Abstract
Background & Aims
Alcoholic liver disease is a leading cause of mortality. Chronic alcohol consumption is accompanied by intestinal dysbiosis, and development of alcoholic liver disease requires gut-derived bacterial products. However, little is known about how alterations to the microbiome contribute to pathogenesis of alcoholic liver disease.
Methods
We used the Tsukamoto-French mouse model which involves continuous intragastric feeding of isocaloric diet or alcohol for 3 weeks. Bacterial DNA from the cecum was extracted for deep metagenomic sequencing. Targeted metabolomics assessed concentrations of saturated fatty acids in cecal contents. To maintain intestinal metabolic homeostasis, diets of ethanol-fed and control mice were supplemented with saturated long-chain fatty acids (LCFA). Bacterial genes involved in fatty acid biosynthesis, amounts of lactobacilli, and saturated LCFA were measured in fecal samples of non-alcoholic individuals and patients with active alcohol abuse.
Results
Analyses of intestinal contents from mice revealed alcohol-associated changes to the intestinal metagenome and metabolome, characterized by reduced synthesis of saturated LCFA. Maintaining intestinal levels of saturated fatty acids in mice resulted in eubiosis, stabilized the intestinal gut barrier and reduced ethanol-induced liver injury. Saturated LCFA are metabolized by commensal Lactobacillus and promote their growth. Proportions of bacterial genes involved in fatty acid biosynthesis were lower in feces from patients with active alcohol abuse than controls. Total levels of LCFA correlated with those of lactobacilli in fecal samples from patients with active alcohol abuse but not in controls.
Conclusion
In humans and mice, alcohol causes intestinal dysbiosis, reducing the capacity of the microbiome to synthesize saturated LCFA and the proportion of Lactobacillus species. Dietary approaches to restore levels of saturated fatty acids in the intestine might reduce ethanol-induced liver injury in patients with alcoholic liver disease.
Keywords: metagenomics, metabolomics, microbiome, microbiota
Introduction
Liver cirrhosis is the 10th leading cause of mortality in the United States; more than 40% of all cirrhosis-associated deaths are related to alcohol1. Alcoholic liver disease can progress from simple hepatic steatosis to cirrhosis and even hepatocellular carcinoma in some individuals2.
Chronic alcohol consumption is associated with qualitative and quantitative (overgrowth) dysbiotic changes in the intestinal microbiota3–7. Alcoholic dysbiosis is characterized by reduced proportions of commensal probiotic bacteria such as Lactobacillus species in animal models and humans with cirrhosis3, 8. A common and important feature of disease pathogenesis is leakiness of the intestinal barrier, resulting in translocation of microbial products such as lipopolysaccharide from the intestinal lumen to the liver9. Development of alcoholic liver disease depends on microbial translocation; mice that express nonfunctional toll-like receptor 4 are resistant to experimental alcoholic liver disease10. Furthermore, mice that are protected from bacterial overgrowth have decreased alcohol-induced liver disease despite leakier guts4, 11. Administration of the probiotic Lactobacillus rhamnosus GG to alcohol-fed mice restored intestinal integrity, decreased intestinal translocation of microbial products, and reduced features of alcoholic liver disease12, 13, providing evidence that targeting dysbiosis improves disease.
Although alcohol-associated changes in the enteric microbiome and subsequent disruption of the intestinal barrier are required for development and progression of liver disease in animal models and humans14, 15, little is known about the mechanisms of this process. We used metagenomic and metabolomic analyses to investigate the effects of chronic ethanol administration on metabolism by the intestinal microbiota.
Materials and Methods
Alcohol feeding
Male C57BL/6J mice (age 8 weeks) were purchased from Jackson Laboratory. All mice received humane care in compliance with institutional guidelines. Continuous intragastric infusion of ethanol was performed as previously described3, 4, 16. In brief, mice were anesthetized by injection of ketamine and xylazine, and underwent surgical implantation of a long-term gastrostomy catheter made of Tygon and silastic tubings with Dacron felt under sterile conditions. The use of a swivel allows free movement of the mouse in a micro-isolator cage. After 1 week acclimatization period during which mice were given intragastric infusions of a control diet, ethanol infusion was initiated at 22.7g/kg/day on days 1 and 2, 24.3 g/kg/day on days 3 and 4, 26 g/kg/day on days 5 and 6, and 27.5 g/kg for day 7. For mice fed ethanol for 3 weeks, the dose increased to 29.2 g/kg/day for days 9–14 and 30.9 g/kg/day for week 3. At the initial ethanol dose, total caloric intake was set at 533 Cal/kg; the percentages of calories from ethanol, dietary carbohydrate (dextrose), protein (lactalbumin hydrolysate), and fat (corn oil) were 29%, 13%, 23%, and 35%, respectively. A vitamin, salt, and trace mineral mix was included at the recommended amounts by the Committee on Animal Nutrition of the National Research Council (AIN-76A, 4.42 g/L and 15.4 g/L, respectively; Dyets Inc.). Mice were individually caged and control mice received an isocaloric amount of dextrose instead of ethanol.
