. Currently, we demonstrate greater sensitivity of ethanol inhibition of NAc dopamine signaling in C57 compared to DBA mice. The reduced sensitivity to ethanol inhibition in DBA mice may contribute to the overall greater ethanol-induced dopamine signaling and related behaviors observed in this strain. NAc cholinergic activity is known to potently modulate terminal dopamine release. Additionally, ethanol is known to interact with multiple aspects of nicotinic acetylcholine receptor activity. Therefore, we examined ethanol-mediated inhibition of dopamine release at two ethanol concentrations (80 and 160mM) during bath application of the non-selective nicotinic receptor antagonist mecamylamine, as well as compounds selective for the β2- (DhβE) and α6- (α-conotoxin MII [H9A; L15A]) subunit-containing receptors. Mecamylamine and DhβE decreased dopamine release and reduced ethanol's inhibitory effects on dopamine in both DBA and C57 mice. Further, α-conotoxin also reduced the dopamine release and the dopamine-inhibiting effects of ethanol at the 80mM, but not 160mM, concentration. These data suggest that ethanol is acting in part through nicotinic acetylcholine receptors, or downstream effectors, to reduce dopamine release during high-frequency activity.
Keywords: Ethanol vulnerability, phasic dopamine, voltammetry, C57BL/6 DBA/2 mice, nucleus accumbens, mecamylamine
1. Introduction
The mesolimbic dopamine system, which sends projections from the ventral tegmental area (VTA) to limbic structures such as the nucleus accumbens (NAc), has been implicated in the rewarding and reinforcing properties of ethanol and other drugs of abuse (for review see Sulzer, 2011). Electrophysiological and electrochemical studies have shown that acute ethanol increases VTA dopamine cell firing rates and dopamine release in the NAc (Brodie and Appel, 1998; Imperato and Di Chiara, 1986; Mereu and Gessa, 1984). Additionally, behavioral studies have shown that pharmacological or genetic manipulations which diminish dopamine activity also inhibit ethanol consumption, ethanol preference (El-Ghundi et al., 1998; Ikemoto et al., 1997; Phillips et al., 1998), conditioned place preference for ethanol (Cunningham et al., 2000; Risinger et al., 2001; Young et al., 2013), and the acquisition of ethanol self-administration (Risinger et al., 2000).
Although it is clear that dopamine activity in the NAc is involved in ethanol reinforcement, its exact contribution has been difficult to resolve due to the opposing effects of low vs. high-dose ethanol on dopamine neurotransmission. For example, microdialysis studies have found that intraperitoneal (I.P.) administration of low to moderate doses (1–2.5 g/kg) of ethanol results in increases in NAc dopamine levels (Ericson et al., 1998; Weiss et al., 1993; Blomqvist et al., 1993; Imperato and Di Chiara, 1986; Yoshimoto et al., 1992). These ethanol-induced increases in dopamine levels reach their peak at doses around 1 g/kg. However, at higher doses (2–5 g/kg I.P.), dopamine responses are reduced or reversed to decreases (Blanchard et al., 1993; Imperato and Di Chiara, 1986). Voltammetry studies have also illustrated the dose-dependent, biphasic effects of ethanol. For instance, excitatory effects of ethanol have been identified using voltammetry in freely-moving rats, where non-contingent ethanol administration (0.125–2.0 g/kg I.P.) increased the frequency of naturally occurring spontaneous dopamine release events (Cheer et al., 2007; Robinson et al., 2009). Furthermore, in anesthetized rats and mice, very low doses of ethanol (0.1 g/kg) increase the amplitude of electrically evoked NAc dopamine release (Pelkonen et al., 2010; Yavich and Tiihonen, 2000). However, ethanol has been shown to inhibit electrically evoked dopamine at low to high doses (0.5–5 g/kg) in vivo (Budygin et al., 2001a; Jones et al., 2006; Pelkonen et al., 2010; Yavich and Tiihonen, 2000) and at supraphysiological concentrations in brain slices from mice and rats (100–200 mM; Budygin et al., 2001b; Mathews et al., 2006). Together, these studies suggest that ethanol can have both excitatory and inhibitory effects on dopaminergic activity and NAc dopamine levels, depending on the dose / concentration of ethanol used in the study.
Not only are the biphasic effects of ethanol on dopamine release dose-dependent, but we have recently shown that the inhibitory effect of ethanol on dopamine release is dependent upon the frequency of stimulation (Yorgason et al., 2014). High and low frequency stimulations are often used to model two distinct modes of firing that occur in VTA dopamine neurons and which have differential effects on dopamine release at terminals. Tonic, low frequency firing of VTA dopamine neurons occurs in a pacemaker fashion at 1–10 Hz (Grace and Bunney, 1984; Hyland et al., 2002; Overton and Clark, 1997; Panin et al., 2012). In the presence of salient stimuli, such as rewards or reward-predicting cues, dopamine neurons shift to a phasic firing mode with bursts of action potentials occurring at 14–22 Hz (Grace and Bunney, 1984; Hyland et al., 2002; Overton and Clark, 1997; Panin et al., 2012). We have recently shown that that dopamine release is particularly sensitive to the inhibitory effects of ethanol under high frequency stimulation parameters in the NAc (Yorgason et al., 2014).
C57BL/6J (C57) and DBA/2J (DBA) mice are two mouse strains that have been identified for their divergent dopamine and related behavioral responses to ethanol. Although C57 and DBA mice have similar baseline VTA dopamine firing rates (Brodie and Appel, 2000; McDaid et al., 2008) and NAc dopamine levels (Kapasova and Szumlinski, 2008), they show very different sensitivities to ethanol’s behavioral and neurochemical effects. While DBA mice do not consume ethanol as readily as C57s, possibly due to taste aversion in DBAs (Blizard, 2007; Grahame and Cunningham, 1997; McCool and Chappell, 2012), they exhibit greater ethanol sensitivity in dopamine related behaviors such as conditioned place preference (CPP), hyperlocomotion, and locomotor sensitization (Cunningham and Noble, 1992; Gremel et al., 2006; Melon and Boehm, 2011; Phillips et al., 1994; Rose et al., 2013). Additionally, DBA mice are more sensitive to ethanol’s excitatory effects on VTA dopamine firing activity (Brodie and Appel, 2000) and elevations in NAc dopamine levels (Kapasova and Szumlinski, 2008; but see Zapata et al., 2006). These previous electrophysiological and neurochemical studies suggest that DBA mice are more susceptible to the dopamine-increasing effects of ethanol. Since phasic dopamine release is important for ethanol reinforcement learning, and DBA mice are more susceptible to the reinforcing effects of ethanol, we hypothesized that DBA mice may be less sensitive to ethanol’s inhibitory effects on dopamine release in response to high frequency stimulations. Therefore, we examined ethanol inhibition of dopamine release in these strains across a range of stimulation frequencies.
