Abstract
Objective
To assess if ezetimibe (EZE), a sterol-absorption inhibitor, improves platelet (PLT) count and size relative to its effect on plasma plant sterol (PS) in patients with sitosterolemia (STSL).
Study design
Patients with STSL (5 males, 3 females, 16 to 56 years of age) receiving EZE intervention as part of their routine care participated in this study. EZE was discontinued for 14 weeks (off) and then resumed for another 14 weeks (on). Hematology variables along with plasma and red blood cells (RBC) PS and total cholesterol (TC) levels were measured at the end of each phase.
Results
EZE increased PLT count (23 ± 9%) and decreased mean PLT volume (MPV; 10 ± 3%, all P < .05). In patients off EZE, PLT counts inversely correlated (r = − 0.96 and r = − 0.91, all P < .01) with plasma and RBC PS to TC ratio (PS/TC), and MPV positively correlated (r = 0.91, P = .03 and r = 0.93, P = .02) with plasma and RBC PS/TC. EZE reduced plasma and RBC sitosterol (−35 ± 4 and −28 ± 3%), total PS (−37 ± 4 and −28 ± 3%, all P < .0001) levels and PS/TC (−27 ± 4 and −28 ± 4%, P < .01).
Conclusion
EZE reduces plasma and RBC PS levels, and increasing PLT count and decreasing MPV, and thereby may reduce the risk for bleeding in STSL. Plasma PS levels and ABCG5/ABCG8 genes should be analyzed in patients with unexplained hematologic abnormalities.
Keywords: phytosterols, macrothrombocytopenia, sterol absorption inhibitor
Sitosterolemia (STSL) is a rare autosomal recessive condition caused by mutations in either of ABCG5 or ABCG8, two adenosine triphosphate-binding cassette (ABC) transporter genes located in a head-to-head orientation on chromosome 2p21.1 The transporters are primarily expressed in the intestine and the liver, and participate in the removal and excretion of absorbed dietary sterols, thus preventing their accumulation in blood and tissues.1–3 In STSL, the plasma levels of plant sterols (PS), mainly sitosterol, campesterol and stigmasterol are elevated. In contrast, plasma total cholesterol (TC) levels are usually normal or moderately elevated4, 5 although in some cases TC can be extremely high.6–8 Clinical features of STSL include tendon xanthomas, premature coronary artery disease, hemolytic anemia, macrothrombocytopenia, (large platelets [PLT] present in reduced quantity), and bleeding.2, 9–11 Severe bleeding episodes, resulting from macrothrombocytopenia, have been reported in STSL, which necessitate PLT transfusion.11 Other hematologic abnormalities include hemolytic anemia with stomatocytes; deformed red blood cells (RBC).9
Ezetimibe (EZE), a sterol absorption inhibitor commonly used to treat hypercholesterolemia12 is also used to treat STSL. In STSL, EZE effectively decreases plasma levels of TC, low-density lipoprotein (LDL)-cholesterol,13 and PS.14 Importantly, EZE has been shown to improve thrombocytopenia in ABCG5-knockout (KO) mice,11, 15 although such effects are not consistently observed in patients with STSL.16, 17 Abnormal liver function has been reported in patients with STSL receiving EZE,18 but overall EZE is considered safe in patients with STSL.14, 19, 20 We sought to investigate if EZE improves PLT abnormalities in STSL, and if any effects correlate with PS reduction. The safety of EZE on liver and kidney function was also examined.
METHODS
Eight patients (5 males and 3 females, between 16 and 56 years of age) were recruited from Hutterite colonies in Manitoba, Canada (n = 4) and South Dakota, US (n = 4). All patients were identified as having homozygous ABCG8 S107X mutation (NM_022437.2:c.320C>G) and are related to a proband previously reported by Mymin et al21, 22 and Chong et al.23 All procedures involving human patients were approved by the University of Manitoba Biomedical Ethics board and written informed consent was obtained from all patients. The trial was conducted by investigators of the Sterol & Isoprenoid Research (STAIR) consortium (https://rarediseasesnetwork.epi.usf.edu/STAIR/) after approval by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
The study was a single-site pilot interventional study of 8 patients with STSL conducted at the Richardson Centre for Functional Foods and Nutraceuticals, University of Manitoba. It was designed as a two 14-week phase (off and on EZE) study. After consenting, patients were taken off EZE for 14 weeks with blood samples collected before and at the end of the phase. On the last day of blood draw, patients were instructed to resume EZE (10 mg/day) for another 14 weeks and follow their usual diet. Blood was collected at the end of the 14 weeks treatment period with EZE. Plasma sterol and lipid concentrations, and complete blood counts were measured at baseline, after 14 weeks off EZE and 14 weeks on EZE. Serum, plasma and RBC fractions were separated by centrifugation at 3000 rpm for 20 minutes at 4°C, and stored at −80°C until further analysis. Participants were asked to monitor and report any adverse experiences including headaches, chest pain, or dizziness. Physical examinations and markers of liver and kidney function were measured at baseline and 14 weeks off and on EZE.
