Abstract
Objectives
To determine if prominent intrapulmonary anastomotic vessels (IPAV) or bronchopulmonary “shunt” vessels, can be identified in lungs from infants with fatal congenital diaphragmatic hernia (CDH).
Study design
We performed histology with immunostaining for CD31 (endothelium) and D2-40 (lymphatics), and high-precision 3-dimensional (3D) reconstruction on lung tissue from 9 patients who died with CDH.
Results
Each patient with CDH required mechanical ventilation, cardiotonic support and pulmonary hypertension (PH)-targeted drug therapy. All patients were diagnosed with severe PH by echocardiogram and 5 received extra-corporeal membrane oxygenation therapy. Death occurred at a median age of 24 days (range: 10–150) from refractory hypoxemia with severe PH, pneumonia, or tension pneumothorax. Histology showed decreased alveolarization with pulmonary vascular disease. In each patient, prominent IPAV were identified as engorged, thin walled vessels that connected pulmonary veins (PV) with microvessels surrounding pulmonary arteries (PA) and airways in lungs ipsi- and contralateral to the CDH. Prominent anastomosis between PA and bronchial arteries were also noted. 3-D reconstruction studies demonstrate that IPAV connect pulmonary vasculature to systemic (bronchial) vessels both at the arterial and venous side.
Conclusions
Histology and 3D reconstruction identifies prominent bronchopulmonary vascular anastamoses in the lungs of infants who died with severe CDH. We speculate that IPAV connecting pulmonary and bronchial arteries contribute to refractory hypoxemia in severe CDH.
Keywords: intrapulmonary shunt, congenital diaphragmatic hernia, pulmonary circulation, lung vascular development, pulmonary hypertension, persistent pulmonary hypertension of the newborn, bronchopulmonary anastomotic vessels
Congenital diaphragmatic hernia (CDH) is characterized by lung hypoplasia with pulmonary hypertension (PH) that causes severe respiratory distress shortly after birth (1). Despite recent advances in the care of neonates with CDH, including novel ventilator strategies, aggressive cardiotonic support and PH therapies, mortality remains high (2,3). Two main determinants of morbidity and mortality in CDH include the degree of lung hypoplasia and sustained PH due to decreased pulmonary arterial growth and hypertensive vascular remodeling (1,4–7). Abnormal pulmonary vascular growth and structure include decreased pulmonary arterial number in lungs ipsilateral and contralateral to the CDH, increased muscularization of the pulmonary arterial walls, and abnormalities of adventitial thickening (8,9). Other histologic findings include immaturity of alveolar and interstitial development with fewer alveoli, capillaries, and septae (10–14). Overall, these findings result in a striking decrease in lung surface area for gas exchange in CDH (15,16).
Despite aggressive interventions, many infants with CDH have persistent and refractory hypoxemia due to extra-pulmonary shunt with right to left blood flow across a patent ductus arteriosus (PDA) or patent foramen ovale (PFO), as in persistent pulmonary hypertension of the newborn (PPHN) (17,18). Hypoxemia may also be related to intrapulmonary shunt due to lung hypoplasia with decreased surface area or parenchymal lung disease. Although lung hypoplasia contributes to poor gas exchange in CDH, the exact mechanisms underlying refractory hypoxemia are incompletely understood.
Past studies have described the presence of vascular anastomoses connecting the bronchial and pulmonary circulations in some adults (19–20). Connections from the pulmonary circulation of the lung to the extrapulmonary bronchial circulation may be important because unlike the pulmonary vasculature, the bronchial vasculature is capable of proliferation and angiogenesis in response to disease processes (21). In animal and human fetal lungs pre-acinar intrapulmonary anastomotic vessels (IPAV) connect the pulmonary and systemic (bronchial) circulations (22–24). Recent studies have identified the presence of strikingly prominent IPAV in infants dying with alveolar capillary dysplasia and misalignment of pulmonary veins (ACD/MPV) and bronchopulmonary dysplasia (BPD) (25,26, 41). These anastomoses form vascular pathways through which blood can potentially be directed through pulmonary arteries (PA) away from smaller arteries and capillaries associated with distal airspaces through communications between the bronchial circulation and pulmonary veins (PV), leading to marked hypoxemia (25). IPAV could potentially contribute to refractory hypoxemia, but whether these vessels are present and prominent in infants dying with severe CDH has not been studied.
