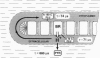
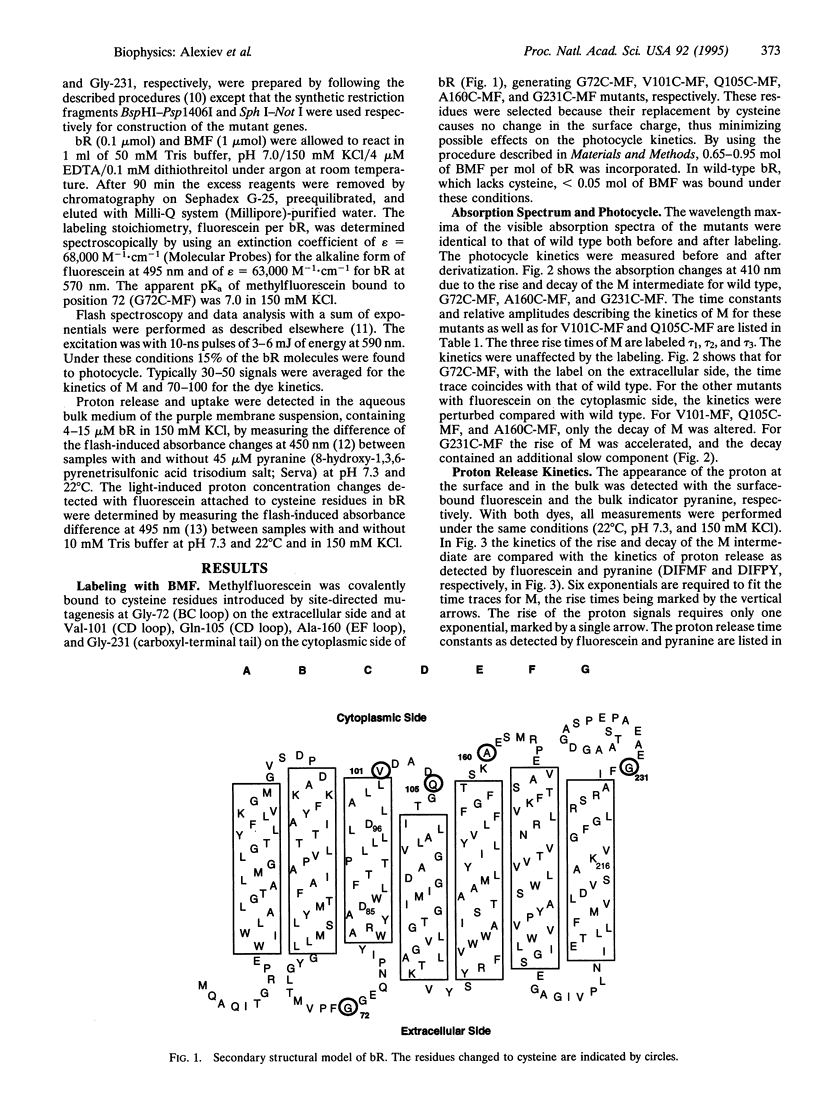
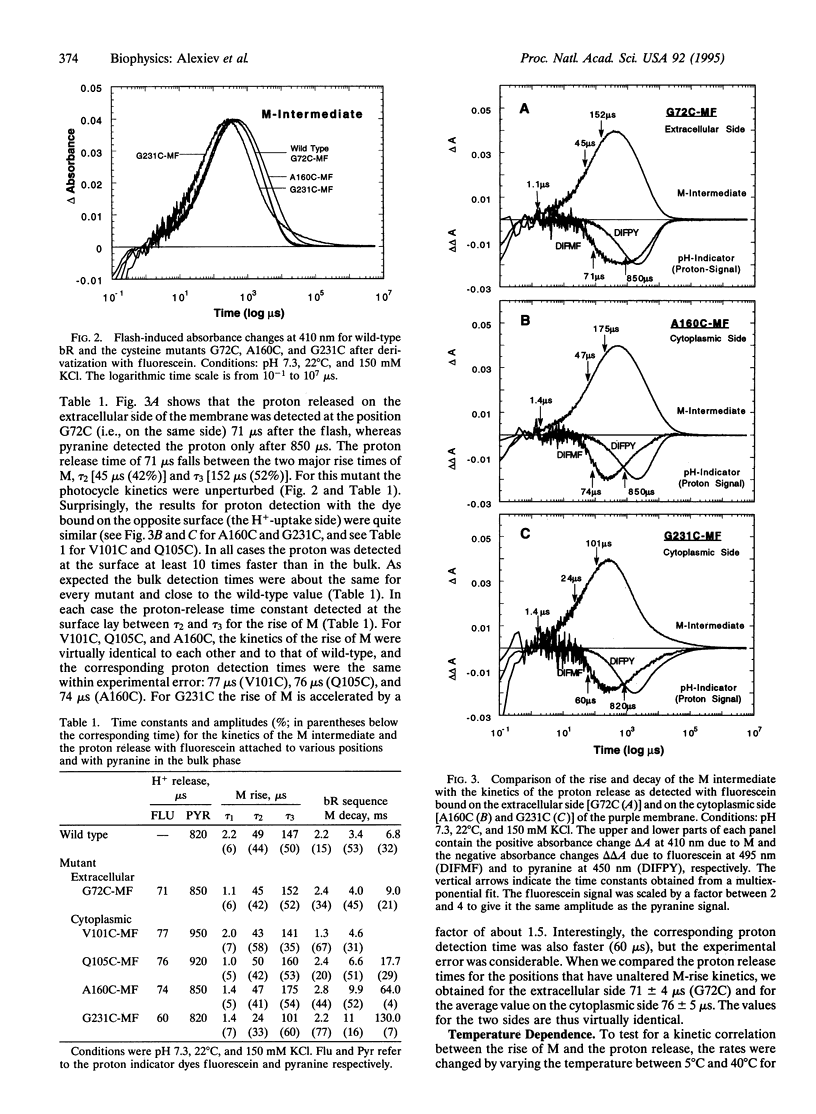
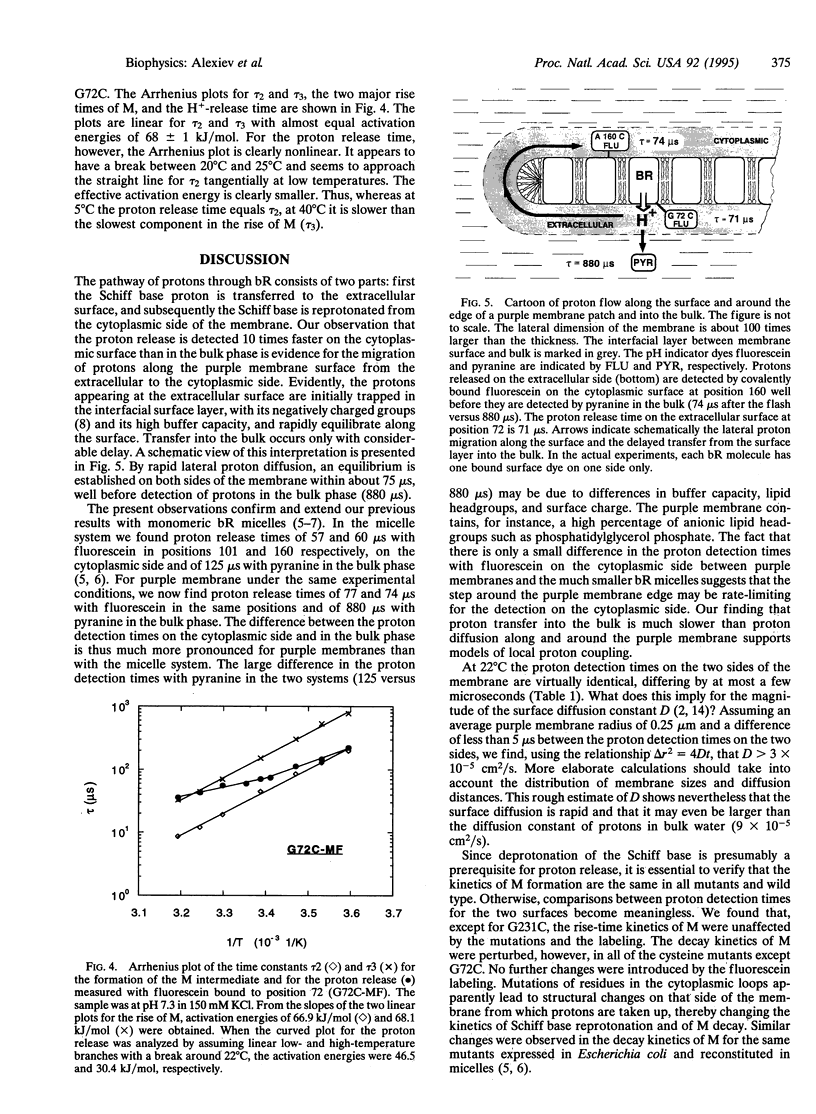
Abstract
The pH-indicator dye fluorescein was covalently bound to the surface of the purple membrane at position 72 on the extracellular side of bacteriorhopsin and at positions 101, 105, 160, or 231 on the cytoplasmic side by reacting bromomethylfluorescein with the sulfhydryl groups of cysteines introduced by site-directed mutagenesis. At position 72, on the extracellular surface, the light-induced proton release was detected 71 +/- 4 microseconds after the flash (conditions: pH 7.3, 22 degrees C, and 150 mM KCl). On the cytoplasmic side with the dye at positions 101, 105, and 160, the corresponding values were 77, 76, and 74 +/- 5 microseconds, respectively. Under the same conditions, the proton release time in the bulk medium as detected by pyranine was around 880 microseconds--i.e., slower by a factor of more than 10. The fact that the proton that is released on the extracellular side is detected much faster on the cytoplasmic surface than in the aqueous bulk phase demonstrates that it is retained on the surface and migrates along the purple membrane to the other side. These findings have interesting implications for bioenergetics and support models of local proton coupling. From the small difference between the proton detection times by labels on opposite sides of the membrane, we estimate that at 22 degrees C the proton surface diffusion constant is greater than 3 x 10(-5) cm2/s. At 5 degrees C, the proton release detection time at position 72 equals the faster of the two main rise times of the M intermediate (deprotonation of the Schiff base). At higher temperatures this correlation is gradually lost, but the curved Arrhenius plot for the proton release time is tangential to the linear Arrhenius plot for the rise of M at low temperatures. These observations are compatible with kinetic coupling between Schiff base deprotonation and proton release.