The levels of plasma and RBC TC and total PS (sitosterol, campesterol, stigmasterol and brassicasterol) were measured by gas-liquid chromatography equipped with a flame ionization detector and an auto sampler system (Varian 430-GC; Agilent Technologies, Santa Clara, CA, US). For sterol quantification, an internal standard of 5α-cholestane was used in combination with sterol standard curve for each individual sterol, using authentic standards (Sigma-Aldrich Ltd. Oakville, ON, Canada) and (MJS BioLynx Inc, Brockville, ON, Canada). Briefly, 5α-cholestane (50 ug) was added to each 0.5 mL plasma or 0.5 g RBC sample, and saponified with 4 mL methanolic potassium hydroxide for 2 h at 100°C. The unsaponifiable portion (sterols) were extracted twice from the mixture with 4 mL petroleum ether, derivatized with 0.1 mL TMS reagent (pyridine–hexamethyldisilazane–trimethylchlorosilane; 9:3:1 by volume), re-suspended in 0.4 mL hexane and injected (1 µL) onto a 30-m SAC-5 column (Sigma-Aldrich Ltd. Oakville, ON, Canada). The column temperature was set to 280°C, with isothermal running conditions maintained for 30 minutes per sample. The injector and detector temperatures were set at 295°C and 300°C, respectively. The carrier gas (helium) flow rate was 1 mL/minute, with the inlet split set at 40:1.24 Complete blood count was measured using automated hematology analyzer. Serum markers of RBC hemolysis including lactate dehydrogenase (LDH) and total bilirubin and serum lipids were measured using automated enzymatic methods. Serum LDL-cholesterol levels were calculated by the Friedewald equation.25
Statistical Analyses
Statistical analyses were performed using SPSS 21.0 (SPSS, Inc., Chicago, IL, US). All data are presented as mean ± SEM. Statistical significance was set at P < .05. Linear mixed-model analysis was used where treatment, sex and site were specified as fixed factors, and age was specified as a covariate in the model. Significant values for treatment effect were analyzed by including the treatment term as a main effect in the linear mixed-model ANOVA. Percentage change from baseline for each phase was analysed using two-tailed paired Student t test. Relationships between two variables were assessed with stepwise multiple linear regression analysis unless otherwise stated. Data that were not normally distributed, as determined by a Shapiro-Wilk test, were log or inverse transformed before statistical analysis.
RESULTS
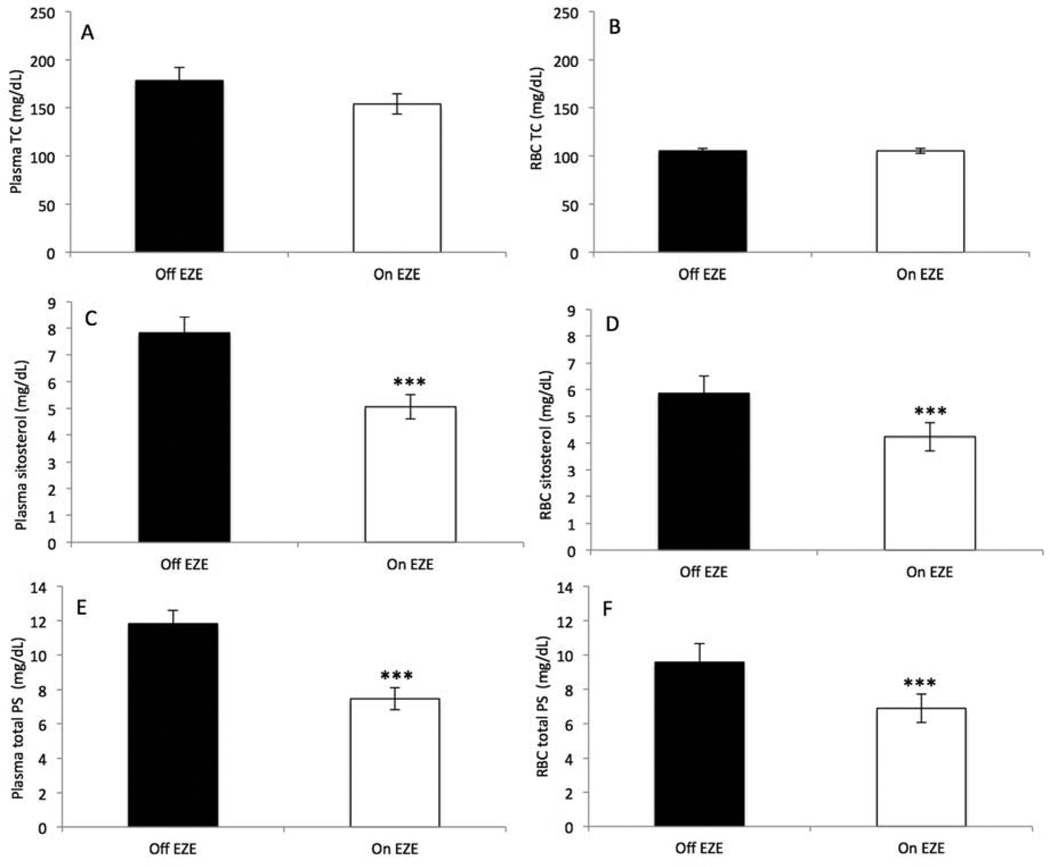
All eight initially recruited patients completed the study (Table I). Mean values of serum TC and LDL-cholesterol indicated mild hypercholesterolemia. All patients had elevated plasma and RBC levels of PS (Table I). The plasma and RBC concentrations of sitosterol and total PS were similar on average (P > .11), but the PS to TC ratio (PS/TC) tended to be higher in RBC than in plasma (P = .08; Table I). One subject had mild anemia [hemoglobin (Hb) = 11.7 g/dL; hematocrit (HCT) = 37.7%], and stomatocytes were noted in another, otherwise