We describe the presence of IPAV in lung tissue from patients who died with severe CDH by utilizing extensive histologic and high fidelity three-dimensional (3-D) reconstruction. The presence of these vessels supports the hypothesis that increased flow through intrapulmonary shunt vessels contributes to hypoxemia in fatal CDH.
Methods
This study was conducted in accordance and with approval of the Institutional Review Board at the University of Colorado Denver Anschutz Medical Center. We used autopsy tissue from 9 infants with CDH who died with severe PH. Clinical data were from the medical record and the autopsy report. The definition of PH was based on standard clinical echocardiographic criteria, which include extrapulmonary right to left shunts, right ventricular systolic pressure greater than 50% of systemic values based on tricuspid regurgitant jet velocity, marked septal flattening, right ventricular dilation, and right ventricular hypertrophy (27).
The streptavidin-biotin peroxidase method with the Ventana Benchmark automatic slide stainer was used for immunohistochemistry. Primary antibodies included CD31 (endothelium) (Ventana Inc, Tucson, AZ) and D2-40 (lymphatics) (Dako, Carpinteria, CA).
Lung tissue from patients with CDH was also studied by 3-D reconstruction, as previously described (25). Tissue samples from infants with CDH were from multiple regions of both the ipsi- and contralateral lungs. Samples from multiple areas of all lobes were serially sectioned at 5 μm intervals. These serial sections were then stacked and stained with hematoxylin and eosin. In order to differentiate the endothelium and lymphatic tissue, every 11th section was stained with CD31, an endothelial marker; the subsequent section was stained with D2-40, a lymphatic specific marker. We then placed the sections onto the Stereo Investigator microscope (Microbrightfield, Williston, VT), which is designed to trace and three-dimensionally reconstruct the tissue (28,29). The sections were then traced and each structure given a distinct color (red: pulmonary artery (PA) endothelium; aqua: PA wall; pink: lymphatic; green: airway; blue: veins; yellow: IPAV). Each section was aligned and traced until the stack was completed and the images could be three-dimensionally constructed.
Results
The average gestational age of the 9 infants was 37.7 weeks ± 1.9 weeks (mean ± SD) and birth weight was 2706 g ± 630g (mean ± SD; Table). Six diaphragmatic defects were left-sided, 2 were right-sided, and one infant had a bilateral defect. All infants developed marked respiratory distress at birth requiring intubation with mechanical ventilation, which included high flow oscillatory ventilation, and treatment with vasopressor or inotropic agents. All infants were diagnosed with PH by echocardiogram, including 6 infants with supra-systemic pulmonary pressures with marked extra-pulmonary right to left shunt across the patent foramen ovale or ductus arteriosus. All infants were treated with inhaled nitric oxide and 5 infants received extracorporeal membrane oxygenation (ECMO). Seven infants had surgical repair of their diaphragmatic defect with a prosthetic patch prior to death. The median age at death was 24 days (range: 10–150). The causes of death included severe PH or withdrawal of support due to severe lung disease and PH (n=6), pneumonia (n=2), and tension pneumothorax (n=1). Eight infants required support with mechanical ventilation throughout their lives.
Table 1.
