Abstract
Alcoholic liver disease (ALD) has been amongst the leading causes of liver cirrhosis and liver-related death worldwide for decades. Early discoveries in alcoholic liver disease identified increased levels of bacterial endotoxin in the portal circulation suggesting a role for gut-derived “toxins” in ALD. Indeed, alcohol consumption can disrupt the intestinal epithelial barrier and result in increased gut permeability that is increasingly recognized as a major factor in ALD. Bacterial endotoxin, LPS, is a prototypic microbe-derived inflammatory signal that contributes to inflammation in ALD through activation of the Toll-like receptor 4 (TLR4). Recent studies also demonstrated that alcohol consumption is associated with alterations in the gut microbiome and the dysbalance of pathogenic and commensal organisms in the intestinal microbiome may contribute to the abnormal gut-liver axis in ALD. Indeed, bacterial decontamination improves ALD both in human and animal models. This short review summarizes recent findings and highlights emerging trends in the gut-liver axis relevant to ALD.
Keywords: gut permeability, inflammation, microbiome, lipopolysaccharide
Introduction to Gut-Liver Interactions
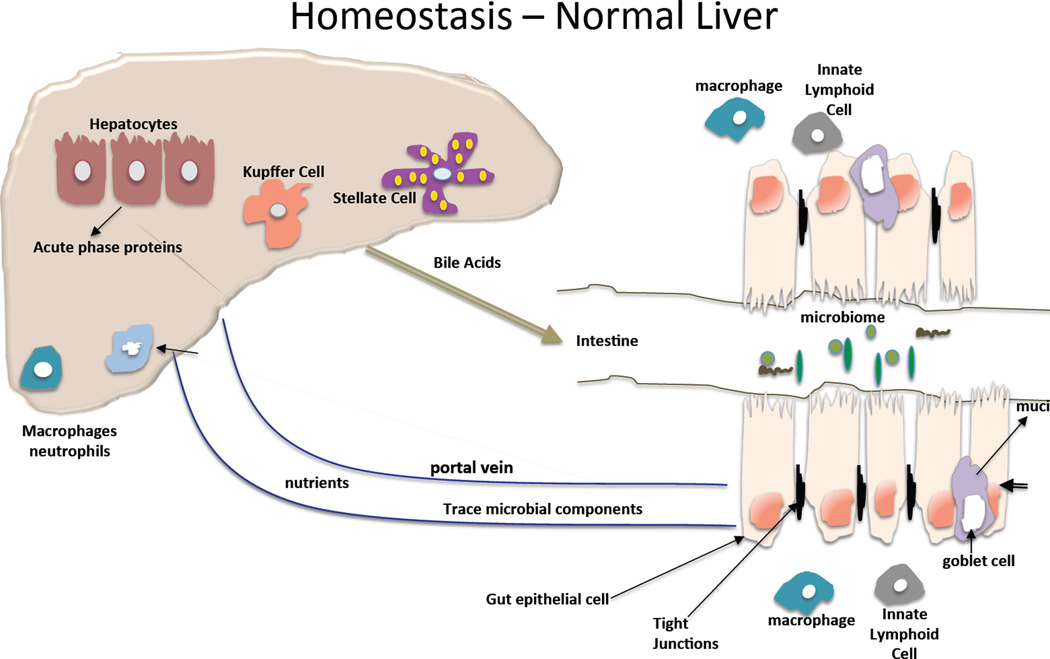
The anatomy of the liver provides its close interaction with the gut where nutrients and the microbiome contribute to the maintenance of a healthy metabolism and liver. Gut derived nutrients and other signals are delivered to the liver via the portal circulation that has several unique features. The slow blood flow in the liver sinusoids permits interactions between gut-derived substances and hepatocytes, other liver parenchymal cells and liver immune cells; this is further promoted by the fenestrated endothelium in the sinusoids1. The liver as the largest immune organ hosts the entire spectrum of immune cell repertoire and has a remarkable capacity to recruit and activate immune cells in response to gut-derived metabolic or pathogen-derived signals. The effects of gut microbiota in liver diseases has been a major interest in recent years2. A recent study places the liver in the center of the intersections between the host and the gut commensal microbiota3. Interestingly, bile acid produced by the liver can also modulate the microbiome as some bacteria utilize bile acids4. The interaction between the microbiome and the host liver is of particular interest in alcoholic liver disease where alcohol was shown to both change the composition of the microbiome and impair intestinal integrity and barrier function5,6.