RBC and white blood cells (WBC) indices were within normal ranges (Table II). In contrast, three subjects had decreased PLT counts (≤140×103/µL), four had increased PLT size (MPV >12 fL) and two had both large PLT size and low PLT counts. One of our subjects had a clinically significant hematologic disorder, namely, a past history of chronic hemolysis, which was no longer manifested at the time of this study. None of the other subjects had any history of bleeding diathesis or atherosclerotic manifestations, heart murmurs, or vascular bruits. Baseline kidney function tests for all patients were within normal limits as measured by serum creatinine levels (Data not shown). Patients lacked evidence of abnormal liver function except two patients who had mild elevations in one liver enzyme (gamma-glutamyltransferase) or all (gamma-glutamyltransferase, alanine transaminase, aspartate transaminase and alkaline phosphatase) (Data not shown). All patients had normal bilirubin levels (0.42 ± 0.06 mg/dL). EZE tends to lower plasma TC (−12 ± 6%, P = .09) after 14 weeks but did not affect RBC TC (0.3 ± 3%, P = 1.0) (Figure 1, A and B). In patients off EZE, RBC TC was negatively correlated to RBC sitosterol (r = −0.77, P = .03), total PS (r = −0.79, P = .02) and PS/TC (r = −0.86, P = .006). Regardless of EZE treatment, RBC consistently had lower (P < .001) TC levels than plasma (−39 ± 3 and −30 ± 4%). EZE dramatically reduced plasma and RBC sitosterol (−35 ± 4 and −28 ± 3%, all P < .0001) and total PS (−37 ± 4 and −28 ± 3%, all P < .0001) (Figure 1, C–F). When total PS were expressed against TC concentrations (i.e., PS/TC ratio) in plasma and RBC these reductions were significant (−27 ± 4%, P = .002 and −28 ± 4%, P = .003). Furthermore, the decrease in the PS/TC in plasma after EZE correlated with that of tissue (RBC). After removing an outlier, the correlation became significantly stronger (r = 0.99, P < .0001) than when the outlier was included (r = 0.72, P = .05).
Table I.
Baseline characteristic of sitosterolemia patients (mean ± SEM)
| Demographics (n = 8) | |
| Age (year) | 28 ± 6 |
| Body weight (kg) | 72 ± 6 |
| Height (cm) | 166 ± 2 |
| Body mass index (kg/m2) | 26 ± 2 |
| Lipid analyses (n = 7)* | |
| TC (mg/dL) | 211 ± 17 |
| LDL-cholesterol (mg/dL) | 131 ± 14 |
| HDL-cholesterol (mg/dL) | 55 ± 3 |
| Triglyceride (mg/dL) | 122 ± 27 |
| Plasma TC¥ (mg/dL) | 156 ± 13 |
| RBC TC¥ (mg/dL) | 101 ± 6 |
| Plasma sitosterol (mg/dL) | 6 ± 0.7 |
| RBC sitosterol (mg/dL) | 5 ± 0.5 |
| Plasma total PS† (mg/dL) | 9 ± 1 |
| RBC total PS (mg/dL) | 8 ± 0.7 |
| Plasma PS/TC ratio | 0.06 ± 0.01 |
| RBC PS/TC ratio | 0.08 ± 0.01 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoproteins; PS, plant sterols; RBC, red blood cells; TC, total cholesterol; PS/TC ratio, plant sterol to cholesterol ratio.
n=7, biochemical data was not available for one patient.
Plasma TC and RBC TC were determined by gas chromatography,
Total PS is the sum of sitosterol, campesterol, stigmasterol and brassicasterol.
Table II.