Study Population
| Patient | Birth Weight (g) | Gestational age (wks) | Sex | Age at death (days) | Cause of death | ECMO | Pulmonary Hypertension | Ventilation at death |
|---|---|---|---|---|---|---|---|---|
| 1 | 1900 | 36 4/7 | m | 112 | tension pneumothorax | no | Y; severe | Y |
| 2 | 3100 | 38 | f | 51 | heart failure; withdrawal of care | no | Y; PAP>systemic | Y |
| 3 | 2845 | 38 4/7 | m | 150 | Pneumonia; cerebral herniation | no | Y | N; 0.25L Nasal Canula |
| 4 | 3680 | 38 | m | 24 | persistent hypoxia leading to hypoxic ischemic encephalopathy | yes | Y; severe | Y |
| 5 | 2040 | 33 | m | 10 | pulmonary hypertension; withdrawal of support | yes | Y; PAP>systemic | Y |
| 6 | 3000 | 38 | m | 16 | pulmonary hypertensive crisis | yes | Y; severe septal flattening | Y |
| 7 | ? | 39 6/7 | f | 150 | pulmonary hypertensive crisis | no | Y; PAP>systemic | Y |
| 8 | 2085 | 38 | f | 20 | pulmonary hypertensive crisis | yes | Y; moderate septal flattening | Y |
| 9 | 3000 | 39 | f | 19 | pulmonary hypertensive crisis | yes | Y | Y |
Histologic evaluation
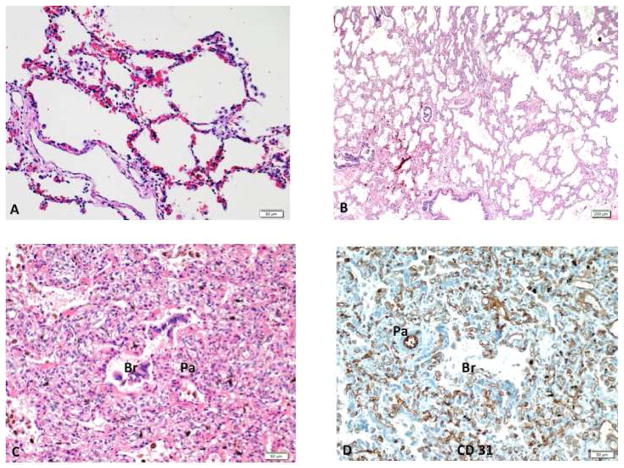
In comparison with lungs from infants who died without respiratory disease, lung histology of the patients with CDH showed decreased alveolarization with a mixture of “BPD –like” changes (large and simplified alveoli with thinned interstitium); thick interstitum containing numerous microvessels; and immaturity (Figure 1). In addition, intrapulmonary vessels linking pulmonary veins (PV) with microvessels surrounding larger PA and airways were readily identified in both the ipsi- and contralateral lungs (Figure 2). These vessels appeared as dilated, thin-walled vascular channels. Vascular connections between BA and PA were also observed. Immunostaining studies demonstrated that these vascular channels were uniformly positive for CD31, an endothelial-specific marker, and negative for D2-40, a lymphatic marker, confirming that these vascular channels are not lymphatic vessels (Figure 2).
Figure 1.
Lung histology from an infant with congenital diaphragmatic hernia shows 3 distinct histologic patterns. The BPD like is characterized by enlarged and simplified alveoli identical to that seen in infants with BPD (A). The immature pattern demonstrates lung tissue composed of immature alveoli with thickened interstitium (B). A “hemangiomatosis” pattern is dominated by numerous capillaries filling up the interstitium, as well as surrounding the airways (Br) and pulmonary artery (PA) (C), highlighted by CD31 immunostaining (D). BPD – bronchopulmonary dysplasia.