In addition to ethanol’s effects on dopamine in C57 and DBA mice, we were also interested in potential mechanisms that explain the differences in these strains. Nicotinic acetylcholine receptors (nAChRs) located on dopamine nerve terminals are known to be powerful modulators of dopamine release (Zhang and Sulzer, 2004; Rice and Cragg, 2004). Additionally, ethanol has been shown to interact with nAChRs to produce changes in dopamine levels (for review see Hendrickson et al., 2013). Because the inhibitory effects of ethanol and nAChR-modulation of dopamine release are both frequency-dependent, we examined nAChR involvement in the dopamine-decreasing effects of ethanol. C57 and DBA mice express a number of differences in cholinergic systems, including enzymatic activity and nAChR sensitivity to ethanol (Iacopino et al., 1986; Butt et al., 2003; Symons et al., 2010). Therefore, in an attempt to investigate potential mechanisms of ethanol’s effects on dopamine release in the two mouse strains, we investigated whether nAChR blockade would reduce ethanol-mediated inhibition of dopamine release under high frequency conditions. Specifically, we examined the effects of ethanol in the presence of the non-selective nAChR antagonist mecamylamine, the more selective β2-nAChR antagonist DhβE, and the α6-nAChR antagonist α-conotoxin MII [H9A; L15A].
2. Material and Methods
2.1. Animals
Male C57BL/6J and DBA/2J mice (Jackson Labs; aged 7–9 weeks) were given ad libitum access to food and water, and maintained on a reverse 12:12-h light/dark cycle (lights on at 15:00 h). All protocols and animal care procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering and the number of animals used in the present study.
2.2. Brain Slice Preparation
Isoflurane (Patterson Veterinary, Devens, MA) anesthetized mice were sacrificed by decapitation and brains were rapidly removed and transferred into ice-cold, pre-oxygenated (95% O2/5% CO2) artificial cerebral spinal fluid (aCSF) consisting of (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4), pH adjusted to 7.4. Tissue was sectioned into 400 µm-thick coronal slices containing the striatum with a vibrating tissue slicer (Leica VT1000S, Vashaw Scientific, Norcross, GA). Brain slices were placed in a submersion recording chamber, and perfused at 1 ml/min at 32 °C with oxygenated aCSF.
2.3. Fast Scan Cyclic Voltammetry
Fast scan cyclic voltammetry (voltammetry) recordings of dopamine signals were performed and analyzed as previously described, using in-house software (Demon Voltammetry and Analysis; Yorgason et al., 2011). The carbon fiber electrode (7 µm × ~150 µm) potential was linearly scanned as a triangular waveform from −0.4 to 1.2 V and back to −0.4 V (Ag vs Ag/Cl) at a scan rate of 400 V/s. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 msec by means of a potentiostat (Dagan Corporation, Minneapolis, MN). Dopamine release was evoked every 3–5 min through a bipolar stimulating electrode (Plastics One, Roanoke, VA) placed 100–200 µm from the carbon-fiber electrode in the NAc core, as previously described (Yorgason et al., 2013). Dopamine release was determined from voltammetry collections, where dopamine oxidation (occurring at ~0.6 V) produces a faradaic current proportional to the amount of dopamine present at the electrode. Current vs time signals were then transformed into µM dopamine concentrations using post-experiment calibration factors, and peak values were used for baseline comparisons. For input/output experiments examining baseline dopamine signals across increasing current stimulations, single pulse baseline dopamine signals were collected (4 ms, 350 µA) until signals were stable across 3 collections, at which point stimulation intensity was decreased to 10 µA, and subsequently increased across a range of intensities (10–500 µA). For baseline frequency response curves, single pulse release was measured until stabilized, at which point stimulated release was measured across multiple frequencies (5, 10, 20, 25, 100 Hz) at 5 pulses. For ethanol frequency response curve experiments, stabilized baseline signals were obtained under high frequency stimulation conditions (20 Hz, 10 Pulses, 350 µA), and tested at 1 pulse, and across a range of frequencies (10, 20, 40, 60 Hz) at 10 pulses before and after 80 mM ethanol application. For additional experiments examining concentration dependent ethanol effects across strains, stimulations were at 20 Hz 10 pulses (350 µA), and ethanol concentrations were increased (20–160 mM). Experiments examining nAChR interactions with ethanol inhibition were performed with high frequency stimulations at 20 Hz 10 pulses (300 µA) with 80 mM and 160 mM concentrations of ethanol. Mecamylamine (Sigma-Aldrich; St Louis, MO), (2R)-amino-5-phosphonovaleric acid (AP-5; Sigma-Aldrich), Dihydro-β-erythroidine hydrobromide (DhβE; Tocris Bioscience; Minneapolis, MN) and α-conotoxin MII [H9A; L15A] (α-Ctx) (synthesis described in McIntosh et al., 2004) were bath applied for similar amounts of time in all experiments to control for relative rundown in dopamine signals across time. Because mecamylamine alone modestly reduced dopamine release, the effects of mecamylamine were subtracted from ethanol + mecamylamine results to isolate ethanol effects.
2.4. Statistical Analysis
Data are shown as mean ± SEM. For experiments examining stimulation amplitude dependent changes in dopamine release (input/output curve), a two-way repeated measures analysis of variance (ANOVA) was performed, with stimulation intensity as the within subjects factor, and strain as the between subject factor. For experiments examining frequency dependent effects of ethanol in both mouse strains, two-way repeated measures ANOVA was performed with stimulation frequency and ethanol as the within subjects factors. Post-tests for these experiments were Tukey’s pairwise comparisons, testing for significant differences between dopamine signals after ethanol to respective frequency baseline signals. For ethanol concentration response curves, two-way ANOVA with ethanol concentration as the within-subject variable, and mouse strain as the between-subject variable, were used to examine ethanol potency between strains. In these experiments, Bonferroni post-tests were performed comparing each drug concentration to their respective baseline to test for significant differences. Experiments examining effects of various nAChR antagonists on dopamine release were analyzed using two-way ANOVAs with drug (i.e. Control, AP-5, MEC, DhβE, and α-conotoxin) and ethanol concentration as the two between-subject variables. All statistics were performed using GraphPad Prism 5 (GraphPad Software, La Jolla CA) and NCSS 8 (NCSS, Kaysville UT).