Alcoholic Liver Disease
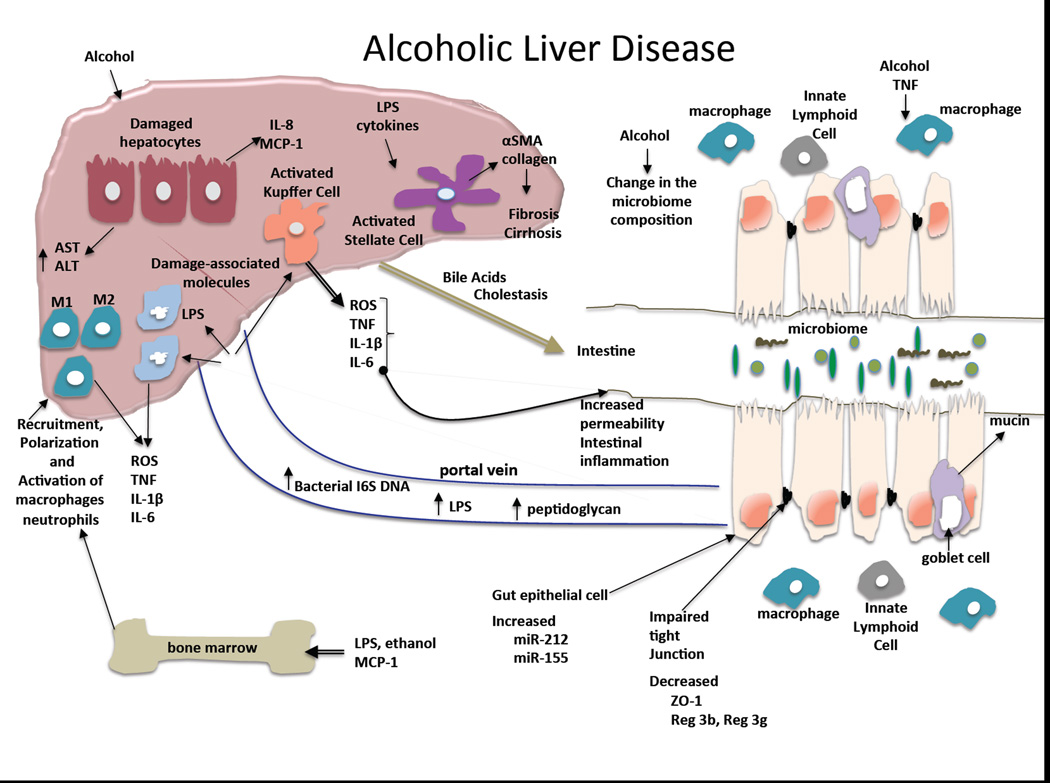
Excessive alcohol use over a prolonged period of time often results in alcoholic liver disease. The spectrum of alcoholic liver disease includes steatosis, steatohepatitis, acute alcoholic steatohepatitis, alcoholic fibrosis and cirrhosis (Laennec’s cirrhosis). Steatosis is and early steatohepatitis are reversible after cessation of alcohol use. Based on current understanding, multiple pathogenic factors are involved in the development of alcoholic liver disease. Alcohol and its metabolites induce reactive oxygen species and hepatocyte injury, though mitochondrial damage and ER stress7–12. There is early activation of chemokines, particularly MCP-1 that contributes to recruitment of macrophages and IL-8 that recruits neutrophil leukocytes in the liver13,14. Activation of Kupffer cells has been identified as a central element in the pathogenesis of ALD15,16. Previous studies demonstrated that KCs and recruited macrophages in the liver are activated by bacterial endotoxin (lipopolysaccharide-LPS) through Toll-like receptor 4 (TLR4) and that the level of LPS is increased in the portal as well as in the systemic circulation after excessive alcohol intake17,18. These observations suggest that gut-derived LPS is a central mediator of inflammation in alcoholic steatohepatitis.
