Abstract
Porphyromonas gingivalis, an important periodontal pathogen, infects primary gingival epithelial cells (GECs). Despite the large number of bacteria that replicate inside the GECs, the host cell remains viable. We demonstrate that P. gingivalis triggers rapid and reversible surface phosphatidylserine exposure through a mechanism requiring caspase activation. However, after 1 day of infection, the bacteria no longer induce phosphatidylserine externalization and instead protect infected cells against apoptosis. Infection exerts its effect at the level of mitochondria, as P. gingivalis also blocks depolarization of the mitochondrial transmembrane potential and cytochrome c release. Interestingly, protein kinase B/Akt is phosphorylated during infection, which can be blocked with the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002. Suppression of the PI3K/Akt pathway following staurosporine treatment results in mitochondrial-membrane depolarization, cytochrome c release, DNA fragmentation, and increased apoptosis of infected GECs. Thus, P. gingivalis stimulates early surface exposure of phosphatidylserine, which could downmodulate the inflammatory response, while also promoting host cell survival through the PI3K/Akt pathway.
Epithelial cells represent the first line of defense against most microbial and protozoan pathogens, but at the same time they are the initial sites of host invasion. An increasing number of bacterial pathogens are known to modulate apoptotic pathways in epithelial cells and tissues during invasion. Although many pathogenic bacteria induce apoptosis after infection of epithelial cells, other bacteria inhibit apoptosis in similar types of cells (16, 44). The strategy of the latter microorganisms could aid in prolonging bacterial growth, evading immune responses, and promoting colonization within the tissue. Porphyromonas gingivalis, a gram-negative anaerobe identified as a predominant colonizer of oral tissues and an etiological agent in the severe forms of adult periodontitis, invades and remains viable for extended periods in primary gingival epithelial cells (GECs) (26, 41). The invasion is swift, without being confined to a membrane-bound vacuole, and the pathogen congregates in large numbers in the perinuclear region after 30 min of infection. P. gingivalis is highly capable of intracellular replication. It also modulates many phenotypic and signaling properties of GECs, such as elevation of intracellular Ca2+, selective phosphorylation of mitogen-activated protein kinase family kinases, reorganization of the actin cytoskeleton, microtubule dynamics, and activation of paxillin and focal adhesion kinase (4, 43, 46). As part of its strategy to evade the host immune response, it also downregulates expression of interleukin-8, a chemokine involved in chemoattraction of neutrophils. Interestingly, despite the burden of large numbers of intracellular bacteria, the infected GECs do not undergo apoptotic cell death and, according to a preliminary report, are resistant to camptothecin-induced cell death (10, 19, 33). P. gingivalis-induced suppression of apoptosis of primary GECs is probably related at least partially to the bacterium's ability to target members of the Bcl-2 family of proteins, Bcl-2 and Bax (33). Infection of P. gingivalis upregulates expression of the proapoptotic molecule Bax at early time points. However, after longer incubation, Bax levels decline, and there is an increase in the expression of the antiapoptotic molecule Bcl-2. These findings suggest that P. gingivalis infection may subdue apoptosis of primary GECs by balancing apoptotic pathways. However, levels of other Bcl-2 family proteins were not measured, and the mechanisms of inhibition of apoptosis and the biochemical and morphological alterations induced by P. gingivalis have not been characterized.
During early stages of apoptosis, phosphatidylserine (PS), a lipid sequestered to the inner leaflet of the plasma membrane of viable cells, is translocated to the outer layer and becomes exposed on the external side of the cell (39). Shortly after infection by microorganisms such as Pseudomonas aeruginosa, Chlamydia spp., Legionella pneumophila, and Actinobacillus actinomycetemcomitans, host cells increasingly externalize PS and display early characteristics of apoptosis (17). In many models of apoptosis, PS exposure is generally accompanied by release of cytochrome c from mitochondria, activation of caspases, and finally endonuclease activation resulting in DNA cleavage and nuclear segmentation (34). However, PS can also be exposed on the cell surface reversibly in cells that are not undergoing apoptosis (17, 27). Surface exposure of PS can take place during vesicle secretion by macrophages (27), and its recognition by specific receptors on macrophages can lead to downmodulation of the inflammatory response (13, 20, 39).
In the present work, we examined whether infection by P. gingivalis alters membrane PS asymmetry, depolarizes the mitochondrial membrane potential (ΔΨm), and evokes cytochrome c release, caspase activation, and ultimately fragmentation of DNA in primary cultures of GECs. Furthermore, we explored the role of phosphatidylinositol 3-kinase (PI3K)/Akt molecules in the P. gingivalis-induced GEC resistance to apoptosis.
MATERIALS AND METHODS
Bacteria and growth conditions.
P. gingivalis ATCC 33277 was cultured anaerobically for 24 h at 37°C in Trypticase soy broth supplemented with yeast extract (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml). All bacteria were grown for 24 h, harvested by centrifugation at 6,000 × g and 4°C for 10 min, washed, and resuspended in Dulbecco's phosphate-buffered saline (pH 7.3) (Sigma, St. Louis, Mo.). The number of bacteria was determined with a Klett-Summerson photometer (25).
Culture of GECs.
Primary cultures of GECs were generated as described previously (25). Briefly, healthy gingival tissue was obtained after oral surgery, and surface epithelium was separated by overnight incubation with 0.4% dispase. Cells were cultured as monolayers in serum-free keratinocyte growth medium (Clonetics, San Diego, Calif.) at 37°C with 5% CO2. GECs were used for experimentation at 75 to 80% confluence and were cultured for 48 h before infection with bacterial cells or exposure to other test reagents in keratinocyte growth medium.
Infection of cells with P. gingivalis and treatment with zVAD-fmk, staurosporine, and PI3K inhibitor.