Hematology indices at baseline, 14 weeks off ezetimibe (off EZE) and 14 weeks on ezetimibe (on EZE) (mean ± SEM)
| Variable | Baseline | 14 week off EZE |
14 weeks on EZE |
Mean % change |
P |
|---|---|---|---|---|---|
| PLT (103/mL)§ | 164.3 ± 11.9 | 143.6 ± 15.8 | 170.3 ± 13.7 | +23 ± 8.9 | .03 |
| MPV (fL) | 13.0 ± 0.4¶ | - | 12.4 ± 0.4§ | −10 ± 2.6 | .04 |
| WBC (103/mL)§ | 5.6 ± 0.5 | 5.6 ± 0.3 | 6.3 ± 0.5 | +11 ± 5.3 | .07 |
| RBC (106/mL)§ | 4.8 ± 0.1 | 4.6 ± 0.2 | 4.7 ± 0.1 | +1 ± 1.4 | .57 |
| Hb (g/dL)§ | 14.2 ± 0.5 | 13.8 ± 0.5 | 14.0 ± 0.6 | +1.3 ± 1.0 | .24 |
| Hematocrit (%)§ | 42.6 ± 1.3 | 41.4 ± 1.5 | 41.4 ± 1.5 | +0.1 ± 1.1 | .98 |
| MCV (fL)§ | 89.7 ± 0.8 | 89.5 ± 0.9 | 88.8 ± 1.2 | −0.8 ± 0.7 | .32 |
| MCH (pg/cell)§ | 30.0 ± 0.4 | 29.8 ± 0.4 | 29.9 ± 0.6 | +0.4 ± 0.8 | .61 |
| MCHC (g/dL)§ | 33.4 ± 0.4 | 33.3 ± 0.3 | 33.7 ± 0.2* | +1.3 ± 0.5 | .03 |
| RDW-SD (fL)† | 43.3 ± 0.8 | 45.0 ± 0.9 | 44.6 ± 0.7 | −0.8 ± 1.0 | .37 |
| RDW-CV (%)§ | 13.6 ± 0.1 | 14.2 ± 0.2 | 14.1 ± 0.4 | −0.8 ± 2.0 | .86 |
| Neutrophils (%)§ | 55.5 ± 2.2 | 56.2 ± 2.0 | 53.7 ± 3.0 | −4 ± 4.0 | .31 |
| Lymphocytes (%)§ | 32.2 ± 2.8 | 33.3 ± 2.1 | 34.5 ± 3.1 | +3 ± 5.9 | .59 |
| Monocytes (%)§ | 9.7 ± 1.4 | 8.1 ± 0.8 | 8.7 ± 0.9 | +10 ± 9.7 | .42 |
| Eosinophils (%)§ | 2.2 ± 1.5 | 2.1 ± 0.6 | 2.6 ± 0.6 | +20 ± 10.7 | .07 |
| Basophils (%)§ | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | +33 ± 19.8 | .36 |
P <.05.
(n = 8),
(n = 5), and
(n = 4).
Because of technical problems MPV could not be determined on the samples obtained at 14 weeks off EZE. MPV measurements were therefore done on the baseline samples, where the mean MPV value represents measurement for five patients.
Abbreviations: Hb, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; RBC, red blood cells; RDW-CV, red cell distribution width coefficient of variation; RDW-SD; red cell distribution width standard deviation; WBC, white blood cells.
Figure 1.
Plasma and RBC total cholesterol (TC), sitosterol and total plant sterols (PS) in patients with STSL (n = 8) after 14 weeks off ezetimibe (Off EZE) and 14 weeks on ezetimibe (On EZE). Data are presented as mean ± SEM. *: P <.05; **: P <.01; ***: P <.0001. Total PS is the sum of sitosterol, campesterol, stigmasterol and brassicasterol values.