Fibrosis is a dynamic and progressive process governed by stellate cell activation from inflammatory cytokines and gut-derived products19. Indeed, microbial components in the portal blood contribute to progression of fibrosis and the development of portal hypertension19,20. Advanced ALD predisposes to hepatocellular cancer where LPS-TLR4 interactions and stem cell Nanog expression seem to have mechanistic roles in animal models21,22.
Gut barrier function – effects of alcohol
The integrity of the intestinal mucosa is determined by the function of several components: protective layer of defensins on the intraluminal surface of the intestinal epithelium; tight junction proteins between intestinal epithelial cells, and the gut immune cells in the intestinal wall5. Alcohol has both direct effects on these functions in the intestine and indirect effects by alcohol and/or its metabolites distributed via the blood stream23. Acute alcohol binge causes cellular damage and death of the intestinal epithelial cells when consumed at high concentrations24. In addition, increase in blood alcohol levels is associated with reduced expression of mRNA levels of important proteins involved in tight junctions between colon epithelial cells25. In Caco-2 intestinal epithelial cells, alcohol decreases the expression of the tight junction proteins, occludin and zona-occludens -1 (ZO-1).25,26 It has been shown that microRNA-221 is involved in the downregulation of the tight junctions proteins in the mouse model of alcohol-induced gut permeability25. Acetaldehyde, a highly toxic metabolite of alcohol, disrupts tight junction thereby contributing to the increased gut permeability caused by chronic alcohol use27. In Caco-2 cells, alcohol also increased the expression of the circadian clock proteins, CLOCK and PER2, via reactive oxygen species (ROS)-induces upregulation of CyP2E1 leading to intestinal hyperpermeability28. Further studies demonstrated that disruption of the circadian clock in mice resulted not only in increased intestinal permeability but also promoted alcohol-induced liver damage and inflammation29. Chronic exposure of Caco-2 cells to alcohol also increased the susceptibility of these epithelial cells to infections by bacterial pathogens30. In vivo, deficiency in intestinal mucin-2 ameliorated ALD in mice and this was linked to increased killing of commensal bacteria and prevention of bacterial overgrowth31.
We recently found that acute alcohol binge drinking in healthy human volunteers resulted in a significant increase in serum endotoxin levels and this correlated with increased bacterial 16S rDNA increase suggesting gut microbial origin32. This is similar to the increased levels of serum endotoxin reported in patients with chronic alcohol consumption and liver disease16,33. In the animal model, we found that both acute alcohol binge and chronic alcohol administration increased serum endotoxin levels in mice24. The acute alcohol binge resulted in minimal inflammation in the proximal intestine while TNFa and NF-kB activation were robust after chronic alcohol feeding. The alcohol-induced intestinal inflammation correlated with reduced mRNA and protein levels of Reg3b and increased expression of microRNA-155 in the small intestine and further studies demonstrated that miR-155-deficien mice were protected from chronic alcohol-induced inflammation in the small intestine. Furthermore, there was no increase in serum endotoxin levels after chronic alcohol feeing in the miR-155-deficient mice suggesting that this microRNA-155 may have a role in alcohol-induced disruption of the gut integrity24. Another recently identified regulator of gut permeability is FoxO4 induced by alcohol34. It was found that alcohol significantly increase FoxO4 that can regulate intestinal permeability34. These recent studies imply the complexity of gut barrier function and highlight the multiple checkpoints where alcohol could interfere with normal function.
Gut microbiome and alcohol
Perhaps the majority of new information on gut-liver axis in recent years is related to understanding the role of the microbiome in human health and disease. The intestinal microbiota has a major role in shaping the host immune response and commensial bacteria shape the integriy of the gut mucosa35. A wide array of human diseases including obesity, insulin resistance and the related metabolic syndrome, and NASH, cancer, chronic inflammatory diseases and infections, and neuroinflammatory processes have been linked to change in the gut microbiome. In case of alcohol use, the microbiome is a very likely target given that in human alcohol consumption alcohol in the intestinal content has direct contact with components of the microbiome. In vitro studies demonstrated that alcohol has direct and selective effects on the growth of bacteria and intestinal overgrowth can produce ethanol that in turn may affect intestinal permeability36.