GECs were infected at a multiplicity of infection of 100 with P. gingivalis 33277 for 30 min, 2 h, 5 h, 7 h, or 24 h at 37°C in a CO2 incubator. All time points for the infections were carried out backwards; i.e., instead of beginning all infections at the same time, infections were initiated at the indicated times before time zero so that all incubations could be stopped at the same time. Thus, all cells (e.g., uninfected and 24-h-infected cells) were examined at the same stage of growth, in order to exclude any effects of GEC aging. For inhibition and induction of apoptosis studies, GECs were preincubated with a broad-spectrum caspase inhibitor, zVAD-fmk (40 μM) (Enzyme Systems Products, Livermore, Calif.), 30 min prior to infection. Cells were treated with a broad-spectrum protein kinase inhibitor, staurosporine (2 μM) (Sigma), for 3 h after 21 h of infection with or without the bacteria. Additionally, after 3 h of infection with P. gingivalis 33277, GECs were treated with the PI3K inhibitor LY294002 (20 μM) (Sigma) for 18 h prior to the 3-h staurosporine treatment. All treatments were performed in GEC culture medium at 37°C in a CO2 incubator.
Flow cytometry. (i) Analysis of apoptosis by Annexin-V and PI staining.
Early apoptotic changes were identified by using fluorescein isothiocyanate (FITC)-conjugated Annexin-V-Fluos (Roche Applied Science, Indianapolis, Ind.), which binds to PS exposed on the outer leaflet of apoptotic cell membranes. Propidium iodide (PI) (Sigma) was used for the discrimination of necrotic cells from the cell cluster positively stained with Annexin-V. Briefly, GECs were grown in six-well plates (Falcon; BD Biosciences, San Jose, Calif.) at a density of 2 × 105 per well, incubated with various treatments as described above, and dissociated with 0.05% trypsin-0.53 mM EDTA (Gibco BRL, Gaithersburg, Md.). The GEC samples were collected, washed in cold phosphate-buffered saline (PBS), and resuspended in 100 μl of Annexin-V-Fluos binding solution containing 20 μl of Annexin-V-Fluos labeling reagent per 1,000 μl of HEPES buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 5 mM CaCl2) and 1 μg of PI per ml. After 15 min of incubation in the dark at room temperature, 400 μl of incubation buffer was added to each sample and the cells were analyzed by flow cytometry with excitation at 488 nm and a 515-nm band pass filter for fluorescein detection and a 585-nm filter for PI detection. Cells incubated in the binding buffer with only Annexin-V or PI separately served as controls (37). For each dye, appropriate electronic compensation of the instrument was performed to avoid overlapping of the two emission spectra.
(ii) Measurement of mitochondrial ΔΨm.
GECs were grown and harvested as described above, and their mitochondrial ΔΨm was assessed by using 3,3-dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes, Inc., Eugene, Oreg.). A total of 2 × 105 cells were incubated with 40 nM DiOC6 in PBS at 37°C for 15 min. The percentage of cells exhibiting a low level of DiOC6 uptake, which reflects loss of mitochondrial ΔΨm, was determined by analyzing cells by flow cytometry as described previously (36).
(iii) Measurement of cytochrome c release.
GECs were harvested and treated with digitonin (50 μl/ml) (Sigma) in PBS with 100 mM KCl for 5 min on ice. The cells were then fixed in 10% neutral buffered formalin for 20 min at room temperature and washed three times in PBS and blocking buffer (3% bovine serum albumin and 0.1% Triton X-100 in PBS) for 1 h. The cells were incubated overnight at 4°C with 1/200 anti-cytochrome c monoclonal antibody conjugated to FITC (eBioscience, San Diego, Calif.) in blocking buffer and washed three times. Cells were then analyzed by flow cytometry for detection of FITC fluorescence levels (3).
Immunoblot analysis.
Untreated control and treated GECs were grown in six-well plates (Falcon), washed in ice-cold PBS, and lysed in a buffer containing 50 mM Tris-HCI (pH 7.2), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, and protease inhibitor cocktail (10 μl/ml) (Sigma). The amount of protein in each sample was determined by bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, Ill.), and equal amounts of protein were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. Proteins were blotted onto nitrocellulose membranes, blocked in 5% dried milk solution diluted in Tris-buffered saline containing 0.01% Tween 20 incubated with anti-Akt/protein kinase B (pS473) phosphospecific antibody at a dilution of 1:2,000 (Biosource International, Camarillo, Calif.), and treated with horseradish peroxidase-conjugated secondary antibody at 1:20,000 (Biosource International). Results were visualized with an ECL detection kit (Amersham Biosciences, Piscataway, N.J.). The blots were then stripped and reprobed with Akt1/2 antibody (1:2,000) (Santa Cruz Biotechnology, Santa Cruz, Calif.) to determine total Akt protein in the samples. Densitometric scanning was quantitated by using National Institutes of Health Image analysis.
Fluorescence microscopy. (i) Detection of cytochrome c release.
GECs were grown on four-well chambered slides (Nalge-Nunc International, Rochester, N.Y.), washed with ice-cold PBS, and fixed in 10% neutral buffered formalin for 20 min. The cells were permeabilized for 10 min with 0.1% Triton X-100 at 4°C, and the same slides were incubated with anti-cytochrome c monoclonal antibody at 1:100 (BD PharMingen) and anti-P. gingivalis 33277 rabbit polyclonal antibody at 1:2,500 in PBS containing 0.1% Tween and 3% bovine serum albumin overnight at 4°C. After washing with PBS, samples were stained with rhodamine red-X-labeled goat anti-mouse immunoglobulin G (heavy plus light chains) (Molecular Probes) and Oregon green 488 goat anti-rabbit immunoglobulin G (heavy plus light chains) (highly cross-absorbed) (Molecular Probes) at 1:400 for 45 min at room temperature. The same samples were incubated with 4′,6′-diamidino-2-phenylindole (DAPI) (1 μg/ml) for 15 min for nuclear staining. Finally, slides were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) and examined with an epifluorescence microscope (Zeiss Axioskope) equipped with band pass optical filter sets appropriate for tetramethyl rhodamine isocyanate, FITC, and DAPI dyes. The images were captured by multiple exposures with a cooled charge-coupled device camera controlled by QCAPTURE software, version 1394.
(ii) DNA fragmentation visualized by TUNEL assay.