For fatty acids supplementation experiments, the total caloric intake was reduced by 30%, to 373 Cal/kg; the percentage of calories from ethanol, dietary carbohydrate (dextrose), and protein (lactalbumin hydrolysate) were 29%, 13%, and 23%, respectively. Liquid diets were also supplemented with a small amount of corn oil (5% of total calories) to avoid deficiency in essential fatty acids. Most of the dietary fat component was fed via pellets in an isocaloric amount, because saturated fatty acids have a wax-like consistency at room temperature and cannot be provided as liquid diet. The unsaturated fatty acid [USF] group received pellets made of corn oil (#401150; Dyets Inc.), whereas the saturated fatty acid [SF] group received pellets made of hydrogenated soy glyceride (#D404363; Dyets Inc.). Corn oil contains predominately unsaturated fatty acids (60% polyunsaturated fatty acids [linoleic acid] and 26% monounsaturated fatty acids [oleic acid]; Supplemental Table 1). 80-S hydrogenated soya glyceride contains mostly saturated fatty acids (12% palmitic acid and 85% stearic acid; Supplemental Table 2). Comparable amounts of USF and SF pellets were consumed by mice in both groups (Supplemental Figure 1). Following 3 weeks of intragastric alcohol feeding and dietary supplementation with fatty acids, single-caged mice were sacrificed for analysis.
Other materials and methods are described in the Supplementary Materials and Methods section.
Results
Chronic administration of ethanol inhibits biosynthesis of saturated fatty acids by the intestinal microflora
We first determined the effect of chronic alcohol intake on the intestinal metagenome. We used individually housed mice given intragastric infusion of ethanol-containing diet as a model of alcohol-induced liver disease. This model is ideal for well-controlled microbiomic and metabolic studies, because the same liquid diet is administered to all mice at the same rate, with the same amount of calories and volume. Control mice received infusion of an isocaloric amount of diet with dextrose instead of ethanol. Mice continuously fed ethanol for 3 weeks developed severe liver injury and steatosis as described by us3.
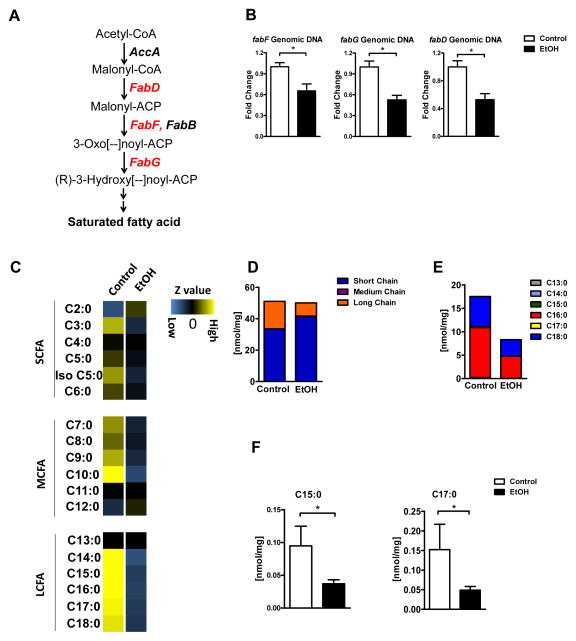
The cecal microbiota from mice fed ethanol for 3 weeks had a lower proportion, compared with controls, of bacterial genes involved in the biosynthesis of saturated fatty acids including malonyl CoA:ACP acyltransferase (FabD; [EC:2.3.1.39]), 3-oxoacyl-[acyl-carrier-protein] synthase II (FabF; [EC:2.3.1.179]) and 3-oxoacyl-[acyl-carrier protein] reductase (FabG; [EC:1.1.1.100]), determined by metagenomic sequencing (Figure 1A). Quantitative (q)PCR confirmed these results (Figure 1B). To further explore whether ethanol reduces saturated fatty acid biosynthetic gene abundance independently from the host, we performed ex vivo analysis by incubating cecal contents from control mice with ethanol under anaerobic conditions. Ethanol decreased the abundance of fabF, fabG and fabD genes (Supplemental Figure 2A), suggesting that ethanol exerts a direct effect on the microbiota. We further assessed bacterial gene expression on the transcriptional level. The mRNA level of all three fab genes showed a similar reduction as the genomic DNA after ethanol incubation (Supplemental Figure 2B), suggesting that ethanol changes microbial communities rather than having a direct inhibitory effect on bacterial gene transcription. Phylogenetic analysis of fab genes derived from metagenomic assemblies lacked representation in certain clades of Bacteroides, Clostridium, and Akkermansia spp., suggesting that some phylotypes were more sensitive to the effects of ethanol; therefore, reducing the copy number of these genes in the intestine (Supplemental Figure 2C).
Figure 1. Chronic ethanol administration reduces bacterial biosynthesis of saturated LCFA.
C57BL/6 mice were fed intragastric isocaloric (control) or alcohol diets for 3 weeks. (A) Fatty acid synthesis pathway: Metagenomic sequencing revealed a reduced proportion of bacterial genomic DNA (shown in red) containing genes involved in biosynthesis of saturated fatty acids. (B) Fold changes in genomic DNA containing malonyl CoA:ACP acyltransferase (fabD), 3-oxoacyl-[acyl-carrier-protein] synthase II (fabF) and 3-oxoacyl-[acyl-carrier protein] reductase (fabG), based on real-time PCR (n = 5). (C) Z value of each single fatty acid in the cecum of control (n = 3) and ethanol fed mice (n = 7). (D) Levels of saturated short-, medium-, and long-chain fatty acids in the cecum of control (n = 3) and ethanol-fed mice (n = 7). (E and F) Total levels of saturated LCFA, C15:0 and C17:0, in the cecum of control and ethanol-fed mice (n = 3 for control; n = 7 for ethanol). *p<0.05.