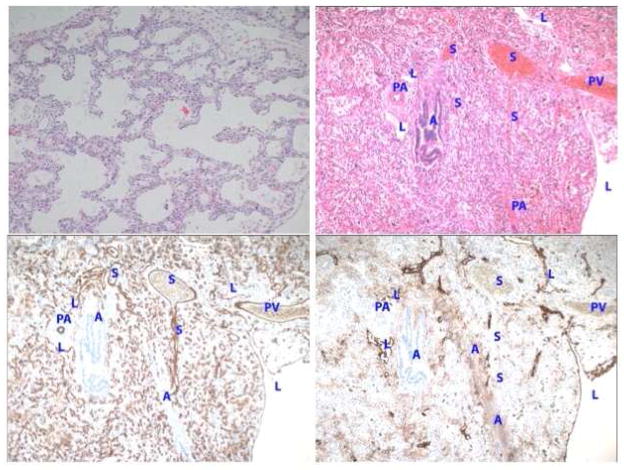
Figure 2.
Intrapulmonary anastomotic vessels (IPAV) (or shunt vessels; designated as “S”) are readily identified as thin walled vessels that bridge pulmonary veins and microvessels surrounding pulmonary arteries and airways in infants with severe CDH Prominent and dilated IPAV are often located within the broncho-arterial bundle in the distal lung (top panels). Immunostaining of vessels for CD31 and D2-40 demonstrate strong staining for CD31 (endothelial marker) (bottom left) but not stain for D2-40 (lymphatic marker) in IPAV (bottom right panel). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus.
3-D reconstruction studies
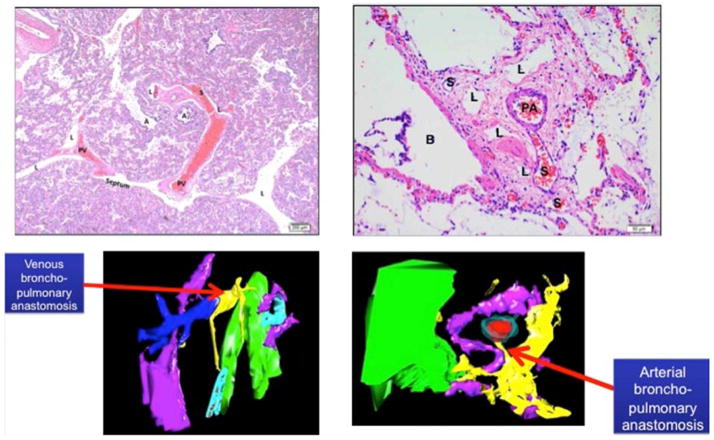
High-precision, computerized 3-D reconstruction was performed to further evaluate the IPAV in CDH lung tissue, providing the ability to track the course of these vascular channels (Figure 3 and Video x; Video x available at www.jpeds.com,). Vascular channels appear in two different patterns: venous broncho-pulmonary anastomoses and arterial broncho-pulmonary anastomoses. These two patterns are shown by histology and through high fidelity 3-D reconstruction (Figure 3 and Video x; Video x available at www.jpeds.com). The venous broncho-pulmonary anastomoses appear to travel from the periphery, the normal location of the pulmonary veins, and originate directly from the pulmonary veins, traveling towards the bronchopulmonary bundles and connect with the surrounding microvessels. Direct PA-PV connection is not observed (Figure 3). The arterial broncho-pulmonary anastomoses are noted between the PA and BA. Branches of BAs travel towards bronchial wall (Figure 3 and Video x; Video x available at www.jpeds.com).
Figure 3.
Venous Intrapulmonary anastomotic vessels (IPAV) (or shunt vessels; designated as “S”) are recognized as thin walled vessels that bridge pulmonary veins and microvessels surrounding pulmonary arteries and airways (top left panel). Arterial IPAV are connections between PA and BA (upper right panel) Lower panels show 3D reconstruction images of venous (left bottom) and arterial (right bottom) bronchopulmonary anastomotic or “shunt” vessels (yellow). (Color key: yellow: shunt vessel; red: PA endothelium; aqua; PA wall; pink: lymphatic; green: airway). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus).