DNA strand breaks induced by apoptosis were identified by using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit according to the protocol of the manufacturer (Roche Applied Science). Briefly, GECs were grown on four-well chambered slides, washed with ice-cold PBS, and fixed with 10% neutral buffered formalin for 1 h at room temperature. After being washed twice with PBS, the cells were treated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. Samples were then washed twice with PBS and incubated with the TUNEL reaction mixture, containing FITC-labeled dUTP and terminal deoxynucleotidyltransferase, for 1 h at 37°C. Control cells were incubated in the absence (negative control) or presence (positive control) of DNase (Roche Applied Science), in addition to control samples incubated without deoxynucleotidyltransferase. After incubation, the cells were washed twice with PBS and analyzed by using the epifluorescence microscope equipped with the cooled charge-coupled device camera, as described above.
RESULTS
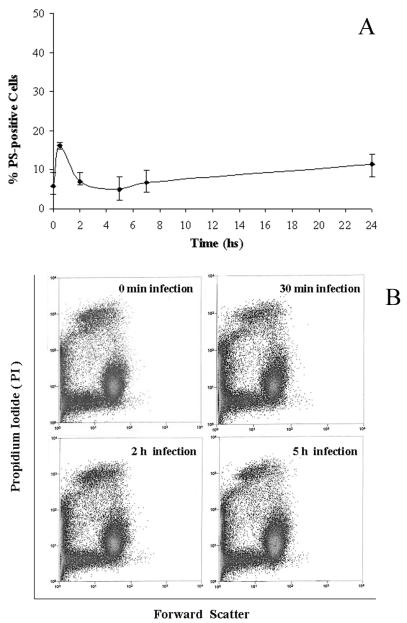
Extensive P. gingivalis infection does not result in apoptosis of epithelial cells, yet it elicits reversible caspase-dependent surface exposure of PS shortly after infection. To characterize the effects of P. gingivalis infection on host cell death, we measured the percentage of apoptotic cells as a function of time after infection by flow cytometry with Annexin V-FITC staining combined with the PI viability assay (36). Annexin V binds strongly to PS that has been externalized, thereby allowing recognition of dying cells or cells exposing PS on the cell surface transiently. As seen in Fig. 1A, incubation of GECs with P. gingivalis (strain 33277) for up to a day did not markedly enhance PS surface exposure on GECs compared to the uninfected controls. Up to 94% of uninfected control cells failed to bind Annexin-V immediately after incubation with P. gingivalis, while approximately 87% of the infected cells still remained negative after 24 h of infection. However, about 16% of P. gingivalis-infected cells displayed Annexin-V staining, which was already detectable and maximal after 30 min of infection. The PS exposure induced by P. gingivalis had declined by 2 h postinfection, reached steady-state levels by 5 h postinfection, and remained stable throughout the rest of the infection. No cell death or cell size changes were observed during the first 5 h of infection, as measured by flow cytometry (Fig. 1B), and the cells appeared morphologically normal (not shown), suggesting that the PS surface exposure is transient and unrelated to cell death.
FIG. 1.
Kinetics of PS externalization during a 24-h infection with P. gingivalis as measured by flow cytometry with the Annexin-V-PI assay. (A) The analysis showed that early stages of infection generated early PS exposure. However, the percentage of PS-positive cells decreased later in the infection. (B) Early P. gingivalis-activated PS externalization is unrelated to cell death. Flow cytometric dot plots for samples at 0 min, 30 min, 2 h, and 5 h of infection, displaying forward light scatter versus PI-positive cells, demonstrated a lack of change in cell size and viability. Data are expressed as means from at least three separate experiments, and the values represent the means and standard deviations of separate measurements.
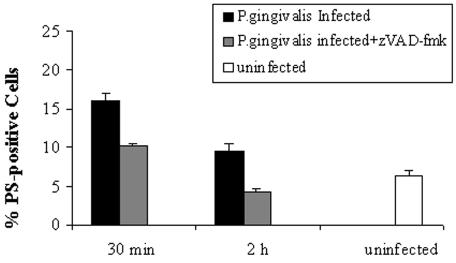
In order to characterize the host response elicited by P. gingivalis invasion, GECs were incubated with a broad-spectrum caspase inhibitor, zVAD-fmk, at 40 μM for half an hour prior to the bacterial infection, and the cells were analyzed by flow cytometry. The pretreatment of host cells with zVAD-fmk significantly inhibited the generation of externalized PS induced by 30 min and 2 h of infection with P. gingivalis (Fig. 2). These results indicate that the rapid surface exposure of PS could be mediated by the activation of caspases at early time points of infection. However, PS exposure is reversible, and early caspase activation does not lead to host cell death at longer times of infection with P. gingivalis.
FIG. 2.
zVAD-fmk partially abolishes P. gingivalis-induced early PS exposure, as determined by Annexin-V-PI staining and analyzed by flow cytometry. GECs were either uninfected (control) or infected for 30 min or 2 h in the absence or presence of 40 μM zVAD-fmk. The bars show the percentages of GECs stained for PS externalization under the different conditions. Data are expressed as the means from at least two separate experiments, and the values represent the means and standard deviations of separate measurements.
P. gingivalis inhibits staurosporine-induced apoptosis through the PI3K pathway.
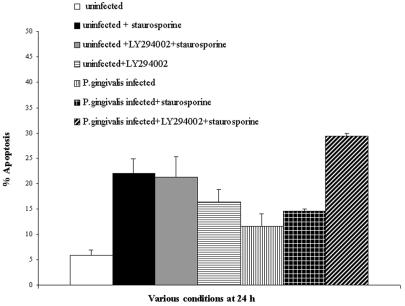
Previous studies have shown that P. gingivalis is capable of blocking campothecin-induced nucleosome release from primary GECs. Campothecin is an antitumor agent that damages DNA by targeting DNA topoisomerase I (33). We therefore treated infected and uninfected GECs with staurosporine, a potent proapoptotic inducer, and analyzed cell death by cytofluorimetry with cells double stained with Annexin-V and PI. Both the control uninfected and 21-h-infected GECs were treated for 3 h with 2 μM staurosporine. Apoptosis levels were 22% (±3%) in staurosporine-treated uninfected epithelial cells, whereas staurosporine-treated infected epithelial cells displayed 14% (±0.51%) apoptosis (Fig. 3). These results, compared with 6 and 12% apoptosis for uninfected and infected cells, respectively, that had not been treated with staurosporine, imply that infection with P. gingivalis inhibits staurosporine-induced apoptosis of GECs. Thus, our results are consistent with previous studies (31, 33, 35).