Targeted metabolomic studies using tandem mass spectrometry determined the concentrations of the free saturated fatty acids C2:0 to C18:0 in cecal contents. With the exception of C10:0, which was lower in ethanol-fed mice, the concentrations of saturated short-chain fatty acids (SCFA; C2:0–C6:0) and medium-chain fatty acids (MCFA; C7:0–C12:0) did not differ significantly between control and alcohol-fed mice. In contrast, concentrations of almost all saturated long-chain fatty acids (LCFA; C13:0–C18:0) were markedly decreased in ethanol-fed mice, compared with control mice (Figure 1C; Supplemental Figure 3).
In control and ethanol-fed mice, the concentrations of SCFA were the highest, when absolute amounts of all saturated fatty acids were compared. Although there was a slight, but not significant increase in the total level of SCFA following ethanol feeding (which is due to an increase in acetate as metabolite from ethanol), the total amount of LCFA was significantly lower in ethanol-fed mice than in control mice (Figure 1D). Palmitic acid (C16:0) and stearic acid (C18:0) are the predominant LCFA in cecal contents of mice; levels of both are significantly reduced following 3 weeks of alcohol administration (Figure 1E). These data support our results from metagenomic analyses and indicate that bacterial synthesis of LCFA is greatly reduced after alcohol ingestion.
Fatty acids from diet or synthesized by the host could contribute to the differences observed in our studies. However, ethanol-fed mice and controls, fed isocaloric diets, received the exact same amount of fat via the gastric feeding tube. Furthermore, expression levels of genes involved in the synthesis of fatty acids (Srebp1, Fasn, Acc1, and Scd1) were similar between groups in tissues collected along the entire intestinal tract (Supplemental Figure 4). Therefore, difference in host gene expression patterns cannot account for differences observed in luminal levels of saturated fatty acid.
Additional support for bacteria-induced changes comes from the analysis of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0), which are LCFA produced by only bacteria17. Although the levels of pentadecanoic and heptadecanoic acid are low in intestines of control mice, ethanol feeding was associated with significantly lower intestinal levels (Figure 1F). Therefore, we observed clear functional differences in the ability of the microbiota to synthesize saturated LCFA following chronic administration of ethanol to mice.
Dietary supplementation of saturated LCFA reduces features of ethanol-induced liver disease in mice
Our findings suggested that chronic alcohol intake reduces the capacity of the intestinal microflora to synthesize saturated LCFA, resulting in lower intestinal levels of palmitic and stearic acid. We therefore investigated whether dietary supplementation to restore intestinal levels of palmitic and stearic acid, which are saturated LCFA, could prevent the development of ethanol-associated liver disease in mice. The diets of ethanol-fed and control mice were supplemented with 12% palmitic acid and 85% stearic acid (the saturated fatty acid [SF] group), which mimic intestinal levels of LCFA in control mice. Calories from fat were predominantly derived from unsaturated fatty acids oleic acid (27%) and linoleic acid (60%) in the unsaturated fatty acid [USF] group. The SF and USF groups were pair-fed and received equal amount of calories from fat.
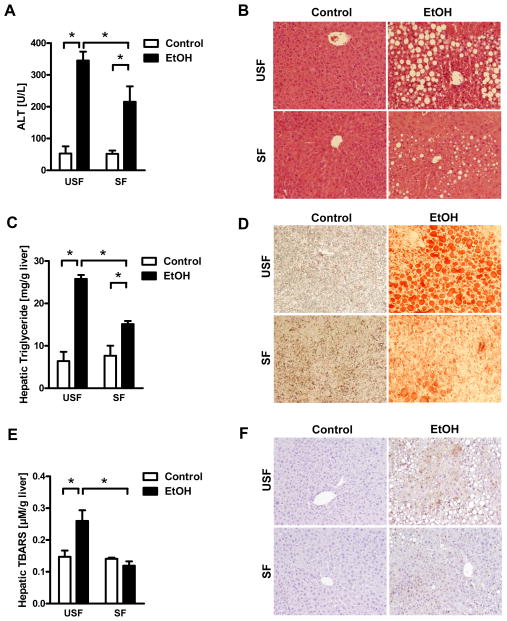
Ratios of liver to body weight did not differ between SF and USF groups of ethanol-fed mice (Supplemental Figure 5). However, the SF group developed less-severe ethanol-associated liver disease; these mice had reduced levels of liver injury (based on level of alanine aminotransferase [ALT]) and hepatic steatosis than mice in the USF group (Figure 2A, B). This was confirmed by measurement of hepatic triglyceride levels (Figure 2C, D). Ethanol administration caused hepatic oxidative stress, indicated by increased levels of thiobarbituric acid-reactive substances (TBARS) in ethanol-fed vs control mice; hepatic oxidative stress was reduced in ethanol-fed mice given palmitic and stearic acid (Figure 2E). Immunohistochemical analysis of 4-hydroxynonenal (4-HNE), a marker for oxidative stress, confirmed these results (Figure 2F). Mice in the SF group showed a significantly reduced hepatic gene expression of chemokines including Ccl2, Ccl3 and Cxcl2 after alcohol feeding compared with USF mice (Supplemental Figure 6).
Figure 2. Supplementation of saturated fatty acids reduces ethanol-induced liver disease.
