Abstract
In an effort to develop combination vaccines for biodefense, we evaluated a ricin subunit antigen, RiVax, given in conjunction with an anthrax protective antigen, DNI. The combination led to high endpoint titer antibody response, neutralizing antibodies, and protective immunity against ricin and anthrax lethal toxin. This is a natural combination vaccine, since both antigens are recombinant subunit proteins that would be given to the same target population.
Keywords: toxin, biodefense, vaccine, antigen
1. Introduction
The Department of Health and Human Services and the Centers for Disease Control and Prevention (CDC) have designated more than 30 biological agents and toxins as having the potential to pose a severe threat to human health should they be deliberately released into the public sphere (http://www.selectagents.gov/Select%20Agents%20and%20Toxins%20List.html). In many instances, especially in the case of toxins like ricin, the onset of morbidity can occur within a matter of hours and there may be little if any opportunity to intervene therapeutically [1]. In other instances, like the dissemination of anthrax spores through the US Postal Service, the scope of an event could overwhelm public health response capabilities. For this reason, there is a concerted effort by Public Health and Department of Defense officials to develop prophylactic vaccines that could be administered to at-risk populations, including emergency first responders, medical care staff, and laboratory personnel. With a number of candidate biodefense vaccines now in Phase I and II clinical trials [2], it is an opportune moment to consider the possibility of combination vaccines as a means to ultimately minimize the total number of injections and office visits necessary to achieve protective immunity against more than one pathogen or toxin.
Ricin is a ribosome-inactivating protein (RIP) and a member of the AB family of protein toxins [3]. Ricin’s enzymatic subunit (RTA) is an RNA N-glycosidase that cleaves ribosomal RNA, leading to protein synthesis arrest and cell death. Ricin’s binding subunit (RTB) mediates binding to cell surfaces via terminal galactose and N-acetyl galactosamine moieties on glycoproteins and glycolipids. Because of ricin’s recent history as a biothreat agent, the development of countermeasures, including a vaccine, are of significant public health importance [2, 4]. One of the leading vaccine antigen candidates is RiVax™, a recombinant derivative of RTA whose enzymatic activity has been largely eliminated through a point mutation in a key active site residue and also contains a mutation in the site attributed to the induction of vascular leak syndrome (VLS) [5]. Parenteral vaccination of RiVax, either as an adjuvant-free formulation or adsorbed to aluminum salts, elicits RTA-specific serum IgG antibodies that are associated with both systemic and mucosal immunity to ricin in mice and non-human primates (C. Roy and R. Brey, unpublished results)[6–8]. Furthermore, the results of two Phase I clinical trials have indicated that RiVax is safe and immunogenic in humans[9, 10]. A second RTA-based antigen known as RVEc is also in Phase I clinical trials and has been shown to be as effective as RiVax in eliciting immunity to ricin i n a mouse model [11]. However, because of the ready availability of GMP-grade RiVax, we chose RiVax over RVEc for this specific study.
When considering a putative combination vaccine for biodefense, we postulated that RiVax could be combined with a recombinant protective antigen (PA) vaccine antigen aimed at eliciting immunity to Bacillus anthracis infection. PA is an 83 kDa protein secreted by B. anthracis that forms hepatmers on host cell surfaces and then non-covalently assembles with two other secreted bacterial proteins, edema factor (EF) and lethal factor (LF), to form edema toxin (ET) and lethal toxin (LT), respectively. ET and LT are the major virulence determinants of B. anthracis and blocking their action is essential in counteracting the effects of inhalational anthrax [12]. Indeed, PA is one of the primary antigenic components of the currently licensed anthrax vaccine known as Biothrax™, which consists of formalin-fixed culture filtrates of a nonencapsulated strain of B. anthracis that have been adsorbed to aluminum salts adjuvant. With the impending phase out of Biothrax™ in favor of more defined vaccine formulations, there are ongoing efforts to identify recombinant derivatives of PA that are safe (i.e., minimally reactogenic), immunogenic and stable [13]. One possible candidate is dominant negative inhibitor (DNI) or VeloThrax™, which is a derivative of PA with two point mutations (K397D, D425K) that impede the capacity of the protein to undergo conformational changes necessary for translocation of EF and LF into host cells [14]. DNI has been shown in mice to be effective at inducing the onset of PA-specific ET and LT neutralizing antibodies [14]. Moreover, clinical grade lots of DNI are remarkably stable in lyophilized form at high temperatures [15]. Thus, DNI constitutes a promising candidate antigen for a second-generation anthrax vaccine.