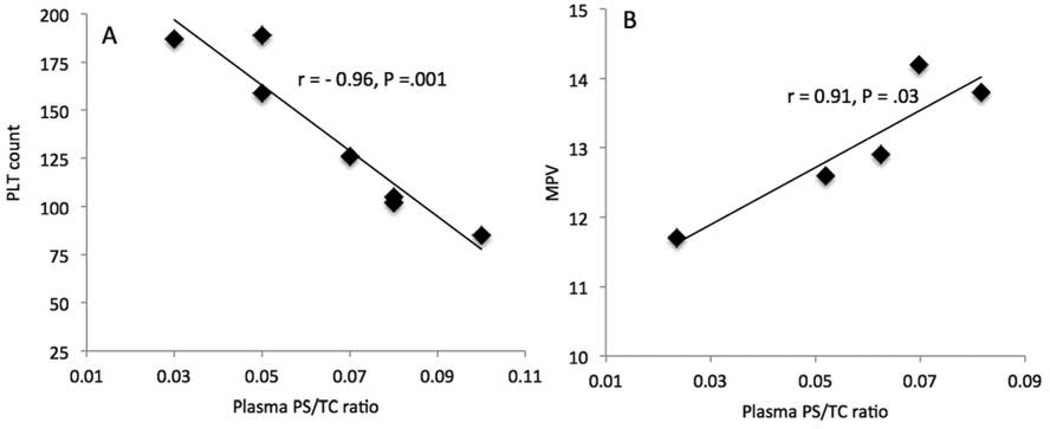
EZE increased (P = .03) PLT count (23 ± 9%) compared with 14 weeks off EZE (170 ± 14 vs 144 ± 16 × 103/µL) (Table II). In patients off EZE, PLT count showed insignificant inverse relationships with plasma and RBC total PS (r = −0.47, P = .24 and r = −0.68, P = .06) and PS/TC (r = −0.64, P = .09 and r = −0.68, P = .06). However, after removing the data for one patient who had the highest PLT counts, these correlations became statistically significant for PS (r = −0.77, P = .04 and r = −0.91, P = .004) and PS/TC (r = −0.96, P = .001, and r = −0.91, P = .005) (Figure 2, A). Likewise, EZE reduced MPV (−10 ± 3%) compared with baseline (12 ± 0.4 vs 13 ± 0.4 fL, P = .04) (Table II). Because of technical problems MPV could not be determined on samples obtained at 14 weeks off EZE. These measures were therefore only conducted on baseline samples, where the MPV for five patients was measured. Baseline plasma and RBC PS/TC were positively correlated with MPV (r = 0.91, P = .03 and r = 0.93, P = .02, n = 5) (Figure 2, B). After 14 weeks on EZE no significant differences were observed in RBC indices including Hb, HCT, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width standard deviation (RDW-SD) and red cell distribution width coefficient of variation (RDW-CV), and WBC, neutrophils, lymphocytes, monocytes, eosinophils and basophils compared with off EZE (Table II). However, MCHC, which reflects the proportion of Hb in RBC, slightly increased (P = .03) after EZE relative to off EZE (34 ± 0.2 vs. 33 ± 0.3 g/dL). MCHC inversely associated with RBC sitosterol (r = −0.86, P < .01), total PS (r = −0.91, P < .01), and PS/TC (r = −0.88, P < .01) before EZE. Similarly, plasma sitosterol (r = −0.51, P = .19), total PS (r = −0.68, P = .07) and PS/TC (r = −0.90, P = .003) were negatively correlated with MCHC in patients off EZE, suggesting that when plasma PS increase in patients off EZE, MCHC decreases.
Figure 2.
A and B, Correlations of PLT counts and MPV with plasma PS/TC. C, Correlation of PLT counts with plasma PS/TC (Off EZE, n = 7, one patient who had the highest PLT counts was an outlier and not included). D, Correlation of MPV with plasma PS/TC (baseline, n = 5).
DISCUSSION
The major finding of this study is that blocking intestinal absorption of PS with EZE in patients with STSL increases PLT number and decreases PLT size, and measures were correlated with reductions in blood PS concentrations. The minimal effect of EZE on blood cholesterol suggests that the improvements in PLT count and size were primarily due to improved PS homeostasis. The effect of EZE on PLT may potentially ameliorate the bleeding tendency in STSL.
Mutations in ABCG5/8 genes cause STSL, which is characterized by increased tissue retention of PS. We previously showed that the presence of intact ABCG5/8 protects against PS accumulation and hemolysis in hamsters26 and hypercholesterolemic subjects.27 Total PS represents 0.2% of total plasma sterols in healthy subjects28 but these levels increase 30-fold in STSL. The ABCG5/8 genes are not expressed in blood cells, and therefore these cells could be a target for the toxic effect of PS.29 Macrothrombocytopenia represents a clinical hematologic feature of STSL, which can lead to clinically problematic bleeding episodes.11, 30, 31
Bile acid sequestrants reduce PS levels but do not normalize macrothrombocytopenia in patients with STSL who presented with moderate bleeding.30 EZE is a sterol absorption inhibitor that has been used successfully in STSL,32, 33 but new data on improvement in hematologic variables have been reported.16 In a mouse model of STSL, EZE reversed macrothrombocytopenia34 compared with bone marrow transplantation, indicating that low PLT count in STSL is caused by the build-up of PS rather than intrinsic hematopoietic defects. Recently, EZE failed to decrease plasma PS levels and improve thrombocytopenia but corrected hemolytic anemia after one year in a patient with STSL, suggesting that EZE may improve some but not all phenotypic features of STSL.16 In the current study, plasma and RBC total PS (free and esterified) and PS/TC were reduced by approximately 30%. Furthermore, our analysis of free and esterified sterols showed that most of the plasma PS are esterified (85 ± 3% of total PS) but those of RBC are free (57 ± 5% of total PS), similar to previous reports.35,36 EZE reduced both plasma free (−32 ± 4%, P =. 006) and the esterified form of PS (−38 ± 7, P =. 009). In RBC, EZE decreased free PS (−35 ± 4%, P <. 0001) but not the ester form (−20 ± 9%, P =. 10) (data not shown). The decrease in plasma PS/TC strongly correlated with the decrease in RBC PS/TC ratio, suggesting tissue stores of PS represented by RBC sterols were reduced with EZE. Measurement of PS in plasma reflects PS levels from the most recent meals, and PS levels in RBC indicate a longer-term average of plasma levels and better reflection for tissue stores. Moreover, PS are carried in plasma by lipoproteins, mainly LDL-cholesterol, and any change in plasma TC concentration will also change PS levels. Thus, PS levels were expressed either as absolute concentrations or expressed as ratios relative to TC. EZE increased PLT count by 23% and decreased MPV by 10%. This is of clinical importance because decrease in PLT count may increase bleeding, however EZE therapy in patients with STSL may normalize PLT function through improved PLT count and size. It is known that build-up of PS in arterial endothelium impairs endothelial function and increases risk of atherosclerosis. Overall, EZE treatment was effective in lowering plasma and RBC PS levels with a favorable increase in PLT count.