C57BL/6 mice were fed saturated fatty acids (SF) or unsaturated fatty acids (USF) and received an intragastric isocaloric (n=3) or ethanol-containing (n=6–7) diet for 3 weeks. (A) Plasma levels of ALT. (B) Representative H&E stained liver sections. (C) Hepatic levels of triglyceride. (D) Representative Oil Red O-stained liver sections. (E) Hepatic levels of TBARS. (F) Representative liver sections stained for 4-HNE. *p<0.05.
Fecal total triglyceride content was similar between ethanol-fed SF and USF groups, indicating that there was no fat malabsorption (Supplemental Figure 7). Cecal concentrations of palmitic and stearic acid were, as expected, higher in the SF group, confirming adequate supplementation (Supplemental Figure 8). Total plasma levels of palmitic and stearic acid were slightly lower in the SF than the USF group (Supplemental Figure 9), as were plasma levels of total triglycerides (data not shown), indicating that systemic lipid profiles were similar between groups.
To determine how LCFA feeding affects ethanol-induced liver injury, we investigated intestinal absorption and hepatic metabolism of ethanol. Plasma levels of ethanol were comparable between the SF and USF groups following 3 weeks of intragastric ethanol administration (Supplemental Figure 10A). Alcohol dehydrogenase (Adh) and cytochrome p450 enzyme 2E1 (CYP2E1) are main hepatic enzymes that metabolize ethanol and convert it to acetaldehyde4. Levels of Adh mRNA level did not differ significantly between the SF and USF groups (Supplemental Figure 10B). Microsomal CYP2E1 protein was similarly upregulated in both groups following ethanol administration (Supplemental Figure 10C). These results demonstrate that the ability of palmitic and stearic acid to reduce ethanol-associated liver disease does not involve changes in fat absorption or intestinal absorption or hepatic metabolism of alcohol. We propose that these LCFA restore intestinal metabolic homeostasis to prevent alcohol-associated liver disease.
Saturated LCFA prevent alcohol-induced gut leakiness
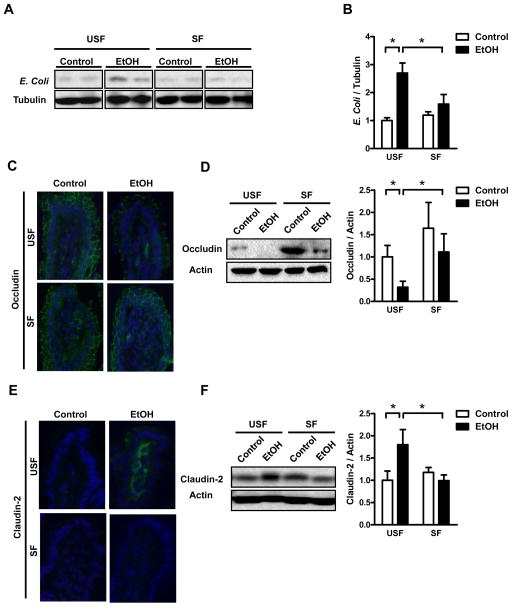
Development of alcoholic liver disease involves increased translocation of microbial products from the intestinal lumen to the liver, facilitated by a disruption of the epithelial barrier18. Following ethanol administration, levels of gut-derived and translocated Escherichia coli proteins increased significantly in liver tissues from USF but not SF mice (Figure 3A, B). Plasma endotoxin levels were significantly lower in alcohol-fed SF mice than alcohol-fed USF animals (Supplemental Figure 11). These results indicate that ethanol feeding increases bacterial translocation, and that palmitic and stearic acid block this process. Changes in levels of tight junction proteins contribute to paracellular leakage; we compared levels of intestinal tight junction proteins in small intestine tissues of mice from both groups. Following ethanol administration, levels of occludin decreased in ethanol-fed vs control mice. However, ethanol-fed mice in the SF group had significantly higher levels of occludin than those of the USF group (Figure 3C, D). Claudin-2 forms pores to increase barrier leakiness19. Levels of claudin-2 increased following ethanol feeding of USF mice, but not SF mice (Figure 3E, F).
Figure 3. Saturated fatty acid supplementation suppresses alcohol-induced intestinal leakiness.
C57BL/6 mice were fed saturated fatty acids (SF) or unsaturated fatty acids (USF) and received an intragastric isocaloric (n=3) or ethanol-containing (n=5–7) diet for 3 weeks. (A) Immunoblot analyses of hepatic tissues for E. coli. (B) Quantification of E. coli proteins normalized to α-tubulin. (C) Representative images of immunofluorescence analysis of occludin (green) in distal small intestine. Nuclei were stained with Hoechst (blue). (D) Immunoblot of occludin in the distal small intestine and quantification of occludin protein, normalized to β-actin. (E) Representative images of immunofluorescence analysis of claudin-2 (green) in distal small intestine. Nuclei were stained with Hoechst (blue). (F) Immunoblot of claudin-2 in the distal small intestine and quantification of claudin-2 protein, normalized to β-actin. *p<0.05.
We investigated whether increased cell death of small intestinal enterocytes contributed to bacterial translocation, but did not find a significant increase in apoptosis of the lining epithelial cells between control and alcohol mice in both groups (Supplemental Figure 12A). The morphology of intestinal villi was normal in all groups (Supplemental Figure 12B). Alcohol induces intestinal inflammation which could contribute to increased intestinal permeability20. SF feeding reduced intestinal inflammation as characterized by lower gene expression of Ccl2 and TNFα in the ileum (Supplemental Figure 13A, B). The number of TNFα+ monocytes and macrophages was significantly decreased in the ileum of SF mice as compared to USF mice following alcohol feeding (Supplemental Figure 13C–F). Therefore, the difference between ethanol-fed USF and SF groups appeared to be stabilized gut barrier, reduced intestinal inflammation and reduced microbial translocation in the SF group. These factors might protect the SF mice from ethanol-induced liver injury.