FIG. 3.
A PI3K inhibitor abolishes P. gingivalis-mediated protection against staurosporine-induced apoptosis. Twenty-four-hour-infected GECs, treated with 20 μM LY294002 (21 h) and then coincubated with 2 μM staurosporine (3 h), are compared with cells with 21 h of infection and 3 h of staurosporine treatment, cells with 24 h of infection only, and uninfected control cells. A significant increase in the amount of cells going through apoptosis is observed for the LY294002-treated cells, as determined by flow cytometry with the Annexin-V-PI assay. Data are expressed as the means from at least two separate experiments, and the values represent the means and standard deviations of separate measurements.
In parallel experiments, we investigated the role of PI3K signaling in the bacterium-induced protection against apoptosis by utilizing LY294002, a specific inhibitor of PI3K. Following 18 h of treatment with 20 μM LY294002, the 21-h-infected and uninfected GEC monolayers were incubated with 2 μM staurosporine for 3 h. The incubations were then stopped, and samples were assayed for apoptosis by cytofluorimetry as described above. Interestingly, inhibition of the PI3K pathway with LY294002 significantly abolished P. gingivalis-dependent protection of GECs (Fig. 3). The P. gingivalis-infected and LY294002-treated cells had the highest level of apoptosis (approximately 30%) after the addition of staurosporine. LY294002 itself had a considerably lesser proapoptotic effect on the uninfected control cells (Fig. 3). Yet, the PI3K inhibitor itself did not induce a higher level of apoptosis in the infected GECs than the staurosporine treatment (data not shown). Thus, the protection of infected GECs against apoptosis induced by external ligands is likely regulated by PI3K signaling pathways.
Akt activation is an event downstream from PI3K signaling during P. gingivalis infection.
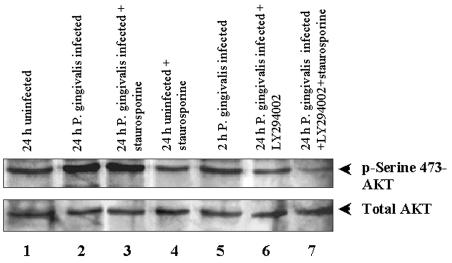
To elucidate the underlying mechanisms of the inhibition of apoptosis induced by P. gingivalis and the ability of the PI3K inhibitor LY294002 to block this inhibition, we tested the status of an essential survival-signaling event downstream of PI3K, serine/threonine kinase Akt phosphorylation, in the infected GECs. Akt can be phosphorylated and activated by PI3K to induce survival in various cell types (5, 11). We performed immunoblotting with Akt (Ser473)-specific phosphorylation antibody to evaluate Akt activity in the primary GECs for the various treatments indicated in Fig. 4. The levels of Akt phosphorylation of P. gingivalis-infected samples were substantially elevated in both the presence and absence of staurosporine. Densitometric analysis of the bands indicated a 70% increase for infected cells over the uninfected cells and a 50% increase in infected cells that had been treated with staurosporine over the uninfected cells. However, the addition of LY294002, a PI3K inhibitor, significantly decreased the amount of Akt phosphorylation in the infected cells. Particularly, the sample treated with staurosporine had a much smaller amount of phosphorylated Akt than the baseline levels (52%) (Fig. 4). The findings suggest that LY294002 inhibits the antiapoptotic effects induced by the bacteria due to Akt phosphorylation, which contributes to the resistance of infected cells to apoptosis.
FIG. 4.
Activation of the Akt/PI3K pathway is required for P. gingivalis-induced inhibition of apoptosis. Infected (24 h) and uninfected cells were further treated with staurosporine or LY294002 for periods similar to the ones for Fig. 3. Samples were subjected to Western blot analysis for Akt (Ser473) phosphorylation. Membranes were probed sequentially with an anti-phospho-Akt (pS473) antibody (upper panel) and an anti-Akt1/2 protein antibody (lower panel) to confirm that each lane received similar amounts of Akt. The blot shown is representative of two independent experiments.
Comparison of mitochondrial depolarization (ΔΨm) and cytochrome c release in P. gingivalis-infected epithelial cells.
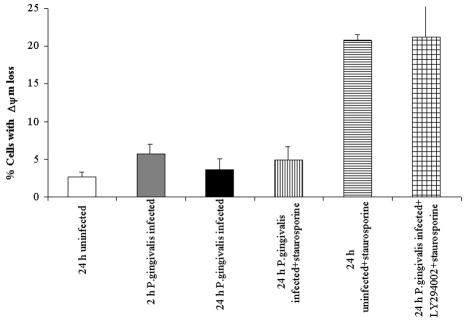
Given the effects of the PI3K pathway on host cell survival, we characterized other cardinal apoptotic parameters, such as the status of the mitochondrial ΔΨm and the distribution of cytochrome c, and the effect of the PI3K pathway on these features. First we analyzed the time course of changes in the state of ΔΨm and cytochrome c distribution during the 24-h infection process. ΔΨm was assessed by using a fluorescent potentiometric probe, DiOC6, by flow cytometry. Consistently, the staurosporine-treated uninfected cells exhibited a collapsed mitochondrial ΔΨm, which was about 21% of the viable population, yet the P. gingivalis-infected, staurosporine-treated cells displayed no significant increase in the percentage of cells with reduced ΔΨm (approximately 5%) compared to the uninfected control cells that had not been treated with staurosporine (approximately 3%) (Fig. 5). The 2-h bacterial infection did not decrease the mitochondrial ΔΨm noticeably (about 6%), but it was still higher than the levels found after a 24-h infection (about 4%) or in the uninfected control cells (3%) (Fig. 5). However, the addition of LY294002 to the P. gingivalis-infected, staurosporine-treated cell monolayers significantly decreased the ΔΨm and enhanced the depolarized cell percentage to 22%. These observations suggest that the early PS exposure was most likely transient and does not lead to a considerable loss of the ΔΨm at 24 h of infection.
FIG. 5.