Metagenomic analysis of alcohol-induced alteration in the intestinal microbiome revealed that alcohol feeding in mice decreases the bacterial diversity and shifts the phylum representation over time37. Compared to pair fed mice where the majority of bacteria were in the Bacteriodes and Firmicutes phylum, alcohol feeding dramatically increased the presence of Actinobacteria and increased the proportion of Firmicutes over altered Bacteriodes37. In another study in a mouse model of alcoholic liver disease, bacterial translocation was found before changes in the microbiome and the bacterial traslocation was associated with reduced expression of the bactericidal c-type lectins, Reg3b and Reg3g in the small intestine38.
Chronic alcohol also alters the metabolic composition in the gastrointestinal content that changes the source of nutrition for microbes in the GI tract39. For example, alcohol feeding resulted in a decrease in all amino acids and branched chain amino acids in the gut39. These deteriorations indicate that chronic alcohol use directly and indirectly changes the composition of the gut microbiota.
Microbial products, pattern recognition receptors, and the immune system
Pathogen-associated molecular patterns (PAMPs) are sensed by pattern recognition receptors including Toll-like receptors (TLRs), Nod-like receptors (NLRs), helicase receptors and others40,41. The microbiome contains a broad variety of PAMPs and owed to the intestinal barrier, these PAMPs don’t reach the systemic circulation. The most studied gut-derived PAMP in the circulation is bacterial lipopolysaccharide (LPS) that is a component of Gram-negative bacterial wall. Many studies demonstrated that chronic alcohol consumption increases LPS levels in the portal as well as in the systemic circulation without entering the brain. This was found in human alcoholics with or without liver disease as well as in rats and mice23,33. In mice, a single acute alcohol gavage can increase serum LPS levels and administration of a 5% alcohol containing diet also increases serum LPS as early as in one week42. A recent study showed that acute alcohol binge in normal volunteers results in a rapid increase in serum LPS as well as in bacterial 16S DNA levels suggesting disruption of intestinal barrier function. In addition ot LPS and 16S bacterial DNA, peptidoglycan, a component of Gram-positive bacteria was also detected in human alcoholics43. It has also been shown that TLR4 or CD14 deficient mice that have disruption of the LPS receptor complex, are protected from alcoholic liver disease17,18,44. Consistent with this, sterilization of the gut also attenuated alcohol-induced liver disease in animal models45.
Repeated engagement of TLR4/CD14 with LPS results in TLR tolerance in macrophages46,47. However, in the alcoholic liver environment with increase portal blood LPS, KCs and macrophages become sensitized to LPS47 leading to increased TNFα production48,49.
Activation of the TLR4 receptor complex by alcohol-induced LPS results in downstream activation of the NFκ-B pathway and induction of pro-inflammatory cytokines and chemokins. Amongst those are TNFα and IL-1β that have been shown to increase gut permeability thereby potentially amplifying the alcohol-induced initial gut leakiness and liver disease process50. Locally in the intestinal mucosa, chronic alcohol results in increased expression of TNFα an IL-1β24. Recent reports described that IL-22 is a cytokine that regulates gut epithelia cells and immune functions. In a burn injury model, alcohol addition amplified reduction in IL-22 that correlated with increased gut permeability. In the same study, IL-22 administration prevented the increased gut permeability caused by the combined insult of alcohol plus burn injury51. In another study, IL-22 administration ameliorated alcoholic liver injury raising the question whether IL-22 acts directly on the liver or via the gut-liver axis in ALD52. IL-22 is produced by innate lymphoid cells (ILC) that reside in the bowel wall5,53. In the intestinal wall the interaction of immune cells is an important component in maintenance of the host and microbiome balance. Recent studies suggest that microbiota can dictate the crosstalk between macrophages and innate lymphoid cells (ILC)3 and this promotes intestinal homeostasis54. ILCs balance immunity, inflammation and tissue repair in the intestine, and may play a role in regulation of the gut-liver axis in ALD55.