The slight increase in RBC MCHC index, together with its negative correlations with plasma and RBC PS suggest that EZE may have increased MCHC by reducing PS levels. Similarly, a high RDW reflects an increased variability in the size of RBC (anisocytosis) and indicates body's iron status. RDW increases with accumulation of PS levels.31, 37 Plasma and RBC total PS levels in patients on EZE tend to positively correlate with anisocytosis (r = 0.64, P = .09 and r = 0.67, P = .07, respectively), denoting a possible lower variation in RBC size after EZE.
EZE tended to lower plasma TC but did not affect RBC TC levels. In this study, similar to previous reports,35,36 most of the plasma cholesterol is esterified (90 ± 2% of TC), and that of RBC is free (56 ± 6% of TC). EZE decreased free (−24 ± 5% and −18% ± 6, all P<. 05) cholesterol but not the ester form (−11 ± 9%, P=. 11 and +16±13, P=. 56) in plasma and RBC, respectively (data not shown). RBC TC concentrations are similar to plasma TC levels in healthy humans.38 Despite EZE treatment, the current patients with STSL had at least 30% lower TC levels in RBC than plasma on both study phases. RBC TC levels inversely correlated with RBC sitosterol and total PS, suggesting reciprocity between cholesterol and PS. Decreased RBC TC levels are likely due to increased RBC membrane PS incorporation39 and may indicate decreased tissue cholesterol content.40 Miettinen et al showed that RBC and plasma TC values were not related to each other in hypercholesterolemic children compared with the high respective correlations of PS.41 Herein, RBC TC tends to correlate with plasma TC after EZE (r = 0.71, P = .05), and RBC PS were strongly correlated with plasma PS (r = 0.90, P = .003), and had a linear relation when adjusted for TC (r = 1.0, P < .0001). Comparable PS levels in plasma and RBC indicate a rapid exchange of PS between the two compartments, suggesting that large amounts of PS in plasma could probably replace cholesterol in the RBC membrane,42 weaken interactions between the molecules,43 and increase cell fragility.9, 10, 37 A mouse model of STSL has shown that PS incorporate directly into the PLT membrane, disrupting lipid asymmetry, resulting in the formation of hyper-activatable PLT that lose the ability to aggregate.11
A number of limitations should be noted regarding the present study. The number of patients was relatively small; however, it should be noted that STSL is an extremely rare disease with fewer than 100 cases reported worldwide. Furthermore, this study included patients of only a single ethnic group, the Hutterites, all with a single genotype and, as such, the results from this study cannot be generalized to other populations. The current study was also limited by short duration. Long-term follow-up is needed to fully assess the ability of EZE to further reduce plasma and tissue PS levels. Given that RBC have a long life span (e.g., 120 days) compared with PLT, which have an estimated turnover of 9–10 days44 longer duration of treatment may be required to see a marked effect on RBC indices. Also three patients were on EZE prior to the start of the study, which may have blunted the effect despite the washout period. Similar to previous reports,17 EZE therapy over 14 weeks was well tolerated with no adverse effects on serum markers of kidney and liver functions, or muscle damage (data not shown). Overall, the study findings indicate that EZE is effective in reducing plasma and RBC PS levels and improving PLT indices in patients with STSL, thereby potentially ameliorating bleeding tendency in STSL. The data also suggest that plasma PS levels and ABCG5/A BCG8 genes should be analyzed in patients with unexplained hematologic abnormalities.
ACKNOWLEDGMENTS
We are grateful to the patients for their loyal support.