Discussion
Infants with severe CDH present with profound hypoxemia shortly after birth, which is largely due to extra-pulmonary right to left shunting of blood through the PFO or PDA. However, mechanisms responsible for hypoxemia in the absence of extrapulmonary shunt at the PFO or PDA remain poorly understood. Based on past reports in ACD/MPV and BPD (25,26,41), we studied lung histology from 9 infants who died with severe CDH and found striking vascular channels that connect bronchial (systemic) and pulmonary vessels, providing the anatomic basis for intrapulmonary shunt. These intrapulmonary anastomotic vessels (IPAV) appeared as thin walled vascular channels and were present in both the ipsi- and contralateral lungs all infants with fatal CDH. The presence and prominence of these vessels may provide insights into mechanisms contributing to the pathophysiology of hypoxemia in severe CDH.
We directly demonstrate the presence of prominent IPAV that can serve as potential pathways through which blood flows away from the alveolar capillary bed through dilated, pre-existing anastomoses that connect the bronchial and pulmonary circulations. Severe CDH is characterized by recalcitrant hypoxemia and PH that persist despite mechanical ventilation, cardiotonic support, PH-specific therapies, and EMCO. Our novel findings suggest that intrapulmonary shunt through prominent IPAV can potentially contribute to sustained hypoxemia in CDH. These intrapulmonary shunt vessels in severe CDH appear similar to “misaligned pulmonary veins” that are commonly recognized in ACD/MPV and as recently described in fatal cases of severe BPD (25,26,30,41). As observed with severe CDH, ACD/MPV is characterized by recalcitrant hypoxemia with lung hypoplasia and severe PH (31). The presence of these shunt vessels in CDH suggests a potential mechanism of recalcitrant hypoxemia as recently suggested in ACD/MPV and BPD (25,26,41).
Previous studies have characterized abnormalities of the pulmonary circulation in CDH, which include a decreased number of pulmonary arteries in both lungs of infants with CDH, hypertensive remodeling of small pulmonary arteries and adventitial thickening, along with immature alveolar and capillary development (8–14). Mechanisms underlying altered pulmonary vascular development in CDH are incompletely understood. Abnormal pulmonary blood flow and endothelial signaling are known to impair lung growth and alveolarization (32). Pulmonary artery endothelial cells have impaired growth and angiogenic capacity in an experimental model of CDH (33). However, mechanisms that disrupt coordinated growth of the distal lung and vasculature and contribute to prominent IPAV in CDH are not clear.
Mechanisms through which IPAV, or “shunt” vessels are augmented in severe CDH are unclear. There is evidence of the presence of IPAV in the late gestation fetus and these vessels were originally thought to disappear in the neonatal period (22–24). However, there have been several reports of intrapulmonary shunt vessels in normal adults, and evidence for physiologic shunt has been elicited during catecholamine infusion, hypoxia or exercise (34–40). Our findings demonstrate prominent IPAV in infants with severe CDH but lack direct physiologic evidence of their clinical impact. We previously suggested that “misaligned pulmonary veins” that are associated with ACD/MPV are enlarged bronchial veins that appear more prominent due to increased shunting of blood from the pulmonary to bronchial circulations through IAPV (26; 41). Our finding of both venous and arterial broncho-pulmonary anastomoses in infants with CDH suggests that IPAV are also prominent in CDH, as previously observed in ACD/MPV and BPD (25,26,41). We speculate that the persistence and prominence of these vessels are due to the marked obstruction and elevated vascular resistance in the distal pulmonary arterial microvasculature in CDH, which then favors greater flow through the anastomotic vessels.