3. Results
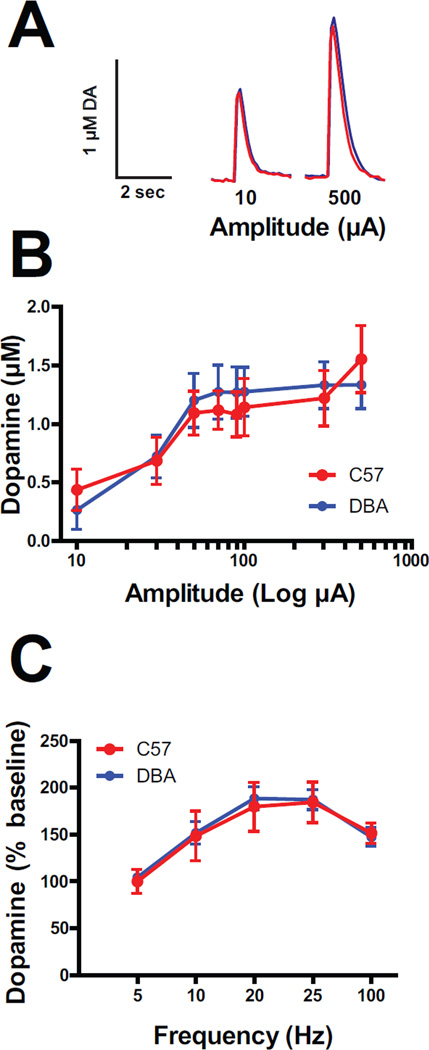
In order to determine whether dopamine release was different between C57 mice and DBA mice under drug free (baseline) conditions, we monitored electrically-stimulated dopamine release across a range of stimulation intensities and frequencies. Figure 1A shows no difference in the peak height of evoked dopamine between C57 (red traces) and DBA (blue traces) mice, using both low (left panel) and high (right panel) amplitude, single-pulse stimulations. Grouped data in Figure 1B shows a clear amplitude response, F(11,7) = 23.58, p<0.0001, that is not different between the two strains of mice (p>0.05). Similarly, Figure 1C shows a significant effect of frequency (5 P @ 300µA) on dopamine release, F(4,8) = 28.51, p<0.0001, that does not differ between C57 and DBA mice (p>0.05).
Figure 1. High and low frequency dopamine (DA) release is similar in C57 and DBA mice.
A) Raw DA traces from single pulse stimulations at low (10 µA) and high (500 µA) stimulation intensities in C57 (red) and DBA (blue) mice. B) Mean (±SEM) input/output curve for DA release across increasing stimulation intensities (10–500 µA) under single pulse stimulation conditions in C57 (red) and DBA (blue) mice. C) Mean (±SEM) DA release in C57 (red) and DBA (blue) mice across a range of stimulation frequencies, from 5–100 Hz (5 pulses, 350 µA).
Given the well-documented differences between C57 and DBA mice in their sensitivity to ethanol’s effects on dopamine dependent behaviors, we tested whether an 80 mM concentration of ethanol would differentially affect sub-second dopamine release in brain slices from C57 vs. DBA mice. We recently showed that low concentrations of ethanol robustly inhibit dopamine release in response to high, but not low, frequency stimulations. Therefore, we monitored dopamine release across multiple low and high frequency stimulations after application of 80 mM ethanol in both strains of mice. Representative dopamine traces demonstrate a robust inhibition of dopamine only in C57 mice (Figure 2A). Similar to our earlier work, this inhibition only occurs at stimulation frequencies greater than or equal to 20 Hz. Indeed, a two-way mixed ANOVA with dopamine release from C57 mice as the dependent variable (Figure 2B) showed a main effect of frequency, F(4,7) = 5.07, p<0.01, a main effect of ethanol, F(1,6) = 22.87, p<0.01, and a Frequency × Ethanol Interaction, F(4,12) = 3.52, p<0.05. Tukey’s post-hoc analyses demonstrated that ethanol significantly blunted dopamine release at the 20 Hz (p<0.001) and 60 Hz (p<0.05) frequencies. The same analysis in DBA mice (Figure 2C) showed only a main effect of frequency, F(4,6) = 15.66, p<0.0001, with no effect of ethanol (p>0.05), and no Frequency × Ethanol Interaction (p>0.05).
Figure 2. Ethanol (EtOH) inhibits dopamine (DA) release under high stimulation frequencies in C57 mice.
A) Raw evoked DA signals in C57 and DBA mice under single pulse conditions, and across increasing stimulation frequencies (10–60 Hz; 10 pulses), with baselines in black, and 80 mM EtOH in red (C57) and blue (DBA). B–C) Group data (mean ±SEM) of DA release evoked from increasing stimulation frequencies from 1 pulse, and 10 pulse (10–60 Hz) experiments in C57 (B) and DBA (C) mice during baseline (closed circles) and 80 mM EtOH (open circles) bath application normalized to baseline single pulse signals. *,p<0.05; ***,p<0.001.