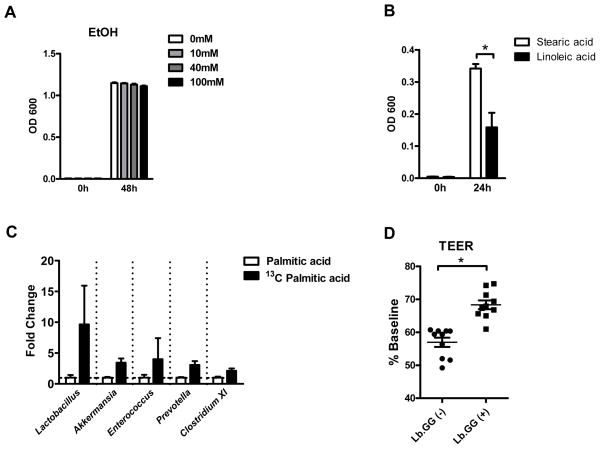
To investigate whether saturated LCFA have a direct effect on intestinal epithelial tight junctions, we used differentiated intestinal epithelial cells to functionally analyze tight junction dynamics. Acetaldehyde, a product of ethanol metabolism, disrupts tight junctions. We analyzed tight junction function in cultured Caco-2 cells by measuring transepithelial electrical resistance (TEER). Incubation of cells with acetaldehyde along with unsaturated fatty acids (oleic acid and linoleic acid) or saturated fatty acids (palmitic acid and stearic acid) did not restore TEER after acetaldehyde exposure (Supplemental Figure 14). The ability of the saturated LCFA palmitic and stearic acid to stabilize the intestinal barrier in mice therefore does not appear to involve direct stabilization of intestinal epithelial cell tight junctions.
Eubiosis is restored following supplementation with saturated LCFA
Our findings indicate that dietary supplementation with saturated LCFA protects the liver through its effects in the intestine. Chronic ethanol administration results in intestinal bacterial overgrowth and dysbiosis, which are associated with intestinal inflammation and dysfunction of the intestinal barrier20. To determine whether saturated LCFAs affect the composition of the intestinal microbiome, we analyzed changes in the microflora by 16S ribosomal RNA (rRNA) gene sequencing.
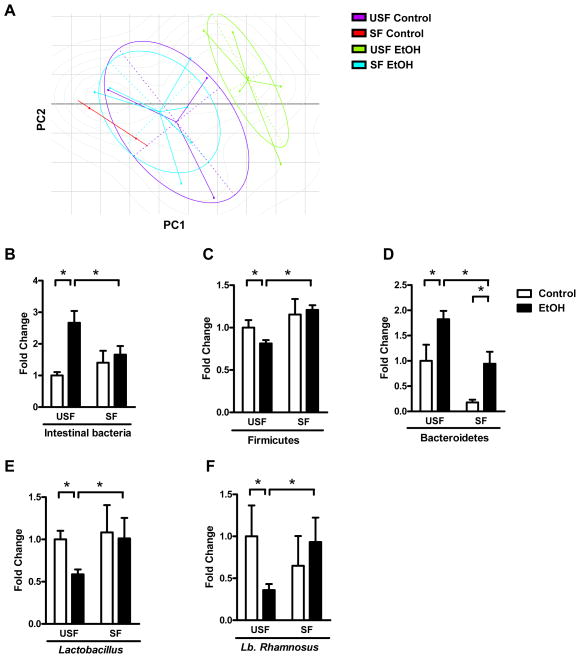
Comparisons of bacterial 16S rRNA data sets demonstrated that in the USF group, the microbiota of ethanol-fed mice clustered separately from that of control mice, based on principal component analysis (PCA); these findings are consistent with previous publications3. However, in the SF group, the microflora of ethanol-fed and control mice did not cluster separately. In fact, the microbiota of ethanol-fed mice in the SF group overlapped significantly with that of the control mice in the USF group (Figure 4A, Supplemental Figure 15). These findings indicate that dietary supplementation with saturated LCFA prevents changes in the composition of intestinal microbiota following chronic ethanol exposure. These findings were confirmed using qPCR with 16S rRNA-specific primers.
Figure 4. Supplementation of diet with saturated fatty acid reverses alcohol-induced dysbiosis.
C57BL/6 mice were fed saturated fatty acids (SF) or unsaturated fatty acids (USF) and received intragastric isocaloric (n=2–3) or ethanol-containing (n=5–7) diets for 3 weeks. (A) Clusters of groups, based on 16S rRNA gene sequence analysis. Principal component analysis was performed on weighted and normalized P1 vs P2 UniFrac distances. PC: principal component. (B–F) Fold-differences between groups in proportions of the intestinal bacteria Firmicutes, Bacteroidetes, total Lactobacillus species and Lactobacillus (Lb.) rhamnosus in cecum of mice. *p<0.05.