Although the histologic and 3D images are striking, we currently lack specific physiologic data to support the actual contribution of these intrapulmonary shunt vessels to persistent hypoxemia in severe CDH. However, as previously reported, we failed to identify IPAV with similar histologic and 3D reconstruction studies of lung tissue from infants who died without respiratory or cardiac disease (25). Recent work with lung tissue from two infants with died with ACD/MPV helps provide indirect evidence of the physiologic impact of these shunt vessels (41). We recently demonstrated striking intrapulmonary vascular pathways linking the systemic and pulmonary circulations that bypass the alveolar capillary bed in ACD/MPV (41). Future study of autopsy tissue will aim to more precisely define and quantify the anatomic distribution of these vessels, clinical and prenatal factors associated with IPAV development, and mechanisms that regulate blood flow through these vessels in CDH. Despite these limitations, describe the presence of arteriovenous shunt vessels in CDH, and provide a novel explanation for the refractory hypoxemia often seen in severe CDH.
In conclusion, we have demonstrated histologic evidence of the presence of IAPV or “bronchopulmonary shunt” vessels in patients dying with severe CDH. The finding of these abnormal vascular channels was confirmed by high-resolution 3-D reconstruction of distal lung tissue. We speculate that these shunt vessels contributes to the refractory hypoxemia and respiratory failure which often characterizes severe CDH.
Supplementary Material
Video 1: Arterial intrapulmonary anastomotic vessels (IPAV) (or shunt vessels) are connections between pulmonary arteries and bronchial arteries. 3-dimensional reconstruction of arterial IPAV. (Color key: yellow: shunt vessel; red: PA endothelium; aqua; PA wall; pink: lymphatic; green: airway). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus).
Video 2: Venous intrapulmonary anastomotic vessels (IPAV) (or shunt vessels) are recognized as thin walled vessels that bridge pulmonary veins and microvessels surrounding pulmonary arteries and airways. 3-dimensional reconstruction of arterial IPAV. (Color key: yellow: shunt vessel; red: PA endothelium; aqua; PA wall; pink: lymphatic; green: airway). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus).
Acknowledgments
Supported by the National Institutes of Health (HL68702 [to S.A.], HL085703 [S.A.], T32 HL07670 [to S.A.], and DK096996 [S.S.-L.]).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus. Neonatology. 2010;(98):354–364. doi: 10.1159/000320622. [DOI] [PubMed] [Google Scholar]
- 2.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: The true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;(112):532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- 3.Balayla J, Abenhaim HA. Incidence, predictors and outcomes of congenital diaphragmatic hernia: A population-based study of 32 million births in the United States. J Matern Fetal Neonatal Med. 2013 Oct 25; doi: 10.3109/14767058.2013.858691. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;(39):307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000;(35):1187–1197. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- 6.Suda K, Bigras JL, Bohn D, Hornberger LK, McCrindle BW. Echocardiographic predictors of outcome in newborns with congenital diaphragmatic hernia. Pediatrics. 2000;(105):1106–1009. doi: 10.1542/peds.105.5.1106. [DOI] [PubMed] [Google Scholar]
- 7.van den Hout L, Sluiter I, Gischler S, De Klein A, Rottier R, Ijsselstijn H, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;(25):733–743. doi: 10.1007/s00383-009-2425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Buys Roessingh AS, Dinh-Xuan AT. Congenital diaphragmatic hernia: current status and review of the literature. Eur J Pediatr. 2009;(168):393–406. doi: 10.1007/s00431-008-0904-x. [DOI] [PubMed] [Google Scholar]