Clinical aspects of the impaired gut-liver axis in ALD
Excessive alcohol use in most cases is associated with alcohol dependence56. The role of intestinal permeability and inflammation has received recent attention in the biological and behavioral control of alcohol dependence57. A recent study found that intestinal permeability and LPS were increased in alcohol-dependent non-cirrhotic subject at hospitalization for detoxification compared to 3 weeks later after successful detoxification. Inflammatory cytokine increase was correlated with depression and alcohol craving suggesting that gut-brain axis may play a role in the pathogenesis of alcohol dependence58.
Alcoholic hepatitis often arises in the setting of liver fibrosis raising the question whether the fibrotic liver was more susceptible to alcohol and/or gut-derived PAMPs induced by alcohol. It has been shown that in patient with portal hypertension the gut barrier function is impaired leading to increased gut “leakiness”59. Increased levels of LPS entering the liver has multiple biologic effects. First, LPS induces recruitment and activation of inflammatory cells and pro-inflammatory cytokine production. Second, LPS modulates hepatocyte functions and results in cholestasis60. Third, LPS and pro-inflammatory cytokines induce production of acute phase reactants by hepatocytes in the liver including serum amyloid A, LPS binding protein (LBP), fibrogen, C-reactive protein, Il0-6 and ceruloplasmin61–63. It has been proposed that normal hepatocytes have a role in “detoxification” of the portal blood including elimination of LPS64,65. Altered production of LBP, soluble CD14 and anti-LPS antibodies that all act by binding circulating LPS and modulate the biologically active form of LPS that leads to inflammation. The capacity of hepatocytes that are constantly exposed to alcohol is unlikely to be contact in the role of LPS detoxification, however, only a few studies have evaluated this question66. Indeed, levels of LBP and soluble CD14 were found to be elevated in advanced liver diseases67,68. Consistent with increased gut permeability, changes in gut bacterial populations and their translocation to the liver and ascites was found in alcoholic liver cirrhosis in various studies69. One of the potential clinical implications of the gut-liver axis in advanced ALD is promotion of bacterial infections often manifested as sub-acute bacterial peritonitis, hepatic encephalopathy, or severe systemic infections70. Selective gut decontamination with Rifaximin has improved hepatic encephalopathy raising the possibility of beneficial effects of selective gut decontamination in advanced ALD71.
Emerging therapeutic approaches that target the gut-liver axis
Given that alcohol disrupts the gut barrier function, it is attractive to explore therapeutic interventions that could prevent alcohol-induced gut “leakiness” and/or restore alcohol-induced defects. For example, it has been shown that alcohol-induced zinc deficiency contributes to the impaired gut barrier function72. More important, administration of zinc in mice with chronic alcohol feeding restored the alcohol-induced gut dysfunction73.
Another approach is to modify the microbiome dysbalance associated with alcohol use. To this end, a recent study evaluated the effect of VSL3 in mice with chronic alcohol feeding and found improvement in gut permeability after VSL3 administration74. In a different study, administration of Lactobacillus rhamnosus GG in mice on chronic alcohol diet resulted in a decrease in fecal pH, attenuated serum endotoxin levels and attenuation of alcohol-induced liver damage (ALT and steatosis)37. These beneficial effects of Lactobacillus rhamnosus GG treatment during continued alcohol intake in mice was associate with improved gut permeability based on tight junction protein expression.37. Notably, Lactobacillus rhamnosus GG treatment also improved markers of intestinal barrier function and provided protection against non-alcoholic fatty liver induced by high fat diet in mice75.
Fecal transplantation as a therapeutic approach demonstrated benefits in C. difficile infection76. Based on observation indicating altered composition of the intestinal microbiome in ALD, it is tempting to speculate that fecal transplantation may modulate the outcome or severity of ALD.