We found that ethanol administration results in intestinal bacterial overgrowth, reduced proportions of the phylum Firmicutes, increased numbers of the phylum Bacteroidetes, and reduced proportions of Lactobacillus species and Lactobacillus rhamnosus. These findings are consistent with previous reports that chronic ethanol administration induces dysbiosis in mice3. Here, we report that administration of saturated LCFA reverses most of these effects (Figure 4B–F), to restore intestinal eubiosis.
Lactobacillus metabolizes saturated LCFA
Lactobacillus species have important roles in the pathogenesis of alcoholic liver disease. The probiotic Lactobacillus rhamnosus GG prevents ethanol-induced steatohepatitis in rodents12, 13. Prebiotics increase numbers of commensal Lactobacillus species and reduce ethanol-induced liver damage in mice3. However, ethanol does not affect the growth of Lactobacillus rhamnosus GG in culture (Figure 5A), so the lower levels of lactobacilli observed in the intestines of mice after chronic ethanol feeding is not a direct effect. Lactobacilli might, however, metabolize saturated fatty acids. Accordingly, the lower levels of saturated fatty acids in the intestine after chronic ethanol administration might limit the proliferation of lactobacilli.
Figure 5. Lactobacillus rhamnosus metabolizes saturated fatty acids in vivo and prevents disruption of monolayers of Caco-2 cells by acetaldehyde.
(A) Effect of ethanol on Lactobacillus rhamnosus growth in vitro (n = 3). (B) Effect of fatty acids on Lactobacillus rhamnosus growth in vitro (n = 4). (C) qPCR amplification of extracted C13-labeled DNA following gavage with unlabeled palmitic acid or [1-13C] palmitic acid in vivo (n = 5–6). (D) TEER of monolayers of polarized Caco-2 cells incubated with acetaldehyde in the absence or presence of supernatants from cultures of Lactobacillus rhamnosus (n = 10). *p<0.05.
To test this hypothesis, Lactobacillus rhamnosus GG were cultured, anaerobically, in medium containing either stearic acid (the saturated fatty acid given to the SF group) or linoleic acid (the unsaturated fatty acid given to the USF group). Consistent with our hypothesis, medium containing stearic acid increased proliferation of Lactobacillus rhamnosus GG, compared with linoleic acid (Figure 5B). Stable isotope tracing studies provided further support for the concept that Lactobacillus species requires saturated LCFA in vivo. Only actively metabolizing and dividing microbes incorporate heavy isotope tracers into their DNA. Mice were gavaged with 13C-labeled palmitic acid once daily for 3 consecutive days, and DNA that contained 13C was extracted from luminal contents of whole small intestine and cecum. Analysis by qPCR showed strong amplification of Lactobacillus genomic DNA, indicating that Lactobacillus species metabolizes palmitic acid in vivo. Other intestinal bacteria such as Akkermansia, Enterococcus, Prevotella and Clostridium cluster XI also had some ability to metabolize palmitic acid, but at a much lower level than lactobacilli (Figure 5C).
To link dysbiosis with intestinal barrier function, we tested the effects of supernatant from lactobacilli cultures on differentiated intestinal epithelial cells. Supernatant from cultures of Lactobacillus rhamnosus GG maintained in MRS broth, protected monolayers of polarized Caco-2 cells against barrier disruption by acetaldehyde (Figure 5D). These findings indicate that ethanol-induced intestinal dysbiosis is characterized by lower proportions of Lactobacillus species, possibly because levels of saturated fatty acids are reduced. Lactobacilli produce factors that maintain tight barrier function in intestinal epithelial cells.
Alcohol abuse changes the intestinal metagenome and metabolome in humans
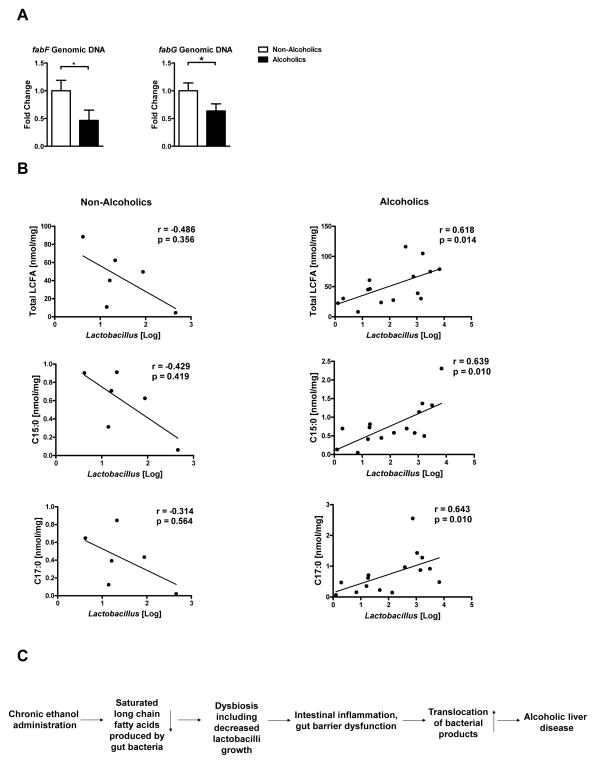
To determine how our findings relate to humans, we amplified bacterial genes involved in fatty acid biosynthesis in fecal samples of non-alcoholic volunteers and patients with active alcohol abuse (Supplemental Table 3). Although fabD could not be amplified from human feces, the proportions of the genes fabF and fabG were significantly lower in feces from patients with active alcohol abuse than controls (Figure 6A). Levels of lactobacilli have been reported to be reduced in fecal samples from patients with liver disease8. We therefore measured amounts of fecal lactobacilli and LCFA. Total levels of LCFA, C15:0 and C17:0 (two LCFA produced by only bacteria) are significantly correlated with those of lactobacilli in fecal samples from patients with active alcohol abuse but not in controls (Figure 6B). These findings support a model in which chronic alcohol abuse reduces the capacity of the microbiome to synthesize saturated fatty acids, leading to reductions in intestinal Lactobacillus species.