- 9.Shehata SM, Tibboel D, Sharma HS, Mooi WJ. Impaired structural remodeling of pulmonary arteries in newborns with congenital diaphragmatic hernia: a histological study of 29 cases. J Pathol. 1999;(1):112–118. doi: 10.1002/(SICI)1096-9896(199909)189:1<112::AID-PATH395>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Arrechom W, Reid L. Hypoplasia of lung with congenital diaphragmatic hernia. Br Med J. 1963;(1):230–233. doi: 10.1136/bmj.1.5325.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askenazi SS, Perlman M. Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Chil. 1979;(54):614–618. doi: 10.1136/adc.54.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin DL. Morphologic analysis of the pulmonary vascular bed in congenital diaphragmatic hernia. J Pediatric. 1978;(92):805–809. doi: 10.1016/s0022-3476(78)80162-0. [DOI] [PubMed] [Google Scholar]
- 13.Yamataka T, Puri P. Pulmonary artery structural changes in pulmonary hypertension complicating congenital diaphragmatic hernia. J Pediatr Surg. 1997;(32):387–390. doi: 10.1016/s0022-3468(97)90587-x. [DOI] [PubMed] [Google Scholar]
- 14.Ionocco JA, Ciley RE, Mauger DT, Krummel TM, Dillon PW. Postnatal pulmonary hypertension after repair of congenital diaphragmatic hernia; predicting risks and outcome. 1999;(32):349–353. doi: 10.1016/s0022-3468(99)90207-5. [DOI] [PubMed] [Google Scholar]
- 15.George DK, Cooney TP, Chiu BK, Thurlbeck WM. Hypoplasia and immaturity of the terminal lung unit (acinus) in congenital diaphragmatic hernia. Am Rev Respir Dis. 1987;(136):947–950. doi: 10.1164/ajrccm/136.4.947. [DOI] [PubMed] [Google Scholar]
- 16.Boyden EA. The structure of compressed lungs in congenital diaphragmatic hernia. Am J Anat. 1972;(134):497–508. doi: 10.1002/aja.1001340407. [DOI] [PubMed] [Google Scholar]
- 17.Kinsella JP, Ivy DD, Abman SH. Pulmonary vasodilator therapy in congenital diaphragmatic hernia: acute, late, and chronic pulmonary hypertension. Semin Perinatol. 2005;29(2):123–128. doi: 10.1053/j.semperi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Muratore CS, Kharasch V, Lund DP, Sheils C, Friedman S, Brown C, Utter S, Jaksic T, Wilson JM. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg. 2001;36(1):133–140. doi: 10.1053/jpsu.2001.20031. [DOI] [PubMed] [Google Scholar]
- 19.Marcel-Dekkar Cudkowicz L. Vol 14 of the Lung Biology in Health and Diseases series. 1979:111–232. [Google Scholar]
- 20.Charan NB. Textbook of Respiratory Disease. 1984:1226–1235. [Google Scholar]
- 21.Charan NB, Baile EM, Pare PD. Bronchial vascular congestion and angiogenesis. Eur Respir J. 1997;10:1173–1180. doi: 10.1183/09031936.97.10051173. [DOI] [PubMed] [Google Scholar]
- 22.Robertson B. Anastomoses in the human lung: postanatal formation and obliteration of arterial anastomoses in the human lung: a microangiographic and histologic study. Pediatrics. 1969;43:971. [PubMed] [Google Scholar]
- 23.Wilkinson MJ, Fagan DG. Postmortem demonstration of intrapulmonary arteriovenous shunting. Arch dis Child. 1990;65:435–437. doi: 10.1136/adc.65.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullan DM, Hanley FL, Cohen GA, Portman MA, Riemer RK. Pulmonary arteriovenous shunting in the normal fetal lung. J Am Coll Cardiol. 2004;44:1497–1500. doi: 10.1016/j.jacc.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Galambos C, Sims-Lucas S, Abman SH. Histologic evidence of intrapulmonary anastomoses by three-dimensional reconstruction in severe bronchopulmonary dysplasia. Ann Am Thorac Soc. 2013;10(5):474–481. doi: 10.1513/AnnalsATS.201305-124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galambos C, Sims-Lucas S, Abman SH. Three-Dimensional reconstruction identifies misaligned pulmonary veins as intrapulmonary shunt vessels in alveolar capillary dysplasia. J Pediatr. 2014;164:192–195. doi: 10.1016/j.jpeds.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mourani PM, Abman SH. Pulmonary vascular disease in BPD: physiology, diagnosis and treatment. In: Abman SH, editor. Bronchopulmonary dysplasia. NY: Informa; 2010. pp. 347–363. [Google Scholar]