Analysis of ΔΨm in P. gingivalis infection. The conditions were similar to those for Fig. 4. Twenty-four-hour-infected and uninfected (control) cells were incubated with or without LY294002 for 18 h before treatment with staurosporine for 3 h (21 h postinfection). Samples (24 h postinfection) were stained with DiOC6 (40 nM) to measure mitochondrial ΔΨm and analyzed by flow cytometry. The bars represent the percentages of cells with loss of ΔΨm. Data are expressed as means from at least two separate experiments, and the values represent the means and standard deviations of separate measurements.
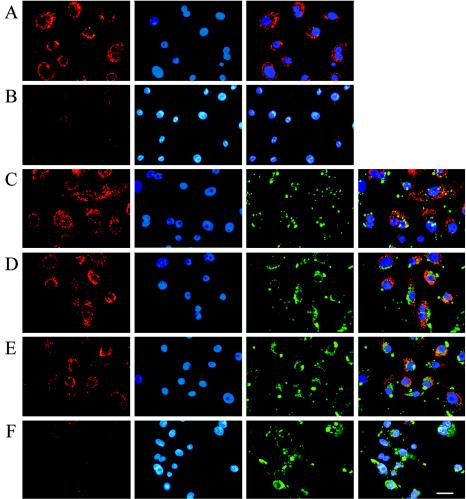
Cytochrome c release was measured by both fluorescence microscopy and flow cytometry. First, the distribution of cytochrome c in the infected and uninfected samples was visualized by using a fluorescent immunostaining technique. The immunofluorescent staining of cytochrome c and the intracellular bacteria was combined with the DAPI staining of nuclei to monitor nuclear condensation and fragmentation events along with the distribution of cytochrome c. Uninfected control cells demonstrated that cytochrome c is localized exclusively in mitochondria around the nucleus in the absence of apoptotic stimuli (Fig. 6A). Addition of staurosporine to the uninfected cells resulted in diffuse and weak punctuated staining of cytochrome c throughout the cytoplasm, indicating that a large amount of cytochrome c was released from mitochondria, as well as the presence of condensed nuclei in the same cells (Fig. 6B). On the other hand, the cells incubated with P. gingivalis for either a short time (2 h) or a long time (24 h) showed no changes in the distribution of cytochrome c, which remained predominantly in the perinuclear space (Fig. 6C and D), and infection with P. gingivalis inhibited staurosporine-mediated cytochrome c release (Fig. 6E). However, incubation of P. gingivalis-infected cells with LY294002 and then with staurosporine led to a significant loss of cytochrome c from the mitochondria. In addition to the depletion of cytochrome c, the same cells showed highly condensed and fragmented nuclei, confirming the apoptotic nature of host cell death (Fig. 6F).
FIG. 6.
Distribution of cytochrome c in P. gingivalis-infected GECs. Cells were treated as for Fig. 5. Samples were examined by fluorescence microscopy. Cells were fixed and permeabilized, followed by immunostaining with a monoclonal antibody for cytochrome c (red fluorescence) and an antibody specific for P. gingivalis (green fluorescence) and were counterstained with DAPI (blue fluorescence) to visualize nuclear morphology. No changes in the cytochrome c localization and immunofluorescence were observed in the 2- and 24-h-infected cells (C and D) and in the 24-h-infected and staurosporine-treated (21 h postinfection) cells (E) compared to uninfected and untreated controls (A). In contrast, 24-h-infected cells coincubated with LY294002 (21 h) and staurosporine (3 h) (F), as well as uninfected cells treated with staurosporine (3 h) only (B), displayed very weak immunofluorescence, indicating significant release of cytochrome c from mitochondria to the cytosol. At least 10 separate fields containing an average of 25 GECs were studied in each of the two independent experiments performed in duplicate. Bar, 10 μm.
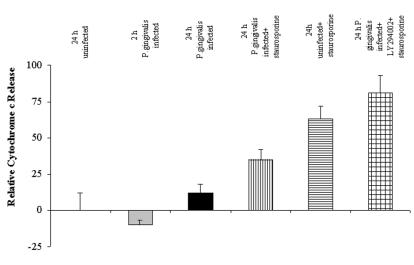
The kinetics of cytochrome c release was also studied by flow cytometry, for which GECs were treated with digitonin prior to fixation, and Triton X-100 treatment followed by incubation with FITC-conjugated anti-cytochrome c monoclonal antibody. Digitonin treatment allowed selective permeabilization of the plasma membrane and diffusion of cytosolic cytochrome c out of the cells, so that the GECs with high cytoplasmic cytochrome c levels have less FITC fluorescence than the cells with intact mitochondria. The results are summarized in Fig. 7, which shows that the percentage of cells with released cytochrome c increased significantly when the P. gingivalis-infected cells were coincubated with LY294002 and staurosporine. Similarly to the uninfected control cells, no significant reduction in the cytochrome c levels was observed in the 24-h-infected cells.
FIG. 7.
Cytochrome c release by infected GECs determined by flow cytometry. The treatment and time course for each condition are the same as for Fig. 6. The number of cells with high fluorescence (intact mitochondria) and the number of cells with low fluorescence (cytochrome c released) were quantified by flow cytometry analysis following staining with FITC-conjugated anti-cytochrome c antibody (details are described in Materials and Methods). Bars represent the percentage of cells in each condition compared to uninfected, untreated controls, which were set at 100%. Twenty-four-hour-infected cells had high fluorescence, as detected by fluorescence microscopy. Significant numbers of LY294002- and staurosporine-treated, infected cells had very low fluorescence, indicating substantial loss of cytochrome c from mitochondria. Data are expressed as means from at least two separate experiments, and the values represent the means and standard deviations of separate measurements.
The data indicate that the PI3K/Akt-dependent inhibition of apoptosis in P. gingivalis-infected cells is associated with the ability to maintain mitochondrial membrane integrity. The disruption of this pathway by LY294002 results in a significant amount of cytochrome c release, followed by nuclear condensation.
Apoptosis of infected cells induced by a PI3K inhibitor is accompanied by DNA fragmentation.