Dietary supplements have received interest in alcoholic liver disease as chronic alcohol use is often associated with deficient intake of nutrients and vitamins. Zinc deficiency is a well established consequence of chronic alcohol consumption72. Studies have shown that zinc deficiency augments alcohol-induced liver injury as well as the negative effects of alcohol on gut permeability72. It was shown that dietary zinc deficiency augmented alcohol-induced increases in serum endotoxin levels as well as most of the pathogenic features of alcohol-induced liver damage and inflammation73. Milk osteopontin, a component of milk was shown to ameliorate alcohol liver damage and serum endotoxin increase in a mouse model of alcoholic liver disease77.
Studies in rat duodenum showed that administration of a 15% alcohol solution or red wine in the intraluminal surface of the duodenum increases duodenal permeability and this could be prevented by administration of melatonin78. Melatonin is produced in the gut enterochromaffin cells and it can act as a potent antioxidant (Stomlanski et al 2012).
New therapeutic approaches to target gut-derived inflammatory signals may consider anti-LPS antibody administration or TLR4 inhibition strategies. Another potential target could be inhibition of miR-155 based on the observation of attenuation of alcohol-induced gut permeability in miR-155 deficient mice24.
Unanswered questions
Although the number of reports on the gut-liver axis in alcoholic liver disease has drastically increased in recent years, there are many remaining questions. Increase in gut permeability is not unique to alcoholic liver disease. In disease conditions such as Crohn’s colitis or HIV infection serum LPS levels are elevated yet there is no liver disease. It appears that increased gut permeability is just one of potentially several factors that contributes to ALD. It is tempting to speculate that alcohol-induced effects on hepatocytes, whether it is induction apoptosis, ER stress, mitochondrial damage and/or modulation of inflammatory cell responses in the liver are fundamental elements in the process of ALD that provide an environment for gut-derived LPS (an/or other PAMPs) to result in the complex pathology of ALD. Nevertheless, most studies suggest that prevention of the alcohol-induced disruption of gut permeability and/or the entry of gut-derived inflammatory signals to the liver have proven beneficial effects on the development of alcoholic liver disease. In conclusion, the interactions between the gut microbiome, intestinal barrier and the liver appear to have a key role in the pathogenesis of alcoholic liver disease and further exploration of the gut-liver axis in ALD deserves attention.
Acknowledgments
This work was supported NIAAA grants AA021907, AA010744 and AA017729. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The author thanks Dr. Pranoti Mandrekar for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Manuscript was written by G. Szabo.
Conflict of interest: Author declares no conflict of interest.
References
- 1.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Gupta M, Aggarwal R. Gut microbiota and liver disease. J Gastroenterol Hepatol. 2014 doi: 10.1111/jgh.12556. [DOI] [PubMed] [Google Scholar]
- 3.Balmer ML, Slack E, de Gottardi A, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6:237ra66. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- 4.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 9.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Dolganiuc A, Thomes PG, Ding WX, Lemasters JJ, Donohue TM., Jr Autophagy in alcohol-induced liver diseases. Alcohol Clin Exp Res. 2012;36:1301–1308. doi: 10.1111/j.1530-0277.2012.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136–2142. doi: 10.3748/wjg.v20.i9.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 13.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–2197. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler MD, Kono H, Yin M, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto N, Ikejima K, Bradford BU, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 17.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 18.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010 doi: 10.1155/2010/710381. 10.1155/2010/710381. Epub 2010 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 20.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 21.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippai D, Bala S, Catalano D, Kodys K, Szabo G. MicroRNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcoholism: Clinical and Experimental Research. 2014 doi: 10.1111/acer.12483. In Press;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction associated proteins. Mol Med Rep. 2014;9:2352–2356. doi: 10.3892/mmr.2014.2126. [DOI] [PubMed] [Google Scholar]