- 28.Sims-Lucas S. Analysis of 3D branching pattern: hematoxylin and eosin method. Methods Mol Biol. 2012;886:73–86. doi: 10.1007/978-1-61779-851-1_7. [DOI] [PubMed] [Google Scholar]
- 29.Sims-Lucas S, Argyropoulos C, Kish K, McHugh K, Bertram JF, Quigley R, Bates CM. Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J Am Soc Nephrol. 2009;20:2525–2533. doi: 10.1681/ASN.2009050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Pediatr Pathol. 1991;11:163–170. doi: 10.3109/15513819109064753. [DOI] [PubMed] [Google Scholar]
- 31.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallen LD, Perry SF, Alston JT, Maloney JE. Morphometric study of the role of pulmonary arterial flow in fetal lung growth in sheep. Pediatr Res. 1990;27:122–127. doi: 10.1203/00006450-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Acker SN, Seedorf G, Abman SH, Nozik-Grayck E, Partrick DA, Gien J. Pulmonary artery endothelial cell dysfunction and decreased populations of highly proliferative endothelial cells in experimental congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2013;305:L943–L952. doi: 10.1152/ajplung.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovering AT, Romer LM, Haverkamp HC, Hokanson JS, Eldridge MW. Excessive gas exchange impairment during exercise in a subject with a history of bronchopulmonary dysplasia and high altitude pulmonary edema. High Alt Med Biol. 2007;8:62–67. doi: 10.1089/ham.2006.0816. [DOI] [PubMed] [Google Scholar]
- 35.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113:1214–1222. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- 36.Bryan TL, van Diepen S, Bhutani M, Shanks M, Welsh RC, Stickland MK. The effects of dobutamine and dopamine on intrapulmonary shunt and gas exchange in healthy humans. J Appl Physiol. 2012;113:541–548. doi: 10.1152/japplphysiol.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stickland MD, Lovering AT. Exercise-induced intrapulmonary arteriovenous shunting and pulmonary gas exchange. Exerc Sport Sci Rev. 2006;34:96–106. doi: 10.1249/00003677-200607000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Eldridge MW, Dempsey JA, Haverkamp HC, Lovering AT, Hokanson JS. Exercise induced intrapulmonary arteriovenous shunting in healthy humans. J Appl Physiol. 2004;97:797–805. doi: 10.1152/japplphysiol.00137.2004. [DOI] [PubMed] [Google Scholar]
- 39.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104:1418–1425. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- 40.Lovering AT, Stickland MK, Amann M, Murphy JC, O’Brien NJ, Hokanson JS, Eldridge MW. Hyperoxia prevents exercise-induced intrapulmonary arteriovenous shunt in healthy humans. J Physiol. 2008;586:4559–4565. doi: 10.1113/jphysiol.2008.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galambos C, Sims-Lucas S, Ali N, Gien J, Dishop MK, Abman SH. Thorax. 2014 Jul 21; doi: 10.1136/thoraxjnl-2014-205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Arterial intrapulmonary anastomotic vessels (IPAV) (or shunt vessels) are connections between pulmonary arteries and bronchial arteries. 3-dimensional reconstruction of arterial IPAV. (Color key: yellow: shunt vessel; red: PA endothelium; aqua; PA wall; pink: lymphatic; green: airway). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus).
Video 2: Venous intrapulmonary anastomotic vessels (IPAV) (or shunt vessels) are recognized as thin walled vessels that bridge pulmonary veins and microvessels surrounding pulmonary arteries and airways. 3-dimensional reconstruction of arterial IPAV. (Color key: yellow: shunt vessel; red: PA endothelium; aqua; PA wall; pink: lymphatic; green: airway). Abbreviations: S, shunt vessel; A, airspace; PV, pulmonary vein; L lymphatic; PA, pulmonary artery; B, bronchus).