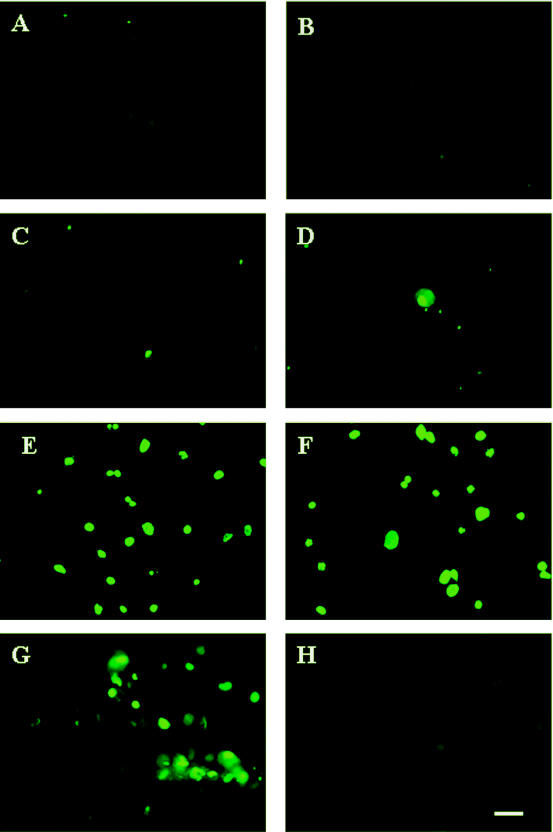
Finally, to determine whether nuclear condensation is accompanied by DNA fragmentation, we examined the DNA fragmentation of primary GECs by use of the TUNEL assay. As shown in Fig. 8A, B, and C, uninfected control cells as well as P. gingivalis-infected cells showed no significant DNA cleavage at both 2 and 24 h postinfection. In contrast, cells incubated with LY294002 (3 h postinfection) and staurosporine (21 h postinfection) showed extensive levels of DNA fragmentation (Fig. 8F), consistent with our preceding results.
FIG. 8.
Detection of apoptosis by TUNEL assay. Cells were treated as for Fig. 7. Samples were fixed and stained with TUNEL reagents and visualized by fluorescence microscopy. Control uninfected cells (A) and 2- and 24-h-infected cells (B and C) stained negative for TUNEL, as displayed by very low (background) green fluorescence. Twenty-four-hour-infected cells treated with staurosporine (D) also displayed staining similar to the samples in panels B and C. However, 24-h-uninfected and staurosporine-treated (E) and 24-h-infected and LY294002- and staurosporine-treated (F) samples exhibited substantial numbers of TUNEL-positive cells. Cells incubated with DNase (G) and without TUNEL enzyme (H) served as controls. Bar, 10 μm.
DISCUSSION
The association of P. gingivalis with apoptosis has been examined in a variety of cell types. Previous studies have indicated that P. gingivalis induces apoptosis in Jurkat T cells, B cells, and human gingival fibroblasts yet inhibits apoptosis in human monocytes, macrophages and neutrophils (15, 18, 31, 35, 42). In particular, a recent study on the apoptotic responses of primary GECs infected with P. gingivalis demonstrated that prolonged bacterial invasion upregulates expression of the antiapoptotic protein Bcl-2 and downregulates that of the proapoptotic Bax protein. Studies have also reported that primary GECs, the preferential host cells for P. gingivalis, do not detach from the substratum and remain capable of excluding trypan blue, hydrolyzing calcein, and maintaining physiologic intracellular calcium ion concentrations up to 48 h postinfection, despite the presence of large numbers of intracellular P. gingivalis bacteria. GECs also remain viable despite marked disassembly and nucleation of the actin and microtubule filaments following 24 h of infection with P. gingivalis (4, 33, 46).
Although P. gingivalis is now known to modulate cell death in several cell types, the biochemical and morphological markers of apoptosis and the mechanisms involved in the modulation have not been characterized. Our study thus examined the relationship between cell death and short-lived exposure of PS, activation of caspases, mitochondrial ΔΨm, and cytochrome c release induced by P. gingivalis infection and focused on the signaling molecules involved in inhibition of apoptosis induced in GECs by P. gingivalis invasion.
The results revealed that P. gingivalis infection does not promote apoptosis of GECs after either short periods or 24 h of infection, yet it elicits rapid and transient cell surface exposure of PS after short periods. The kinetics of PS exposure triggered by P. gingivalis coincides with the kinetics of the invasion, which starts in minutes and reaches a maximum at 30 min (Fig. 1). PS exposure could possibly be associated with an elevation of intracellular calcium, as P. gingivalis infection of GECs leads to a transient increase of the intracellular calcium concentration (26). Externalization of PS has been often described as an early step in apoptosis and as being due to the activation of caspases. However, PS is now known to be externalized transiently even in viable cells (27), and caspase activation is no longer viewed as a necessary and sufficient condition for apoptosis, since activated caspases can also participate in diverse functions such as differentiation of skeletal myoblasts, copper homeostasis, and T-lymphocyte activation (30, 38). In our findings, the inhibition of rapid PS exposure by the broad-spectrum caspase inhibitor zVAD-fmk suggests that P. gingivalis does in fact trigger the activation of caspases at early time points of infection but that neither caspase activation nor PS externalization leads to host cell death.
Thus, externalization of PS may play a role in a nonapoptotic process. Surface exposure of PS could be involved in recognition of infected cells by neighboring epithelial cells or phagocytes, both of which express the PS receptor (12). However, only a minority of infected cells have PS exposed on their surface, which is reversible as the PS distribution across the plasma membrane bilayer reverts to normal within 2 h after infection. In addition, we have not been able to measure a decrease in the number of infected cells after the first 30 min of infection. We therefore favor the possibility that surface-exposed PS on the infected cells may instead contribute to decreasing the inflammatory response to P. gingivalis infection, since ingestion of apoptotic cells or PS liposomes by macrophages induces secretion of anti-inflammatory cytokines and suppression of proinflammatory mediators (20, 39). This is consistent with the observation that P. gingivalis inhibits interleukin-8 secretion by GECs (10), which is thought to reduce the potency of the innate host immune response.