2. Results and Discussion
To assess the feasibility of a RiVax-DNI combination vaccine, groups of female BALB/c mice, 6–8 weeks of ages (n=10/group; Jackson Laboratories, Bar Harbor, ME) were primed on day 0 and then boosted on day 14 with RiVax (10 µg), DNI (10 µg), or a combination of RiVax and DNI (10 µg + 10 µg), administered by intraperitoneal injection. Lyophilized DNI (batch 803918A; Baxter Pharmaceutical Solutions LLC, Bloomington, IN) was reconstituted in sterile water [15]. RiVax (batch 190-100L-GLP-FF-090105) was stored at −20°C in 50% glycerol and 50% histidine buffer (10mM histidine, 144mM NaCl, pH 6.0) [16]. Each antigen was adsorbed to Alhydrogel® (0.85 mg/mL; InvivoGen, San Diego, CA) in histidine buffer for 3 h at 4°C prior to immunization. For the combination vaccine, DNI and RiVax were each adsorbed to Alhydrogel independently and then mixed 1- to-1 (v/v) just prior to injection. Following the second immunization, each group of animals (RiVax, DNI, and dual) was arbitrarily divided into two groups, A and B. The A groups were ultimately challenged with ricin, while the B groups were challenged with LT. Sera were collected from all immunized mice via the lateral tail veins on days 20 and 200, representing snap shots of the early and late (“memory”) responses to the vaccines. All animal experiments were done in strict compliance with protocols approved by the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC). Log transformed paired and unpaired t tests and the Mantel-Cox test were conducted with Graphpad Prism version 5.0 (San Diego, CA).
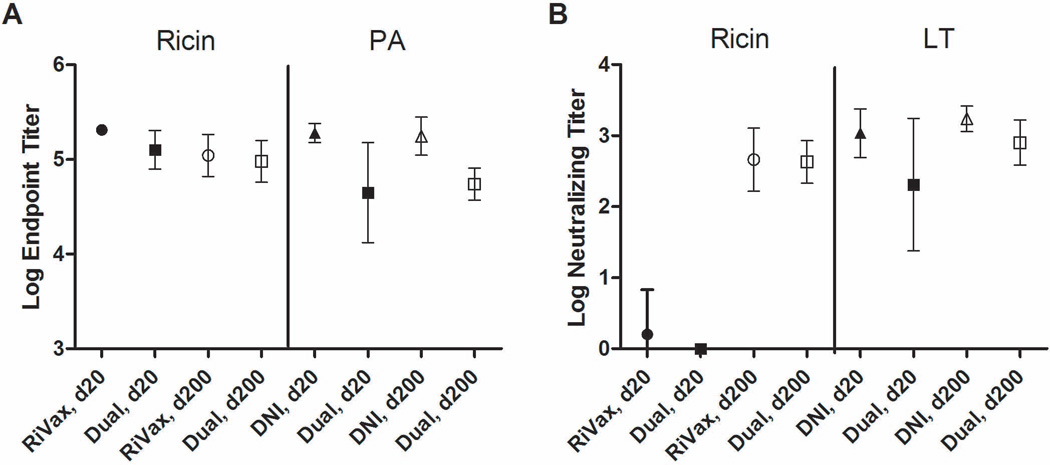
Analysis of sera collected on day 20 indicated that the RiVax-vaccinated mice had anti-ricin holotoxin IgG geometric mean titers (GMT) of >2.0 ×105 and, as expected, no detectable serum antibodies against PA (Figure 1A; Table 1; Table S1). On day 200, the ricin-specific IgG GMT had declined by roughly half, as compared to day 20. On day 20, DNI-vaccinated mice had anti-PA GMT of 1.8 ×105, and no detectable serum antibodies against ricin. Analysis of day 200 sera indicated that PA-specific antibody titers in DNI-vaccinated were unchanged, and in some mice slightly increased, as compared to day 20. Finally, the dual RiVax-DNI vaccinated mice had both ricin- and PA-specific serum antibodies on days 20 and 200, indicating that the subunit antigens were not incompatible with each other (Figure 1A; Table 1; Table S1). However, on day 20, the ricin- and PA-specific serum IgG GMTs in the group of dual vaccinated mice were roughly half of that observed in mice immunized with the individual antigens, demonstrating that the magnitudes of the antibody responses were negatively affected (p<0.01) by the combination of the two antigens. By day 200, anti-ricin antibody titers in the dual immunized mice were still lower than in the mice that received RiVax only, but these differences were not statistically different (p=0.55). In contrast, anti-PA titers remained lower in the dual immunized mice, as compared to DNI immunized animals (p<0.0001), indicating that the immunogenicity of DNI is adversely affected when combined with RiVax.