- 27.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsyth CB, Voigt RM, Shaikh M, et al. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summa KC, Voigt RM, Forsyth CB, et al. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood S, Pithadia R, Rehman T, et al. Chronic alcohol exposure renders epithelial cells vulnerable to bacterial infection. PLoS One. 2013;8:e54646. doi: 10.1371/journal.pone.0054646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann P, Chen P, Wang HJ, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 34.Chang B, Sang L, Wang Y, Tong J, Wang B. The role of FoxO4 in the relationship between alcohol-induced intestinal barrier dysfunction and liver injury. Int J Mol Med. 2013;31:569–576. doi: 10.3892/ijmm.2013.1229. [DOI] [PubMed] [Google Scholar]
- 35.Kamada N, Nunez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477–1488. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986;90:103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- 37.Bull-Otterson L, Feng W, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie G, Zhong W, Zheng X, et al. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12:3297–3306. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737–744. doi: 10.1159/000324281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Petrasek J, Bala S, Csak T, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P. Role of Inflammatory Pathways, Blood Mononuclear Cells, and Gut-Derived Bacterial Products in Alcohol Dependence. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 46.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 47.Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Exp (Warsz) 2012;60:13–18. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 48.Bala S, Marcos M, Kodys K, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3(Suppl 1):S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoseph BP, Breed E, Overgaard CE, et al. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One. 2013;8:e62792. doi: 10.1371/journal.pone.0062792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taube C, Tertilt C, Gyulveszi G, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One. 2011;6:e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortha A, Chudnovskiy A, Hashimoto D, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12:445–457. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trudell JR, Messing RO, Mayfield J, Harris RA. Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol Sci. 2014 doi: 10.1016/j.tips.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajo M, Madamba SG, Roberto M, et al. Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leclercq S, Cani PD, Neyrinck AM, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Mehta G, Mookerjee RP. Breaking Bad - the Two Sides of Gut Microbiota in Portal Hypertension. Liver Int. 2014 doi: 10.1111/liv.12598. [DOI] [PubMed] [Google Scholar]
- 60.Navaneethan U, Jayanthi V, Mohan P. Pathogenesis of cholangitis in obstructive jaundice-revisited. Minerva Gastroenterol Dietol. 2011;57:97–104. [PubMed] [Google Scholar]
- 61.Mackiewicz A, Kushner H. Acute Phase Proteins: Molecular Biology, Biochemistry, and Clinical Applications. Anonymous Boca Raton, FL: CRC Press; 1993. p. 686. [Google Scholar]
- 62.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 63.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 64.Shao B, Lu M, Katz SC, et al. A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J Biol Chem. 2007;282:13726–13735. doi: 10.1074/jbc.M609462200. [DOI] [PubMed] [Google Scholar]
- 65.Jirillo E, Caccavo D, Magrone T, et al. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319–327. doi: 10.1179/096805102125000641. [DOI] [PubMed] [Google Scholar]
- 66.Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res. 2005;29:172S–179S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- 67.Oesterreicher C, Pfeffel F, Petermann D, Muller C. Increased in vitro production and serum levels of the soluble lipopolysaccharide receptor sCD14 in liver disease. J Hepatol. 1995;23:396–402. doi: 10.1016/0168-8278(95)80197-9. [DOI] [PubMed] [Google Scholar]
- 68.Stemerowicz R, Moller B, Martin P, et al. Antibody activity against lipopolysaccharides, lipid A and proteins from Enterobacteriaceae in patients with chronic inflammatory liver diseases. Autoimmunity. 1990;7:305–315. doi: 10.3109/08916939009087590. [DOI] [PubMed] [Google Scholar]
- 69.Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14 doi: 10.1186/1471-230X-14-40. 40-230X-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 71.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 72.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8:e76522. doi: 10.1371/journal.pone.0076522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13 doi: 10.1186/1471-230X-13-151. 151-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritze Y, Bardos G, Claus A, et al. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS One. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 77.Ge X, Lu Y, Leung TM, Sorensen ES, Nieto N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G929–G939. doi: 10.1152/ajpgi.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sommansson A, Saudi WS, Nylander O, Sjoblom M. Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am J Physiol Gastrointest Liver Physiol. 2013;305:G95–G105. doi: 10.1152/ajpgi.00074.2013. [DOI] [PubMed] [Google Scholar]