Besides allowing host cells to survive for long periods of time despite the high bacterial load, the infection also inhibits apoptosis of GECs induced by staurosporine, a potent proapoptotic stimulus. It is widely established that PI3K-mediated signaling pathways can confer resistance to cell death mediated by a variety of apoptotic inducers. We observed that P. gingivalis blocks apoptosis and enhances GEC survival through the activation of Akt, which is mediated by PI3K. Cellular adhesion to extracellular matrix components, mainly through integrin receptors, may result in the activation of PI3K, recruitment of Akt to the plasma membrane and its phosphorylation, and protection against apoptosis (6, 11). Since P. gingivalis-mediated invasion of GECs emanates primarily from the interaction between β-integrin and bacterial fimbriae, the protection against apoptosis might originate from a bacterium-β-integrin signal transduction pathway in GECs (32, 45). Indeed, others have shown that the fimbriae from P. gingivalis have a significant inhibitory effect on apoptosis of human monocytic THP-1 cells (35).
Although further studies should identify the molecular basis of initiation of the protection against apoptosis, our study suggests that signaling via PI3K and its downstream signaling partner Akt exerts a large antiapoptotic effect, possibly through inhibition of mitochondrial permeability changes. Suppression of Akt activation by a broad-spectrum PI3K inhibitor resulted in loss of the mitochondrial ΔΨm, cytochrome c release, and DNA fragmentation in P. gingivalis-infected cells and completely abolished the inhibitory effect of P. gingivalis against apoptosis. However, the PI3K inhibitor did not fully abolish the antiapoptotic effect of P. gingivalis in staurosporine-treated cells after several days of infection (data not shown). These findings suggest that other signaling pathways may also contribute to protection against apoptosis at later times of infection. Many proapoptotic stimuli require a mitochondrion-dependent step involving outer membrane permeabilization and cytochrome c release. Mitochondrial integrity is regulated by the Bcl-2 family of proteins, which includes both antiapoptotic Bcl-2 and proapoptotic Bax family members (1). Although the mechanism responsible for the PI3K/Akt-dependent resistance to apoptosis during P. gingivalis infection has not been fully elucidated yet, it is tempting to speculate that the PI3K/Akt pathway may modulate transcription or posttranslational modification of some of the Bcl-2 family members.
Other bacteria, such as Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, and enteropathogenic Escherichia coli, have also been shown to promote cell survival through the PI3K/Akt pathway (8, 14, 28). Considering the many diverse signaling pathways engaged in apoptosis, it is not surprising that some bacteria inhibit or delay apoptosis while others induce it. For example, P. aeruginosa causes excessive apoptosis to disseminate infection, and Shiga-like toxin-producing E. coli and Staphylococcus aureus also induce apoptosis of epithelial cells. On the other hand, Salmonella, M. tuberculosis, and some Neisseria spp. appear to delay or inhibit apoptosis in epithelial cells. Chlamydia spp. and the gastric pathogen Helicobacter pylori both promote and suppress apoptosis under different conditions (2, 7, 9, 21-24, 29, 37, 40). Thus, depending on the stage of the infection cycle, modulation of apoptosis could enable the bacteria to propagate the infection or to protect infected cells against apoptotic mediators used by the host immune system.
We thus propose that P. gingivalis, having established itself in the nutritionally rich host cell cytosol, blocks mitochondrion-dependent apoptosis in order to maintain its intracellular lifestyle. By inducing transient externalization of PS, the bacteria may also protect the infected cell from the host immune system by diminishing the inflammatory response. This ability may facilitate the microorganism's replication and achievement of high numbers inside the individual cells while protecting the infected cell from cytotoxic mechanisms of the host immune system, thereby permitting the successful spread of the bacteria to adjacent and deeper host tissues. Nevertheless, the ability of P. gingivalis to modulate apoptosis now needs to be characterized in the context of the disease process, where the organism is involved in periodontitis as part of a mixed infection. It is tempting to speculate that the existence of other organisms could enhance or antagonize the effects of P. gingivalis alone.
Acknowledgments
This work was supported by NIDCR grant DE 14168, Université Paris 7, and the Institut Pasteur.
Editor: D. L. Burns
REFERENCES
- 1.Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. [DOI] [PubMed] [Google Scholar]
- 2.Airenne, S., H. M. Surcel, J. Tuukkanen, M. Leinonen, and P. Saikku. 2002. Chlamydia pneumoniae inhibits apoptosis in human epithelial and monocyte cell lines. Scand. J. Immunol. 55:390-398. [DOI] [PubMed] [Google Scholar]
- 3.Bellosillo, B., N. Villamor, A. Lopez-Guillermo, S. Marce, F. Bosch, E. Campo, E. Montserrat, and D. Colomer. 2002. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood 100:1810-1816. [DOI] [PubMed] [Google Scholar]
- 4.Belton, C. M., K. T. Izutsu, P. G. Goodwin, Y. Park, and R. J. Lamont. 1999. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cell. Microbiol. 1:215-223. [DOI] [PubMed] [Google Scholar]
- 5.Brazil, D. P., J. Park, and B. A. Hemmings. 2002. PKB binding proteins: getting in on the Akt. Cell 111:293-303. [DOI] [PubMed] [Google Scholar]
- 6.Cary, L. A., and J. L. Guan. 1999. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 4:D102-D113. [DOI] [PubMed] [Google Scholar]
- 7.Cover, T. L., U. S. Krishna, D. A. Israel, and R. M. J. Peek. 2003. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63:951-957. [PubMed] [Google Scholar]
- 8.Crane, J. K., S. Majumdar, and D. F. R. Pickhardt. 1999. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect. Immun. 67:2575-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danelishvili, L., J. McGarvey, Y. J. Li, and L. E. Bermudez. 2003. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell. Microbiol. 5:649-660. [DOI] [PubMed] [Google Scholar]