Figure 1. Endpoint and toxin-neutralizing serum antibody titers elicited in mice following RiVax, DNI, and dual immunized mice.
Mice were immunized with RiVax, DNI, or the combination of RiVax and DNI adsorbed to aluminum salts on days 0 and 20, as noted in the text. Sera were collected on days 20 and 200 and examined for (A) ricin- and PA-specific IgG antibodies and (B) ricin and LT toxin-neutralizing activities. Symbols and error bars represent means and standard deviations.
Table 1.
Ricin- and LT-specific serum antibody geometric mean titers following immunization
| Ricina | PAa | |||
|---|---|---|---|---|
| Immunization | Day 20 | Day 200 | Day 20 | Day 200 |
| RiVax | 204800 | 109750 | 1 | 1 |
| DNI | 1 | 1 | 189619 | 175564 |
| Dual | 126069 | 95543 | 44572 | 54875 |
, coating antigen used in ELISAs, as described in the text;
, when no antigen-specific serum IgG titers were detectable, an arbitrary value of 1 was assigned for the purpose calculating the geometric mean titers.
We next assessed ricin- and LT toxin-neutralizing activities (TNA) elicited by RiVax, DNI, or the combination of RiVax and DNI vaccination regimens. Ricin TNA was determined using a Vero cell cytotoxicity assay [17]. LT-neutralizing activity was determined using a J774 murine macrophage cell-based assay [15, 18]. Neutralization activity was defined as the highest dilution of serum that protected ≥50% of the cells from toxin-induced death, a value commonly referred to as the TCIC50. In day 20 serum sa mples, the RiVax-vaccinated mice had no detectable TNA, with the exception of one animal that had a neutralizing titer of 100 (Figure 1B; Table 2; Table S1). On day 200, however, the neutralizing GMT in this group of animals was >450, with individual titers ranging from 100–1600. As expected, RiVax-immunized mice had no detectable serum LT-neutralizing activity at either day 20 or 200. DNI vaccination, on the other hand, was highly effective at eliciting the early onset of LT-neutralizing titers, as evidenced by neutralizing GMT of 1087 and 1728 on days 20 and 200, respectively (Figure 1B; Table 2; Table S1). No detectable ricin-neutralizing activity was evident in the sera of DNI-vaccinated mice at either time point, confirming the absence of cross-reactivity between RiVax and DNI.
Table 2.
Toxin-Neutralizing Titers in RiVax-, DNI- and Dual-Immunized Groups of Mice
| Day 20 – LT TNA | Day 200 – LT TNA | Day 200 – Ricin TNA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Total | (A)a | (B) | Total | (A) | (B) | Total | (A) | (B) |
| RiVax | 1b | (1) | (1) | 1 | (1) | (1) | 459 | (528) | (400) |
| DNI | 1087 | (1213) | (951) | 1728 | (1600) | (1903) | 1 | (1) | (1) |
| Dual | 205 | (264) | (159) | 800 | (606) | (1056) | 429 | (696) | (264) |
, Each group of mice (left column) was arbitrarily divided into two subsets, A and B. Shown are the neutralizing titers from sera collected on day 20 or 200 from the all mice in a group (as “Total”) or as the specific A and B subsets;
, mice lacking detectable toxin-neutralizing serum antibody titers were arbitrarily assigned a value of 1 for the purpose of statistical analysis.
The combination of RiVax and DNI was successful at eliciting TNA against both ricin and LT, although the magnitude of the LT response was significantly dampened as compared to mice that received DNI alone (p<0.05; Table 2; Figure 1B; Figure S1). Specifically, the LT-neutralizing GMT in the sera of dual immunized mice was 205 and 800 on days 20 and 200, respectively, as compared to 1087 and 1728 in the sera of mice that received only DNI. The reduction in TNA in the dual immunized mice was proportional to the total PA-specific antibodies, suggesting that the combination vaccine was simply less potent at eliciting PA-specific antibody titers and not altered in its capacity to elicit TNA per se. Interestingly, the combination vaccine did not affect the onset of ricin toxin neutralizing antibodies, as evidenced by the fact that on day 200 the ricin-specific TNA were virtually identical between the RiVax and dual immunized groups of mice (GMT 459 versus 429, respectively; p=0.86). It should be underscored that neither the RiVax or dual immunized groups of mice had any detectable ricin-specific TNA on day 20 (Table 2; Figure 1B)
We next wished to determine whether the dual RiVax and DNI immunization regimen was sufficient to protect mice against lethal dose toxin challenges. Each group of mice that had been immunized with RiVax, DNI, or the combination of RiVax and DNI was randomly divided into two groups (n= 5 per group), A or B, and then challenged on day 202 with 10×LD50 ricin (0.1 mg/kg; Group A) or 2.5×LD100 of lethal toxin (1:1 PA:LF by weight; Group B). Challenge doses of ricin (10×LD50) and LT (2.5×LD100) were based on previous studies from our laboratory and other laboratories [11, 18, 19] and validated (whenever possible) with pilot studies (Figure S1)[11, 18]. As shown in Table 3 and Table S1, RiVax-immunized mice survived ricin toxin challenge, but not LT challenge. Conversely, DNI- immunized mice were protected from LT, but succumbed to ricin toxin. Among the mice that received the combination of RiVax and DNI, 5 of 5 survived ricin challenge, whereas 4 out of 5 survived LT challenge (Figure S2). The single dual-immunized mouse that succumbed to LT challenge had the lowest LT-neutralizing titer (i.e., 200) of all 20 mice that received DNI, suggesting that death was due to the effects of the toxin and not an experimental aberration. As expected, mice that received vehicle only (i.e., aluminum salts) succumbed to ricin and LT challenges (Table S1).