Figure 6. Alcohol abuse decreases bacterial genes that regulate fatty acid synthesis in humans.
(A) Real-time PCR of genomic DNA for 3-oxoacyl-[acyl-carrier-protein] synthase II (fabF) and 3-oxoacyl-[acyl-carrier protein] reductase (fabG) in stool samples of healthy individuals (n = 6) and patients with alcohol abuse (n = 7–8). (B) Total fecal levels of LCFA, C15:0 and C17:0 were correlated with fecal amounts of Lactobacillus species in non-alcoholic individuals (left column; n= 6) and patients with active alcohol abuse (right column; n= 15). One patient sample was excluded from the analysis due to the very high amount of fecal lactobacilli and it was considered as true outlier. (C) Schematic representation of the proposed model: Chronic ethanol feeding reduced the capacity of intestinal bacteria to synthesize saturated LCFA in mice and humans. Dietary supplementation with saturated LCFA maintains eubiosis by preventing ethanol-induced overgrowth of intestinal bacterial and by increasing intestinal levels of probiotic lactobacilli. These bacteria appear to produce factors that promote intestinal barrier integrity. Reduced translocation of bacterial products decreases ethanol-induced liver damage in mice. *p<0.05.
Discussion
Ethanol-induced liver disease is associated with intestinal dysbiosis in animal models and humans3, 7. A prominent feature of alcohol abuse is disruption of the intestinal barrier, leading to translocation of microbial products to the liver. The mechanisms by which alcohol disrupts the intestinal barrier to contribute to alcoholic liver disease are not known. We used metagenomic and metabolomic analyses to investigate the roles of the microbiota in a mouse model of alcoholic liver disease. Chronic ethanol administration reduced the capacity of intestinal bacteria to synthesize saturated LCFA in mice and humans. Dietary supplementation with saturated LCFA and hence restoration of metabolic homeostasis reduced ethanol-induced liver damage in mice. It restored eubiosis by preventing ethanol-induced overgrowth of intestinal bacterial and by increasing intestinal levels of probiotic lactobacilli. These bacteria appear to produce factors that promote intestinal barrier function.
Mice that are protected from bacterial overgrowth were reported to have lower systemic levels of bacterial products and decreased ethanol-induced liver disease, despite leakier guts4. Similarly, rats fed Lactobacillus rhamnosus GG, which increases the load of health-associated bacteria in the intestine and restores eubiosis, develop less-severe liver disease following ethanol administration13. Our findings support the concept that restoration of eubiosis protects against alcohol-induced liver disease.
Saturated fatty acids do not act directly on the intestine to stabilize its barrier. Instead, we showed that commensal lactobacilli metabolize saturated LCFA, in vivo and in cultured cells, to promote their expansion. Saturated fatty acids were reported to serve as vitamin B substitute and promote growth of Lactobacillus species21. Chronic ethanol intake might therefore decrease levels of Lactobacillus species by reducing intestinal levels of saturated fatty acids. Interestingly, in contrast to LCFA, SCFA do not seem to be beneficial for alcoholic liver disease. Although supplementation with butyrate protects from intestinal tight junction disruption, it does not prevent steatohepatitis after chronic alcoholic feeding22.
Lactobacillus might protect against liver damage by directly stabilizing the mucosal barrier. We observed that supernatant from Lactobacillus rhamnosus GG cultures protected monolayers of Caco-2 cells from tight junction disruption by acetaldehyde—the first metabolite of alcohol, which has been shown to induce intestinal leakage18. Lactobacillus casei Shirota restore neutrophil phagocytic capacity in alcoholics with cirrhosis23. L. rhamnosus GG downregulates LPS-induced proinflammatory mediators and decrease systemic inflammation in rats24. These antiinflammatory effects may be related to inhibition of NFκB activation in intestinal epithelial cells25. It is therefore feasible that lactobacilli exert their beneficial effect by inhibition of intestinal inflammation, which we have observed in SF mice. Another important potential protective mechanism of lactobacilli is through reducing oxidative stress. Alcohol ingestion results in hypoxia of intestinal epithelial cells, which in turn can cause intestinal integrity dysfunction through hypoxia-inducible factor (HIF) modulation. Lactobacillus rhamnosus GG increased HIF expression, attenuated alcohol-induced oxidative stress and maintained intestinal integrity12. Restoring intestinal levels of Lactobacilli might also suppress intestinal bacterial overgrowth. Lactobacilli secrete lactic acid to reduce intestinal pH26. A more acidic environment reduces the growth of the commensal microbiota27 and lowers the amount of luminal bacterial products that can translocate to the liver.