Supported by the Canadian Institutes for Health Research. The Sterol and Isoprenoid Research (STAIR) Consortium is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network, supported through collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science, and National Institute of Child Health and Human Development. R.O. was supported by the Libyan Scholarship Program and the Manitoba Health Research Council Graduate Student Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
List of abbreviations
- EZE
ezetimibe
- PS
plant sterols
- STSL
sitosterolemia
- RBC
red blood cells
- MPV
mean platelet volume
- PLT
platelets
- TC
total cholesterol
- MCHC
mean corpuscular hemoglobin concentration
- PS/TC ratio
total PS to total cholesterol ratio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya AK, Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33:945–955. [PubMed] [Google Scholar]
- 5.Lutjohann D, von Bergmann K. Phytosterolaemia: diagnosis, characterization and therapeutical approaches. Ann Med. 1997;29:181–184. doi: 10.3109/07853899708999333. [DOI] [PubMed] [Google Scholar]
- 6.Heum Park J, Chung IH, Hyun Kim D, Ho Choi M, Garg A, Yoo EG. Sitosterolemia presenting with severe hypercholesterolemia and intertriginous xanthomas in a breastfed infant: case report and brief review. J Clin Endocrinol Metab. 2014:jc20133274. doi: 10.1210/jc.2013-3274. [DOI] [PubMed] [Google Scholar]
- 7.Low LC, Lin HJ, Lau KS, Kung AW, Yeung CY. Phytosterolemia and pseudohomozygous type II hypercholesterolemia in two Chinese patients. J Pediatr. 1991;118:746–749. doi: 10.1016/s0022-3476(05)80040-x. [DOI] [PubMed] [Google Scholar]
- 8.McArthur RG, Roncari DA, Little JA, Kuksis A, Myher JJ, Marai L. Phytosterolemia and hypercholesterolemia in childhood. J Pediatr. 1986;108:254–256. doi: 10.1016/s0022-3476(86)80996-9. [DOI] [PubMed] [Google Scholar]
- 9.Neff AT. Sitosterolemia's stomatocytosis and macrothrombocytopenia. Blood. 2012;120:4283. doi: 10.1182/blood-2012-06-429449. [DOI] [PubMed] [Google Scholar]
- 10.Rees DC, Iolascon A, Carella M, O'Marcaigh AS, Kendra JR, Jowitt SN, et al. Stomatocytic haemolysis and macrothrombocytopenia (Mediterranean stomatocytosis/macrothrombocytopenia) is the haematological presentation of phytosterolaemia. Br J Haematol. 2005;130:297–309. doi: 10.1111/j.1365-2141.2005.05599.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanaji T, Kanaji S, Montgomery RR, Patel SB, Newman PJ. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of Sitosterolemia. Blood. 2013 doi: 10.1182/blood-2013-06-510461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 13.Knopp RH, Gitter H, Truitt T, Bays H, Manion CV, Lipka LJ, et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003;24:729–741. doi: 10.1016/s0195-668x(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 14.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruit JK, Drayer AL, Bloks VW, Blom N, Olthof SG, Sauer PJ, et al. Plant sterols cause macrothrombocytopenia in a mouse model of sitosterolemia. J Biol Chem. 2008;283:6281–6287. doi: 10.1074/jbc.M706689200. [DOI] [PubMed] [Google Scholar]
- 16.Quintás-Cardama A, McCarthy JJ. Long-term follow-up of a patient with sitosterolemia and hemolytic anemia with excellent response to ezetimibe. Genet Disor Genet Rep. 2013;2:1. [Google Scholar]
- 17.Salen G, Starc T, Sisk CM, Patel SB. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology. 2006;130:1853–1857. doi: 10.1053/j.gastro.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Castellote J, Ariza J, Rota R, Girbau A, Xiol X. Serious drug-induced liver disease secondary to ezetimibe. World J Gastroenterol. 2008;14:5098–5099. doi: 10.3748/wjg.14.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lutjohann D, von Bergmann K, Sirah W, Macdonell G, Johnson-Levonas AO, Shah A, et al. Long-term efficacy and safety of ezetimibe 10 mg in patients with homozygous sitosterolemia: a 2-year, open-label extension study. Int J Clin Pract. 2008;62:1499–1510. doi: 10.1111/j.1742-1241.2008.01841.x. [DOI] [PubMed] [Google Scholar]
- 20.Musliner T, Cselovszky D, Sirah W, McCrary Sisk C, Sapre A, Salen G, et al. Efficacy and safety of ezetimibe 40 mg vs. ezetimibe 10 mg in the treatment of patients with homozygous sitosterolaemia. Int J Clin Pract. 2008;62:995–1000. doi: 10.1111/j.1742-1241.2008.01786.x. [DOI] [PubMed] [Google Scholar]