Table 3.
Mouse survival following primary and secondary toxin challenges
| 1° CHLa | 2° CHL | ||
|---|---|---|---|
| Groupb | Ricin | LT | Ricin |
| RiVax A | - | 0/5 | - |
| B | 5/5* | - | - |
| DNI A | - | 5/5* | 0/5 |
| B | 0/4c | - | - |
| Dual A | - | 4/5 | 4/4* |
| B | 5/5 | - | - |
| Vehicle A | - | 0/5 | - |
| B | 0/5 | - | - |
CHL, challenge;
Each group of mice (left column) was arbitrarily divided into two subsets, A and B. The A subset received a primary challenge with LT, while the B subset (shaded) received a primary challenge with ricin. The DNI-immunized and the dual-immunized A subsets of animals that survived LT challenge received a secondary challenge with ricin;
A single mouse in this group of animals died of unknown causes prior to the animal’s second immunization.
, indicates statistical significance (p<0.05) based on the Mantel-Cox test.
Finally, to further interrogate the efficacy of the combination vaccine, the four mice that had been immunized with the RiVax and DNI combination and that survived LT challenge were then secondarily challenged two weeks later (day 216) with 10×LD50 ricin. As controls, the DNI-immunized mice that survived a primary LT challenge were also secondarily challenged with ricin. All four dual-immunized mice survived ricin challenge, while all DNI-immunized mice succumbed to ricin intoxication, thereby demonstrating that the combination vaccine did in fact confer specific immunity to both LT and ricin toxins. It should be noted that we also attempted the inverse experiment: mice that had been immunized with the dual vaccine and then challenged with ricin were secondarily subject to an LT challenge. Unfortunately, the challenge was unsuccessful due to insufficient potency of a new lot of LT (data not shown).
In summary, we have demonstrated that DNI and RiVax antigens can successfully be combined following adsorption to aluminum salts adjuvant, into a single vaccine that is capable of eliciting protective immunity to ricin and anthrax LT in mice. The combination vaccine generally resulted in high endpoint serum IgG antibody titers to each antigen, as well as toxin-neutralizing antibodies, which constitute the two critical determinants associated with protective immunity to ricin and B. anthracis. However, the combination of RiVax and DNI did result in significantly lower antitoxin serum antibody titers as compared to mice that received the vaccines individually. While the differences in anti-ricin titers between RiVax alone and dual-immunized mice were not significant on day 200, the differences in anti-PA titers persisted. Moreover, a single mouse that received the combination vaccine succumbed to LT challenge, indicating that the resultant immunity to LT was compromised to some degree when combined with RiVax. It will be critical to determine whether the combination vaccine is in fact sufficient to elicit serum antibodies levels capable of providing protection against an actual B. anthracis spore challenge.
It is unclear whether the dampened antibody response to DNI when combined with RiVax was a result of immunological interference (e.g., B or T cells competing for similar epitopes on RiVax and DNI) or antigen saturation at the level of processing or presentation [20–23]. Considering DNI and RiVax are not similar at the primary sequence level, it is unlikely that direct interference accounts for the difference in serum antibody titers. To address the issue of antigen overload, it will be critical to perform comprehensive dose-response and time course studies with DNI, RiVax and the combination to determine what actually constitutes antigen saturation in this model and at what time points toxin-neutralizing antibodies reach their maximal titers. Finally, it is imperative to examine what effect (if any) the combination of antigens has on the biophysical properties (e.g., deamidation or unfolding) and/or relative bioavailability of DNI or RiVax, which in turn may influence the onset of antigen-specific antibody responses [16, 24–26].