Intestinal permeability increases following chronic alcohol administration. Subsequently, bacterial products translocate from the intestinal lumen to extraintestinal spaces including the systemic circulation and liver. As shown in our study, SF mice showed lower plasma endotoxin and less E.coli proteins in the liver following ethanol feeding. LPS binds to TLR4 on parenchymal and non-parenchymal liver cells resulting in hepatic inflammation and hepatocyte death28, 29. TLR4 is a crucial mediator of experimental alcoholic liver disease10. Stabilizing the gut barrier and decreasing translocation of bacterial products inhibits hepatic expression of inflammatory mediators as shown in our study. Other bacterial products might translocate from the gut to the liver as well, and bind to hepatic TLRs which contribute to alcoholic liver disease. Overall, we propose that probiotic lactobacilli use saturated fatty acids for growth. Lactobacilli, in turn, stabilize the mucosal barrier to prevent translocation of microbial products that contribute to development of liver disease. Blocking translocation of bacterial ligands from the intestine to extraintestinal organs or blocking hepatic TLR signaling inhibits alcoholic liver disease, and these are established pathways resulting in alcoholic liver disease (Figure 6C).
Our findings indicate that alterations in metabolism by the intestinal microbiota contribute to the pathogenesis of alcoholic liver disease. Although studies have described the protective effects of different dietary mixtures of fats containing saturated fatty acids30–35, their relevance to human disease and mechanisms were not determined. We demonstrate the mechanisms by which alcohol disrupts the intestinal microbiome and its metabolome to contribute to liver disease. Diets or agents designed to increase intestinal concentrations of saturated LCFA or their production by the intestinal microbiome might be developed to treat alcohol-induced liver disease.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants K08 DK081830, R01 AA020703, U01 AA021856 and by ABMRF/The Foundation for Alcohol Research (to BS). RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology, a T. Franklin Williams Scholarship Award, Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and grant K23-DK090303. AK received support from NIH grants R01AA020216 and RC2AA019405. JSB is supported by NIH grant R01 AA020203. We thank Akiko Ueno and Raul Lazaro for performing animal studies at the Animal Core facility, Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (ALPD) and Cirrhosis, University of Southern California funded by the National Institute on Alcohol Abuse and Alcoholism (P50AA011999). This work utilized Core Services supported by grant DK097153 of NIH to the University of Michigan.
Abbreviations
- 4-HNE
4-hydroxynonenal
- Adh1
alcohol dehydrogenase 1
- ALT
alanine aminotransferase
- CYP2E1
cytochrome p450 enzyme 2E1
- E.coli
Escherichia coli
- LCFA
long chain fatty acids
- MCFA
medium chain fatty acids
- SCFA
short chain fatty acids
- TBARS
Thiobarbituric Acid Reactive Substances
- TEER
transepithelial electrical resistance
- VDAC1
Voltage-dependent anion-selective channel protein 1
Footnotes
None of the authors has a financial, personal or professional conflict of interest to disclose.
P.C. was responsible for acquisition of data, analysis and interpretation of data, and drafting of the manuscript; M.T., J.D., K.E.N. and D.E.F. were responsible for metagenomic analysis; D.E.F. was responsible for writing the manuscript. K.Z., M.E. and J.T. were responsible for bacterial cultures and stable isotope analysis; P.S., S.B.H., J.S.B., E.A.M. and A.K. were responsible for collection of human samples; J.P.P. was responsible for bacteria growth analysis; R.L. was responsible for statistical analysis; H.T. was responsible for intragastric feeding of mice; B.S. was responsible for the study concept and design, writing the manuscript, and study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim WR, Brown RS, Jr, Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Tilg H, Day CP. Management strategies in alcoholic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:24–34. doi: 10.1038/ncpgasthep0683. [DOI] [PubMed] [Google Scholar]
- 3.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–19. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–92. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–46. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu ZW, Lu HF, Wu J, Zuo J, Chen P, Sheng JF, et al. Assessment of the fecal lactobacilli population in patients with hepatitis B virus-related decompensated cirrhosis and hepatitis B cirrhosis treated with liver transplant. Microb Ecol. 2012;63:929–37. doi: 10.1007/s00248-011-9945-1. [DOI] [PubMed] [Google Scholar]
- 9.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–7. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 10.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 11.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–24. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866–75. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–72. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–7. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 16.Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7:771–81. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–33. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 18.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–4. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 19.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107:8011–6. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirpich IA, Feng W, Wang Y, Liu Y, Beier JI, Arteel GE, et al. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol. Alcohol. 2013;47:257–64. doi: 10.1016/j.alcohol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodicek E. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem J. 1945;39:78–85. doi: 10.1042/bj0390078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res. 2014;38:1489–501. doi: 10.1111/acer.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–51. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Li N, des Robert C, Fang M, Liboni K, McMahon R, et al. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr. 2006;42:545–52. doi: 10.1097/01.mpg.0000221905.68781.4a. [DOI] [PubMed] [Google Scholar]
- 25.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–87. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Drisko JA, Giles CK, Bischoff BJ. Probiotics in health maintenance and disease prevention. Altern Med Rev. 2003;8:143–55. [PubMed] [Google Scholar]
- 27.Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol. 1998;93:2097–101. doi: 10.1111/j.1572-0241.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 28.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–11. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 29.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–18. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanji AA, Sadrzadeh SM, Yang EK, Fogt F, Meydani M, Dannenberg AJ. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology. 1995;109:547–54. doi: 10.1016/0016-5085(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 31.Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao S, et al. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology. 1997;26:1538–45. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- 32.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–12. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 33.Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–46. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong W, Li Q, Xie G, Sun X, Tan X, Jia W, et al. Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G919–32. doi: 10.1152/ajpgi.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–77. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.