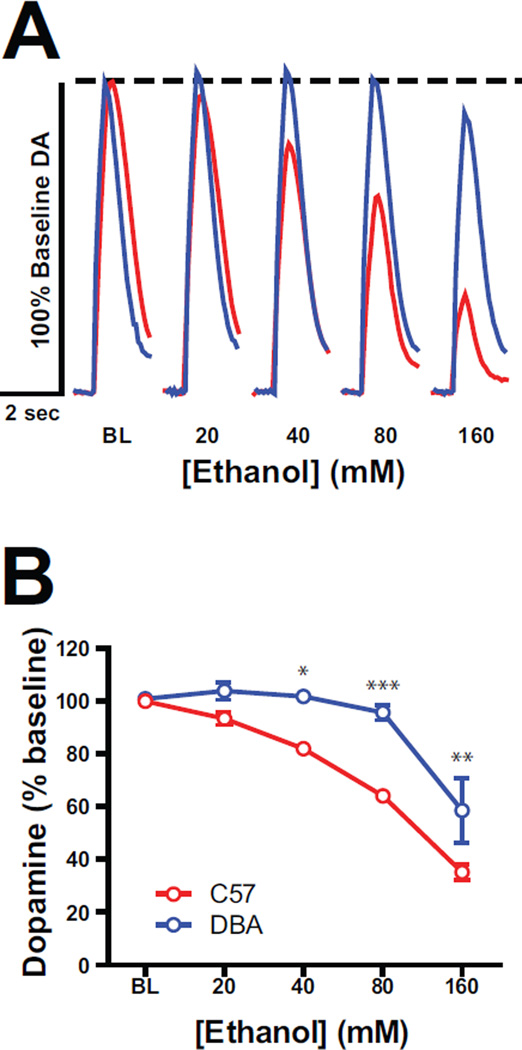
To test whether C57 mice showed greater sensitivity to the dopamine suppressing effects of ethanol across a range of ethanol concentrations, we performed ethanol concentration-response curves using a frequency (10 P @ 20 Hz) shown to be affected by ethanol in C57 mice (Figure 3). Representative dopamine traces demonstrate inhibition of dopamine at moderate to high concentrations (40–160 mM) in C57 mice and only at high concentrations (160 mM) in DBA mice (Figure 3A). A two-way mixed ANOVA with dopamine release from C57 and DBA mice as the dependent variable (Figure 3B) showed a main effect of strain, F(1,10) = 28.44, p<0.001, a main effect of ethanol concentration, F(4,10) = 57.36, p<0.0001, and a Strain × Ethanol Interaction, F(4,40) = 3.96, p<0.01. Bonferroni post-hoc analyses indicated that ethanol significantly blunted dopamine release at the 40 mM (p<0.05), 80 mM (p<0.001), and 160 mM (p<0.01) concentrations. Additionally, Bonferroni comparisons of each ethanol concentration against the pre-drug baseline showed an effect of ethanol at 160 mM (p<0.05), but no effect at 20, 40, and 80 mM ethanol in DBA mice. In C57 mice, however, this comparison yielded no effect at 20 mM, but significant effects of ethanol at 40 (p<0.01), 80 (p<0.001), and 160 mM (p<0.001). Therefore, not only are C57 mice more sensitive to the dopamine inhibiting effects of ethanol, but DBA mice appear to be completely insensitive to the ethanol’s inhibitory effects until high, supraphysiological concentrations are reached.
Figure 3. Ethanol (EtOH) potency for inhibition of dopamine (DA) release is greater in C57 mice.
A) Normalized evoked DA release traces for 20 Hz, 10 pulse stimulation conditions across increasing EtOH concentrations (20–160 mM) in C57 (red) and DBA (blue) mice. B) Mean (±SEM) DA release under high frequency (20 Hz @ 10 pulses) stimulation conditions in C57 (red) and DBA (blue) mice across increasing concentrations of EtOH (20–160 mM). On group data where error bars overlapped with symbols, error bars were removed.*,p<0.05; **,p<0.01; ***,p<0.001.
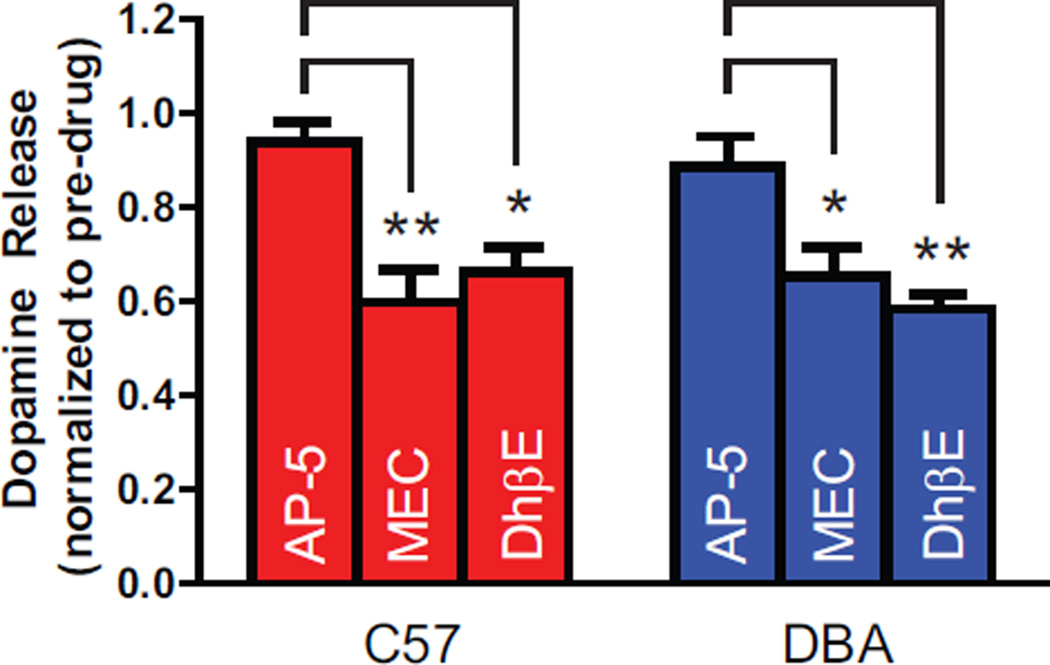
In order to test a differential ability of nAChRs to mediate the effects of ethanol in these two strains, we first investigated whether there were differences in the sensitivity of dopamine release to nAChR blockade. To be consistent with the literature, we used a relatively high concentration of mecamylamine (2 µM) that may also antagonize NMDA receptors (Papke et al., 2001). Therefore, we also included a set of control experiments to examine the effects of the selective NMDA antagonist AP-5 (50 µM) on ethanol-induced inhibition of dopamine release. Mecamylamine and DhβE (100 nM) significantly inhibited high frequency (20 Hz, 10 pulses) dopamine release (Figure 4), F(2,27) = 13.69, p<0.0001, equally in C57 and DBA mice (p>0.05), with no interaction between strain and antagonist effect (p>0.05). Additionally, as expected, AP-5 did not modulate dopamine release in either strain, suggesting mecamylamine-induced decreases in dopamine release are mediated my nAChR, and not NMDA receptor, blockade. We next tested whether ethanol retained the ability to inhibit dopamine release in the presence of the nAChR antagonists, mecamylamine and DhβE, and AP-5. A two-way ANOVA on dopamine release (normalized to pre-ethanol values) when applying 80−160 mM ethanol in C57 mice (Figure 5A) showed a significant main effect of group (i.e., ethanol vs. ethanol + AP-5 vs. ethanol + mecamylamine), F(2,76) = 24.60, p<0.0001, a main effect of ethanol concentration, F(3,76) = 45.99, p<0.0001, and a Drug Group × Ethanol Concentration interaction, F(6,76) = 6.323, p<0.0001. Bonferroni post-hoc analyses demonstrated that the ethanol only group had significantly blunted dopamine release relative to the ethanol + mecamylamine group at the 80 and 160 mM ethanol concentrations. Similar to C57 mice, two-way ANOVA on mecamylamine + ethanol data in DBA mice (Figure 5B) revealed a significant main effect of group (i.e., ethanol vs. ethanol + AP-5 vs. ethanol + mecamylamine), F(2,80) = 5.345, p=0.0066, and a main effect of ethanol concentration, F(3,80) = 18.59, p<0.0001. However, there was no Drug Group × Ethanol concentration interaction, F(6,80) = 1.838, p=0.1022. Bonferroni post-hoc analyses indicated that the APV + ethanol group had significantly blunted dopamine release relative to the ethanol + mecamylamine group. Next, we examined ethanol’s effects in the presence of the β2 subunit containing nAChR antagonist DhβE (Figure 5C–D). For C57 mice (Figure 5C), two-way ANOVA revealed that DhβE significantly attenuated ethanol’s inhibitory effects on dopamine release (F(1,44) = 5.657, p=0.0218). Additionally, there was a main effect of ethanol concentration on evoked dopamine release, F(3,44) = 24.40, p<0.0001, as well as a Group × Ethanol Concentration interaction, F(3,44) = 7.470, p=0.0004. For DBA mice (Figure 5D), there was a main effect of ethanol concentration, F(3,55) = 6.757, p<0.0006, but no main effect of DhβE (p>0.05), and no interaction between DhβE and ethanol concentration (p>0.05).
Figure 4. The nicotinic acetylcholine receptor (nAChR) antagonists mecamylamine (MEC) and dihydro-β-erythroidine hydrobromide (DhβE) reduce dopamine (DA) release similarly in C57 and DBA mice.
Mean (±SEM) pre-drug normalized DA release after bath application of the NMDA receptor antagonist AP-5 (50 µM), or the nAChR antagonists MEC (2 µM) and DhβE (100 nM) in C57 (red) and DBA (blue) mice during 20 Hz, 10 pulse stimulation conditions. *,p<0.05; **,p<0.01.
Figure 5. Nicotinic acetylcholine receptor (nAChR) antagonism attenuates ethanol’s (EtOH) inhibitory effects on dopamine (DA) release.
A–D) Mean (±SEM) baseline (and post-drug) normalized DA release under 20 Hz, 10 pulses stimulation conditions during and after EtOH (80–160 mM) application in C57 (A,C) and DBA (B,D) mice. Experiments were performed in the absence of other drugs (control) and in A–B during bath application of the non-selective nAChR antagonist 2 µM mecamylamine (MEC) or the NMDA receptor antagonist AP-5 (50 µM). In C–D ethanol’s effects were examined during batch application of the β2 specific nAChR antagonist DhβE (100 nM). On group data where error bars overlapped with symbols, error bars were removed. Significance symbols are in relation to Control (*) and AP-5(+) experiments. +, p<0.01; ***,+++,p<0.001.
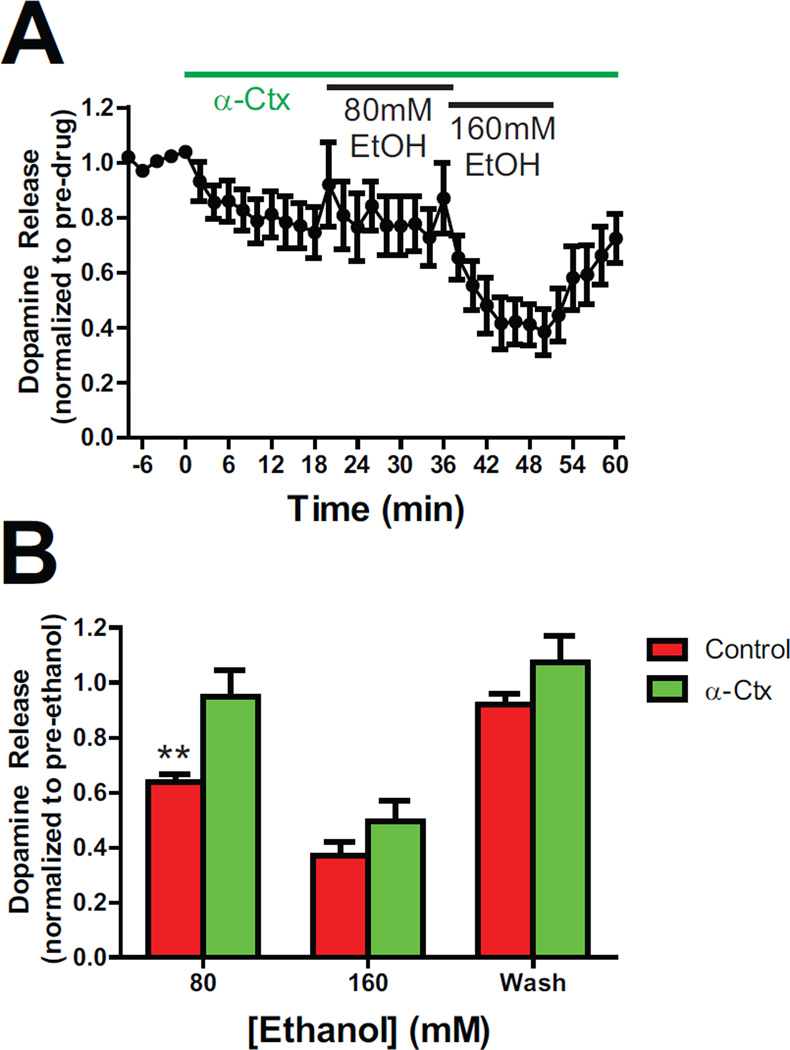
In order to better understand which nAChR subunits are involved in ethanol’s inhibitory effects on dopamine, we bath applied increasing concentrations of ethanol (80 and 160 mM) in the presence of the α6 subunit specific nAChR antagonist, α-Ctx MII [H9A; L15A], in C57 mice (100 nM; Figure 6). Dopamine release under high frequency conditions (20Hz 10p) was reduced by α-Ctx to 77.01% ± 8.132%. This reduction was similar to that produced by mecamylamine and DhβE in C57 mice (p>0.05). Two-way ANOVA examining differences in ethanol sensitivity between α-Ctx and control experiments revealed a main effect α-Ctx treatment, F(2,33) = 40.68, p<0.0001, and a main effect of ethanol concentration, F(2,33) = 40.68, p<0.0001, but no Group X Ethanol Concentration interaction (p>0.05). Also, ethanol’s inhibitory effects on evoked dopamine release washed out at the end of the experiment (p>0.05). Lastly, two-way ANOVA comparing the effects of α-Ctx, DhβE and mecamylamine on ethanol induced inhibition revealed a main effect ethanol concentration, F(2,42) = 12.53; p<0.0001, as well as a nAChR Antagonist X Ethanol Concentration interaction, F(4,42) = 2.671; p=0.0451, and a main effect of nAChR antagonist (F(2,42) = 3.433; p=0.0416).