- 21.Mymin D, Wang J, Frohlich J, Hegele RA. Image in cardiovascular medicine. Aortic xanthomatosis with coronary ostial occlusion in a child homozygous for a nonsense mutation in ABCG8. Circulation. 2003;107:791. doi: 10.1161/01.cir.0000050545.21826.ad. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res. 2004;45:2361–2367. doi: 10.1194/jlr.M400310-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Chong JX, Ouwenga R, Anderson RL, Waggoner DJ, Ober C. A population-based study of autosomal-recessive disease-causing mutations in a founder population. Am J Hum Genet. 2012;91:608–620. doi: 10.1016/j.ajhg.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myrie SB, Mymin D, Triggs-Raine B, Jones PJ. Serum lipids, plant sterols, and cholesterol kinetic responses to plant sterol supplementation in phytosterolemia heterozygotes and control individuals. Am J Clin Nutr. 2012;95:837–844. doi: 10.3945/ajcn.111.028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Demonty I, Ebine N, Jia X, Jones PJ. Fish oil fatty acid esters of phytosterols alter plasma lipids but not red blood cell fragility in hamsters. Lipids. 2005;40:695–702. doi: 10.1007/s11745-005-1432-y. [DOI] [PubMed] [Google Scholar]
- 27.Jones PJ, Raeini-Sarjaz M, Jenkins DJ, Kendall CW, Vidgen E, Trautwein EA, et al. Effects of a diet high in plant sterols, vegetable proteins, and viscous fibers (dietary portfolio) on circulating sterol levels and red cell fragility in hypercholesterolemic subjects. Lipids. 2005;40:169–174. doi: 10.1007/s11745-005-1372-6. [DOI] [PubMed] [Google Scholar]
- 28.Sehayek E, Breslow JL. Plasma plant sterol levels: another coronary heart disease risk factor? Arterioscler Thromb Vasc Biol. 2005;25:5–6. doi: 10.1161/01.ATV.0000151984.34920.aa. [DOI] [PubMed] [Google Scholar]
- 29.Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 1993;105:1806–1813. doi: 10.1016/0016-5085(93)91079-w. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Cao L, Su Y, Wang G, Wang R, Yu Z, et al. Specific macrothrombocytopenia/hemolytic anemia associated with sitosterolemia. Am J Hematol. 2013 doi: 10.1002/ajh.23619. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Cao L, Wang Z, Jiang M, Sun X, Bai X, et al. Macrothrombocytopenia/stomatocytosis specially associated with phytosterolemia. Clin Appl Thromb Hemost. 2012;18:582–587. doi: 10.1177/1076029611435090. [DOI] [PubMed] [Google Scholar]
- 32.Othman RA, Myrie SB, Jones PJ. Non-cholesterol sterols and cholesterol metabolism in sitosterolemia. Atherosclerosis. 2013;231:291–299. doi: 10.1016/j.atherosclerosis.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, et al. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDaniel AL, Alger HM, Sawyer JK, Kelley KL, Kock ND, Brown JM, et al. Phytosterol Feeding Causes Toxicity in ABCG5/G8 Knockout Mice. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin DS, Steiner RD, Merkens LS, Pappu AS, Connor WE. The effects of sterol structure upon sterol esterification. Atherosclerosis. 2010;208:155–160. doi: 10.1016/j.atherosclerosis.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeagle PL. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985;822:267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 37.Ratnayake WM, L'Abbe MR, Mueller R, Hayward S, Plouffe L, Hollywood R, et al. Vegetable oils high in phytosterols make erythrocytes less deformable and shorten the life span of stroke-prone spontaneously hypertensive rats. J Nutr. 2000;130:1166–1178. doi: 10.1093/jn/130.5.1166. [DOI] [PubMed] [Google Scholar]
- 38.Nikolic M, Stanic D, Antonijevic N, Niketic V. Cholesterol bound to hemoglobin in normal human erythrocytes: a new form of cholesterol in circulation? Clin Biochem. 2004;37:22–26. doi: 10.1016/j.clinbiochem.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Naito Y, Nagata T, Takano Y, Nagatsu T, Ohara N. Rapeseed oil ingestion and exacerbation of hypertension-related conditions in stroke prone spontaneously hypertensive rats. Toxicology. 2003;187:205–216. doi: 10.1016/s0300-483x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen LB, Shefer S, Salen G, Ness GC, Tint GS, Zaki FG, et al. A molecular defect in hepatic cholesterol biosynthesis in sitosterolemia with xanthomatosis. J Clin Invest. 1990;86:923–931. doi: 10.1172/JCI114794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ketomaki AM, Gylling H, Antikainen M, Siimes MA, Miettinen TA. Red cell and plasma plant sterols are related during consumption of plant stanol and sterol ester spreads in children with hypercholesterolemia. J Pediatr. 2003;142:524–531. doi: 10.1067/mpd.2003.193. [DOI] [PubMed] [Google Scholar]
- 42.Hac-Wydro K. The replacement of cholesterol by phytosterols and the increase of total sterol content in model erythrocyte membranes. Chem Phys Lipids. 2010;163:689–697. doi: 10.1016/j.chemphyslip.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Kanakaraj P, Singh M. Influence of hypercholesterolemia on morphological and rheological characteristics of erythrocytes. Atherosclerosis. 1989;76:209–218. doi: 10.1016/0021-9150(89)90105-6. [DOI] [PubMed] [Google Scholar]
- 44.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]