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexiev U., Marti T., Heyn M. P., Khorana H. G., Scherrer P. Covalently bound pH-indicator dyes at selected extracellular or cytoplasmic sites in bacteriorhodopsin. 2. Rotational orientation of helices D and E and kinetic correlation between M formation and proton release in bacteriorhodopsin micelles. Biochemistry. 1994 Nov 22;33(46):13693–13699. doi: 10.1021/bi00250a020. [DOI] [PubMed] [Google Scholar]
- Alexiev U., Marti T., Heyn M. P., Khorana H. G., Scherrer P. Surface charge of bacteriorhodopsin detected with covalently bound pH indicators at selected extracellular and cytoplasmic sites. Biochemistry. 1994 Jan 11;33(1):298–306. doi: 10.1021/bi00167a039. [DOI] [PubMed] [Google Scholar]
- Bousché O., Sonar S., Krebs M. P., Khorana H. G., Rothschild K. J. Time-resolved Fourier transform infrared spectroscopy of the bacteriorhodopsin mutant Tyr-185-->Phe: Asp-96 reprotonates during O formation; Asp-85 and Asp-212 deprotonate during O decay. Photochem Photobiol. 1992 Dec;56(6):1085–1095. doi: 10.1111/j.1751-1097.1992.tb09732.x. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Haines T. H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci U S A. 1983 Jan;80(1):160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Riesle J., Thiedemann G., Oesterhelt D., Dencher N. A. Proton migration along the membrane surface and retarded surface to bulk transfer. Nature. 1994 Aug 4;370(6488):379–382. doi: 10.1038/370379a0. [DOI] [PubMed] [Google Scholar]