Figure 6. Ethanol’s inhibitory effects on dopamine (DA) release are through decreased activity at α6 nicotinic acetylcholine receptors (nAChRs).
A) Mean (±SEM) baseline (pre-drug) normalized dopamine responses during high frequency (20 Hz, 10 pulses) stimulation conditions in C57 mice. The α6 subunit specific blocker α-conotoxin MII [H9A; L15A] (α-Ctx) was bath applied at 100 nM, and increasing concentrations of ethanol (80, 160 mM) were bath applied, and subsequently washed out. B) Effects of ethanol (80 and 160 mM) on evoked dopamine release in the absence (control) or presence of α-Ctx (100 nM). **,p<0.01.
4. Discussion
4.1. No Difference in Dopamine Signaling in C57 and DBA Mice
In the present study, we examined dopamine release using multiple stimulation intensities and frequencies in order to better understand NAc dopamine signaling in two strains of mice that have different behavioral and neurochemical responses to ethanol. Specifically, we show here that electrically-evoked dopamine release is similar in C57 and DBA mice, and that these similarities are consistent across a range of stimulation intensities (10–500 µA) as well as frequencies (5–100 Hz). This is consistent with research showing that C57 and DBA mice share similarities in baseline VTA dopamine firing rates (Brodie and Appel, 2000), NAc vesicular monoamine transporter, dopamine transporter, tyrosine hydroxylase expression (D'Este et al., 2007), and basal NAc dopamine levels (Kapasova and Szumlinski, 2008). Therefore, baseline dopamine dynamics do not appear to account for behavioral differences in these strains, which may only be revealed in the context of ethanol administration.
4.2. Differences in Dopamine Signaling and Ethanol Interactions in C57 and DBA mice
Although C57 mice more readily consume ethanol because of a taste preference (McCool and Chappell, 2012), DBA mice are more sensitive to ethanol’s rewarding and dopamine-enhancing effects at low to moderate doses (Cunningham, 1995; Cunningham et al., 1992; Gremel et al., 2006; Brodie and Appel, 2000; Kapasova and Szumlinski, 2008). Results from the current study extend these findings to show a decreased ability of ethanol to inhibit dopamine release in DBA compared to C57 mice at moderate to high (40–160 mM) ethanol concentrations, which overlaps with concentrations/doses used in previous studies (20–160 mM, Brodie and Appel, 2000; 2 g/kg, Kapasova and Szumlinski, 2008). This reduction in the ability of ethanol to inhibit accumbal dopamine release in DBA mice may contribute to the overall increased excitatory effects of ethanol on dopamine levels shown previously. Therefore, the differences in ethanol-mediated dopamine related behaviors in C57 and DBA mice may be attributed, at least in part, to greater sensitivity to excitatory effects at cell bodies in combination with reduced sensitivity to inhibitory effects of ethanol on dopamine terminal release in DBA mice.
4.3. Possible Cholinergic Activity-Related Mechanism of Ethanol Mediated Dopamine Inhibition
Ethanol has many different interactions with the cholinergic system (for review see Hendrickson et al., 2013). In the present study, ethanol and all nAChR antagonists reduced dopamine release under high frequency conditions. Furthermore, nAChR antagonist pre-application attenuated reductions in dopamine release by 80 and 160 mM ethanol. This suggests that ethanol may be acting to reduce either cholinergic interneuron activity/release, nAChR activity on dopamine terminals, or intracellular signaling that influences nAChR activity.
With respect to acetylcholine levels, a recent electrophysiology study has shown that ethanol reduces striatal cholinergic interneuron firing rates, which is attributable to increases in after-spike hyperpolarizations (Blomeley et al., 2011). This report is complemented by studies showing reductions in extracellular acetylcholine levels after ethanol (Erickson and Graham, 1973; Hunt and Dalton, 1976). However, it seems unlikely that ethanol’s inhibitory effects on dopamine release are through decreases in acetylcholine levels, as reductions in cholinergic tone also reduces single pulse dopamine release (Zhou et al., 2001), and single-pulse elicited dopamine release is relatively insensitive to ethanol’s inhibitory effects (Yorgason et al., 2014).