- 10.Darveau, R., C. M. Belton, R. Reife, and R. J. Lamont. 1998. Local chemokine paralysis: a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 66:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.Fadok, V., D. L. Bratton, D. M. Rose, A. Pearson, R. A. B. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 13.Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg, M., R. Blomgran, M. Lerm, E. Sarndahl, S. M. Sebti, A. Hamilton, O. Stendahl, and L. Zheng. 2003. Differential effects of invasion by and phagocytosis of Salmonella typhimurium on apoptosis in human macrophages: potential role of Rho-GTPases and Akt. J. Leukoc. Biol. 74:620-629. [DOI] [PubMed] [Google Scholar]
- 15.Gamonal, J., M. Sanz, A. O'Connor, A. Acevedo, I. Suarez, A. Sanz, B. Martinez, and A. Silva. 2003. Delayed neutrophil apoptosis in chronic periodontitis patients. J. Clin. Periodontol. 30:616-623. [DOI] [PubMed] [Google Scholar]
- 16.Gao, L.-Y., and Y. Abu Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 17.Goth, S. R., and R. S. Stephens. 2001. Rapid, transient phosphatidylserine externalization induced in host cells by infection with Chlamydia spp. Infect. Immun. 69:1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, J. I., R. R. Russell, M. A. Curtis, J. Aduse-Opoku, and J. J. Taylor. 2002. Molecular mediators of Porphyromonas gingivalis-induced T-cell apoptosis. Oral Microbiol. Immunol. 17:224-230. [DOI] [PubMed] [Google Scholar]
- 19.Huang, G. T. J., D. Kim, J. K. H. Lee, H. K. Kuramitsu, and S. K. Haake. 2001. Interleukin-8 and intracellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect. Immun. 69:1364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh, M.-L. N., V. A. Fadok, and P. M. Henson. 2002. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Investig. 109:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jendrossek, V., H. Grassme, I. Mueller, F. Lang, and E. Gulbins. 2001. Pseudomonas aeruginosa-induced apoptosis involves mitochondria and stress-activated protein kinases. Infect. Immun. 69:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, N. L., A. Islur, R. Haq, M. Mascarenhas, M. Karmali, M. H. Perdue, B. W. Zanke, and P. M. Sherman. 2000. Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G811-G819. [DOI] [PubMed] [Google Scholar]
- 23.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knodler, L. A., and B. B. Finlay. 2001. Salmonella and apoptosis: to live or let die? Microbes Infect. 3:1321-1326. [DOI] [PubMed] [Google Scholar]
- 25.Lamont, R. J., A. Chan, C. M. Belton, K. T. Izutsu, D. J. Vasel, and A. Weinberg. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamont, R. J., and O. Yilmaz. 2002. In or out: the invasiveness of oral bacteria. Periodontology 30:61-69. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie, A., H. L. Wilson, E. Kiss-Toth, S. K. Dower, R. A. North, and A. Surprenant. 2001. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 15:825-834. [DOI] [PubMed] [Google Scholar]
- 28.Maiti, D., A. Bhattacharyya, and J. Basu. 2001. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 276:329-333. [DOI] [PubMed] [Google Scholar]
- 29.Massari, P., C. A. King, A. Y. Ho, and L. M. Wetzler. 2003. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell. Microbiol. 5:99-109. [DOI] [PubMed] [Google Scholar]
- 30.Meier, P., and J. Silke. 2003. Programmed cell death: Superman meets Dr. Death. Nat. Cell Biol. 5:1035-1038. [DOI] [PubMed] [Google Scholar]
- 31.Murray, D. A., and J. M. Wilton. 2003. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infect. Immun. 71:7232-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa, I., A. Amano, M. Kuboniwa, N. T., S. Kawabata, and S. Hamada. 2002. Functional differences among FimA variants of Porphyromonas gingivalis and their effects on adhesion to and invasion of human epithelial cells. Infect. Immun. 70:277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakhjiri, S. F., Y. Park, O. Yilmaz, W. O. Chung, K. Watanabe, A. El-Sabaeny, K. Park, and R. J. Lamont. 2001. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 200:145-149. [DOI] [PubMed] [Google Scholar]
- 34.Opferman, J. T., and S. J. Korsmeyer. 2003. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4:410-415. [DOI] [PubMed] [Google Scholar]
- 35.Ozaki, K., and S. Hanazawa. 2001. Porphyromonas gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via extracellular signal-regulated kinase-dependent expression of p21 Cip/WAF1. Infect. Immun. 69:4944-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perfettini, J.-L., M. Gissot, P. Souque, and D. M. Ojcius. 2002. Modulation of apoptosis during infection by Chlamydia. Methods Enzymol. 358:334-344. [DOI] [PubMed] [Google Scholar]
- 37.Perfettini, J. L., V. Hospital, L. Stahl, T. Jungas, P. Verbeke, and D. M. Ojcius. 2003. Cell death and inflammation during infection with the obligate intracellular pathogen, Chlamydia. Biochimie 85:763-769. [DOI] [PubMed] [Google Scholar]
- 38.Perfettini, J. L., and G. Kroemer. 2003. Caspase activation is not death. Nat. Immunol. 4:308-310. [DOI] [PubMed] [Google Scholar]
- 39.Savill, J., and V. Fadok. 2000. Corpse clearance defines the meaning of cell death. Nature 407:784-788. [DOI] [PubMed] [Google Scholar]
- 40.Shirin, H., E. M. Sordillo, T. K. Kolevska, H. Hibshoosh, Y. Kawabata, S. H. Oh, J. F. Kuebler, T. Delohery, C. M. Weghorst, I. B. Weinstein, and S. F. Moss. 2000. Chronic Helicobacter pylori infection induces an apoptosis-resistant phenotype associated with decreased expression of p27(kip1). Infect. Immun. 68:5321-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Socransky, S. S., and A. D. Haffajee. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63:322-331. [DOI] [PubMed] [Google Scholar]
- 42.Wang, P. L., S. Shirasu, M. Shinohara, M. Daito, M. Oido, Y. Kowashi, and K. Ohura. 1999. Induction of apoptosis in human gingival fibroblasts by a Porphyromonas gingivalis protease preparation. Arch. Oral Biol. 44:337-342. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe, K., O. Yilmaz, S. F. Nakhjiri, C. M. Belton, and R. J. Lamont. 2001. Association of mitogen-activated protein kinase pathways with gingival epithelial cell responses to Porphyromonas gingivalis. Infect. Immun. 69:6731-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz, O., K. Watanabe, and R. J. Lamont. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4:305-314. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz, O., P. A. Young, R. J. Lamont, and G. E. Kenny. 2003. Gingival epithelial cell signaling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology 149:2417-2426. [DOI] [PubMed] [Google Scholar]