- Kano K., Fendler J. H. Pyranine as a sensitive pH probe for liposome interiors and surfaces. pH gradients across phospholipid vesicles. Biochim Biophys Acta. 1978 May 18;509(2):289–299. doi: 10.1016/0005-2736(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Mollaaghababa R., Khorana H. G. Gene replacement in Halobacterium halobium and expression of bacteriorhodopsin mutants. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1987–1991. doi: 10.1073/pnas.90.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Dilley R. A. Models of localized energy coupling. J Bioenerg Biomembr. 1986 Feb;18(1):55–64. doi: 10.1007/BF00743612. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A., Junge W. Proton diffusion along the membrane surface of thylakoids is not enhanced over that in bulk water. Biophys J. 1989 Jul;56(1):27–31. doi: 10.1016/S0006-3495(89)82649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer P., Alexiev U., Marti T., Khorana H. G., Heyn M. P. Covalently bound pH-indicator dyes at selected extracellular or cytoplasmic sites in bacteriorhodopsin. 1. Proton migration along the surface of bacteriorhodopsin micelles and its delayed transfer from surface to bulk. Biochemistry. 1994 Nov 22;33(46):13684–13692. doi: 10.1021/bi00250a019. [DOI] [PubMed] [Google Scholar]
- Steinhoff H. J., Mollaaghababa R., Altenbach C., Hideg K., Krebs M., Khorana H. G., Hubbell W. L. Time-resolved detection of structural changes during the photocycle of spin-labeled bacteriorhodopsin. Science. 1994 Oct 7;266(5182):105–107. doi: 10.1126/science.7939627. [DOI] [PubMed] [Google Scholar]
- Teissié J., Gabriel B., Prats M. Lateral communication by fast proton conduction: a model membrane study. Trends Biochem Sci. 1993 Jul;18(7):243–246. doi: 10.1016/0968-0004(93)90171-i. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Váró G., Chang M., Ni B., Needleman R., Lanyi J. K. Pathways of proton release in the bacteriorhodopsin photocycle. Biochemistry. 1992 Sep 15;31(36):8535–8543. doi: 10.1021/bi00151a022. [DOI] [PubMed] [Google Scholar]