Another way ethanol may be acting to reduce dopamine signaling is by reducing nicotinic receptor activity, either through receptor desensitization (for review see Dopico and Lovinger, 2009) or blockade. Mesolimbic dopamine neurons express a number of nAChR subunits, including α3–7 and β2 subunits (Hendrickson et al., 2013). Nicotinic α7 subunits combine to form homopentameric nAChRs, whereas α3–6 subunits are combined in various arrangements with multiple β subunits to form heteropentameric nAChRs (Hendrickson et al., 2013). Homomeric α7 nAChR activity is reduced by ethanol (Cardoso et al., 1999), presenting a candidate for ethanol-induced blockade. However, it should be noted that α7 nAChR antagonists have little to no effect on high frequency stimulated dopamine release ex vivo (Zhou et al., 2001), suggesting that the dopamine-inhibiting ethanol effect may be through interactions with heteromeric β2-containing nAChRs (Exley et al., 2008). Indeed, ethanol’s effects were robustly reduced by the β2 specific antagonist DhβE and the α6 specific conotoxin MII, suggesting that ethanol’s effects in the NAc core are occurring through interactions with nAChRs containing these two subunits. Considering the known heterogeneity of nAChR subunit composition across the striatum (Exley et al. 2012), it would informative to know the extent of ethanol’s inhibitory effects on dopamine throughout these multiple regions. For instance, DhβE attenuates nAChR activity in the NAc core and shell, but MII α-Ctx’s dopamine modulating effects appear to be selective for the NAc core (Schilaty et al., 2014; Exley et al., 2008; Exley et al., 2012). Therefore, if ethanol still inhibits dopamine release in the NAc shell or the CPu (Exley et al., 2008; Exley et al., 2012), this would suggest that the α6 subunit is not essential for ethanol to inhibit dopamine release in this region. It is important to note that while we investigated three nAChR antagonists across multiple concentrations of ethanol, we did not run concentration-response curves of nAChR compounds. However, concentrations used in this investigation are within the range of those used in the literature to investigate the effects of nAChR subunit function on dopamine release (Zhou et al., 2001; Zhang and Sulzer, 2004; Rice and Cragg 2004; Schilaty et al., 2014). Moreover, it is unlikely that nonspecific effects of the antagonists are responsible for the current effects, because DhβE and α-Ctx show high selectivity for β2 and α6 nAChR subunits, respectively, in heterologous expression systems, and we ruled out nonspecific effects of mecamylamine on NMDA receptors. Nevertheless, it appears that while DhβE and mecamylamine attenuate ethanol’s effect at both 80 and 160mM, α-Ctx reduces ethanol’s effects only at 80mM. The difference between these antagonists at the high dose of ethanol might suggest two distinct possibilities. The first is that higher doses of α-Ctx are required for attenuation of ethanol’s effects at the high concentration. The second is that α6 nAChR subunits mediate effects only at lower concentrations of ethanol, while additional, non-α6 nAChR subunits are mediating ethanol’s effects at high concentrations. Additional experimentation will be required to understand both the brain region and subtype specificity of these effects.
Similar to our findings of decreased dopamine release after nAChR blockade, nAChR activation (and possible desensitization) also inhibits electrically evoked dopamine release (Zhou et al., 2001; Zhang and Sulzer, 2004; Rice and Cragg 2004). This suggests that the inhibitory effects of ethanol on dopamine could be either through enhanced activation (and subsequent desensitization) or blockade of nAChRs. The caveat with desensitization of β2-containing nAChRs is that ethanol has been shown to increase nAChR-mediated cation conductance in heteromeric receptors containing specific subunit combinations, including α2β2, α2β4, α4β2 and α4β4 containing receptors (Borghese et al., 2003; Bradley et al., 1984; Cardoso et al., 1999; Zuo et al., 2001). While these previous studies may suggest that ethanol mostly increases heteromeric nAChR activity, ethanol-mediated desensitization of heteromeric nAChRs is still under investigation, and may involve secondary signaling effectors not examined in the previous reports. Most notably, ethanol has been shown to activate protein kinase A (PKA) and protein kinase C (PKC) dependent pathways (Ron and Messing, 2013) that are involved in nAChR desensitization (Huganir and Miles 1989; Marszalec et al., 2005).
Since the present study demonstrates that DBA mice have decreased sensitivity to ethanolmediated dopamine inhibition, and that ethanol-mediated inhibition is reduced by nAChR antagonism, it is notable that C57 and DBA mice have very different striatal cholinergic systems. For instance, compared to C57 mice, DBA mice have increased striatal acetylcholine esterase density and activity (Iacopino et al., 1986). This difference in enzymatic activity may result in greater acetylcholine levels during burst stimulations in C57 mice, and thus increase C57 mouse susceptibility to nAChR desensitization (Giniatullin et al., 2005; Threlfell et al., 2012). Furthermore, C57 and DBA mice differentially express several single nucleotide polymorphisms in nAChR subunits that appear to contribute to ethanol sensitivity (Butt et al., 2003; Symons et al., 2010). While it is not presently known if these differences in nAChR ethanol sensitivity translate into differences in desensitization, it is certainly possible, given the present results. However, we did not observe any noticeable strain differences in sensitivity to nAChR antagonists in the present study. Therefore, future studies examining ethanol’s inhibitory effects on striatal dopamine release may benefit from more directly assaying the respective contributions of ethanol interactions with acetylcholine levels as well as nAChR desensitization on dopamine terminals in these strains.
4.4. Conclusions
We found that dopamine release under baseline conditions is similar in C57 and DBA mice, suggesting that baseline parameters likely do not contribute to differences in ethanol sensitivity of dopamine terminals. Contrary to a lack of effect on baseline measures, ethanol inhibition of high frequency, phasic-like dopamine release is greater in C57 compared to DBA mice. This difference in sensitivity to ethanol inhibition of dopamine release may help explain the large behavioral differences in reward related tasks, such as ethanol CPP and ethanol locomotor stimulation, observed in these mouse strains (Cunningham et al., 1992). Increased sensitivity to ethanol’s rewarding and locomotor activating effects in DBA mice may be influenced by the reduced sensitivity to ethanol’s inhibitory effects on dopamine release, as shown here, resulting in greater ethanol-induced increases in dopamine levels as measured by microdialysis (Kapasova and Szumlinski, 2008). Lastly, ethanol inhibition of dopamine release under higher frequency conditions may be in part due to reduced nAChR activity on dopamine terminals. However, additional studies examining how cholinergic activity, and subsequent changes in rapid dopamine signaling, is influenced by ethanol may elucidate the involvement of ethanol inhibition of dopamine release in reinforcement and reward related behaviors.
Ethanol modulates dopamine levels through a balance of excitation and inhibition.
Ethanol inhibits dopamine release preferentially under high frequency stimulations.
DBA mice are less sensitive to ethanol inhibition than C57 mice.
Nicotinic receptor antagonism blocks ethanol-mediated inhibition of dopamine.
Acknowledgements
We would like to thank Joanne Konstantopoulos and Jason Locke for their technical assistance and for the maintenance of animal colonies. These studies were supported by F31 AA020439 (JTY), T32 DA007262 (JTY), F31 DA035558 (JHR), R01 GM103801 (JMM), P01 GM48677 (JMM), K99 DA031791 (MJF), U01 AA014091 (SRJ) and P01 AA021099 (SRJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Blomeley CP, Cains S, Smith R, Bracci E. Ethanol affects striatal interneurons directly and projection neurons through a reduction in cholinergic tone. Neuropsychopharmacology. 2011;36:1033–1046. doi: 10.1038/npp.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Henderson LA, Bleck V, Trudell JR, Harris RA. Sites of excitatory and inhibitory actions of alcohols on neuronal alpha2beta4 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;307:42–52. doi: 10.1124/jpet.102.053710. [DOI] [PubMed] [Google Scholar]
- Bradley RJ, Sterz R, Peper K. The effects of alcohols and diols at the nicotinic acetylcholine receptor of the neuromuscular junction. Brain Res. 1984;295:101–112. doi: 10.1016/0006-8993(84)90820-5. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001a;297:27–34. [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Wightman RM, Jones SR. Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study. Synapse. 2001b;42:77–79. doi: 10.1002/syn.1101. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Stitzel JA, Balogh SA, Owens JC, Collins AC. A polymorphism in the alpha4 nicotinic receptor gene (Chrna4) modulates enhancement of nicotinic receptor function by ethanol. Alcohol Clin Exp Res. 2003;27:733–742. doi: 10.1097/01.ALC.0000067973.41153.BC. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL. Localization of genes influencing ethanol-induced conditioned place preference and locomotor activity in BXD recombinant inbred mice. Psychopharmacology (Berl) 1995;120:28–41. doi: 10.1007/BF02246142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- D'Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J) J Chem Neuroanat. 2007;33:67–74. doi: 10.1016/j.jchemneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, Liu C, Sibley DR, Westphal H, O'Dowd BF. Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol. 1998;353:149–158. doi: 10.1016/s0014-2999(98)00414-2. [DOI] [PubMed] [Google Scholar]
- Erickson CK, Graham DT. Alteration of cortical and reticular acetylcholine release by ethanol in vivo. J Pharmacol Exp Ther. 1973;185:583–593. [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci. 2012;32:2352–2356. doi: 10.1523/JNEUROSCI.4985-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 1997;21:56–62. [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:783–790. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Guildford MJ, Tapper AR. Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Front Psychiatry. 2013;4:29. doi: 10.3389/fpsyt.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir RL, Miles K. Protein phosphorylation of nicotinic acetylcholine receptors. Crit Rev Biochem Mol Bio. 1989;24:183–215. doi: 10.3109/10409238909082553. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Dalton TK. Regional brain acetylcholine levels in rats acutely treated with ethanol or rendered ethanol-dependent. Brain Res. 1976;109:628–631. doi: 10.1016/0006-8993(76)90043-3. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Iacopino C, Altavista MC, Gozzo S, Albanese A. Quantitative pharmacohistochemistry of acetylcholinesterase in neostriatum of inbred strains of mice. Brain Res. 1986;374:402–408. doi: 10.1016/0006-8993(86)90439-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, McBride WJ, Murphy JM, Lumeng L, Li TK. 6-OHDA-lesions of the nucleus accumbens disrupt the acquisition but not the maintenance of ethanol consumption in the alcohol-preferring P line of rats. Alcohol Clin Exp Res. 1997;21:1042–1046. [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Marsxalek W, Yeh JZ, Narahashi T. Desensitization of nicotinic acetylcholine receptors: modulation by kinase activation and phosphatase inhibition. Eur J Pharmacol. 2005;514:83–90. doi: 10.1016/j.ejphar.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addict Biol. 2012;17:121–131. doi: 10.1111/j.1369-1600.2010.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100:1202–1210. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Gessa GL. Ethanol excites dopamine (DA) neurons and inhibits non-dopamine (non-DA) neurons in the Substantia nigra of rats. Ann Ist Super Sanita. 1984;20:11–15. [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Panin F, Cathala A, Piazza PV, Spampinato U. Coupled intracerebral microdialysis and electrophysiology for the assessment of dopamine neuron function in vivo. J Pharmacol Toxicol Methods. 2012;65:83–92. doi: 10.1016/j.vascn.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Pelkonen A, Hiltunen M, Kiianmaa K, Yavich L. Stimulated dopamine overflow and alpha-synuclein expression in the nucleus accumbens core distinguish rats bred for differential ethanol preference. J Neurochem. 2010;114:1168–1176. doi: 10.1111/j.1471-4159.2010.06844.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational effects of ethanol in DARPP-32 knock-out mice. J Neurosci. 2001;21:340–348. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 2000;152:343–350. doi: 10.1007/s002130000548. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JH, Calipari ES, Mathews TA, Jones SR. Greater ethanol-induced locomotor activation in DBA/2J versus C57BL/6J mice is not predicted by presynaptic striatal dopamine dynamics. Plos One. 2013;8:e83852. doi: 10.1371/journal.pone.0083852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilaty ND, Hedges DM, Jang EY, Folsom RJ, Yorgason JT, McIntosh JM, Steffensen SC. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2014;349:559–567. doi: 10.1124/jpet.113.211490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons MN, Weng J, Diehl E, Heo E, Kleiber ML, Singh SM. Delineation of the role of nicotinic acetylcholine receptor genes in alcohol preference in mice. Behav Genet. 2010;40:660–671. doi: 10.1007/s10519-010-9366-9. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Yavich L, Tiihonen J. Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol. 2000;401:365–373. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–1031. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Ferris MJ, Jones SR. Frequency-dependent effects of ethanol on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2014;38:438–447. doi: 10.1111/acer.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Young EA, Dreumont SE, Cunningham CL. Role of nucleus accumbens dopamine receptor subtypes in the learning and expression of alcohol-seeking behavior. Neurobiol Learn Mem. 2013 doi: 10.1016/j.nlm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Aistrup GL, Marszalec W, Gillespie A, Chavez-Noriega LE, Yeh JZ, Narahashi T. Dual action of n-alcohols on neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:700–711. [PubMed] [Google Scholar]