An interesting facet of the data presented in this report is the notable difference in the onset of toxin-neutralizing antibodies following DNI and RiVax immunizations. On day 20, which corresponds to 6 days after the booster immunization, 95% (19/20) of the mice administered DNI had detectable LT-neutralizing antibodies, whereas on the same day only 5% (1/20) of the RiVax-immunized mice had detectable ricin toxin-neutralizing antibodies. By day 200, toxin-neutralizing antibodies were detected in all DNI and RiVax immunized animals. It is interesting to speculate that the threshold for eliciting neutralizing antibodies may be lower for PA than RTA, due to different mechanisms by which antibodies neutralize LT and ricin. In other words, it may be easier to neutralize LT than ricin. For example, anti-PA antibodies have been shown to neutralize LT by at least five different mechanisms, including interference with receptor attachment, inhibition of furin-mediated cleavage of PA, blocking PA heptamerization or EF/LF engagement, and interruption of pore formation in the endosomal membrane [27, 28]. In contrast, anti-RTA antibodies do not affect toxin attachment or internalization, but rather interfere with intracellular toxin trafficking [29–31]. Moreover, there is evidence to suggest there are only a limited number of “neutralizing” epitopes on the surface of RTA [32], which is in contrast to PA, where neutralizing epitopes have been identified on each of PA’s four domains. If our model is correct, then efforts to accelerate the onset of ricin toxin-neutralizing antibodies may need to be aimed on “focusing” the antibody response to the most relevant epitopes on the surface of RTA [33, 34]. In addition, there may be benefits to complexing RiVax with RTB as a means to elicit toxin-neutralizing antibodies that interfere with ricin-receptor interactions. These studies are ongoing in the laboratory.
Supplementary Material
Highlights.
Evaluated a combination vaccine for ricin and anthrax adsorbed to aluminum salts.
The combination vaccine elicited neutralizing antibodies to ricin and lethal toxin
Mice immunized with combination vaccine were immune to ricin and lethal toxin challenge
Long lasting immunity was achieved after only two immunizations
The combination vaccine may prove useful for biodefense
Acknowledgments
We would like to thank Dr. Karen Chave and Li Zhong of the Wadsworth Center’s Protein Expression Core facility for providing the recombinant PA and lethal factor used in this study. We would also like to acknowledge Dr. Erin Sully for her assistance with animal studies. This work was supported by National Institutes of Health grant U01-A1-08-2210 (Robert N. Brey, principal investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294:2342–2351. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe DN, Florence W, Bryant P. Current biodefense vaccine programs and challenges. Human vaccines & immunotherapeutics. 2013:9. doi: 10.4161/hv.24063. [DOI] [PubMed] [Google Scholar]
- 3.Mantis NJ. Ricin Toxin. In: Liu D, editor. Manual of Security Sensitive Microbes and Toxins. CRC Press; 2014. p. 1024. [Google Scholar]
- 4.Reisler RB, Smith LA. The need for continued development of ricin countermeasures. Adv Prev Med. 2012;2012:149737. doi: 10.1155/2012/149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smallshaw JE, Vitetta ES. Ricin Vaccine Development. Curr Top Microbiol Immunol. 2012;357:259–272. doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- 6.Mantis NJ, Morici LA, Roy CJ. Mucosal Vaccines for Biodefense. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neal LM, McCarthy EA, Morris CR, Mantis NJ. Vaccine-induced intestinal immunity to ricin toxin in the absence of secretory IgA. Vaccine. 2011;29:681–689. doi: 10.1016/j.vaccine.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25:7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006;103:2268–2273. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitetta ES, Smallshaw JE, Schindler J. A Small Phase IB Clinical Trial of an Alhydrogel- Adsorbed Recombinant Ricin Vaccine (RiVax) Clin Vaccine Immunol. 2012 doi: 10.1128/CVI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Hara JM, Brey RN, Mantis NJ. Comparative Efficacy in Mice of Two Lead Candidate Ricin Toxin A Subunit (RTA) Vaccine. Clinical and Vaccine Immunology. 2013 doi: 10.1128/CVI.00098-13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Moayeri M, Leppla SH. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 2014;22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedlander AM, Little SF. Advances in the development of next-generation anthrax vaccines. Vaccine. 2009;27(Suppl 4):D28–D32. doi: 10.1016/j.vaccine.2009.08.102. [DOI] [PubMed] [Google Scholar]
- 14.Yan M, Roehrl MH, Basar E, Wang JY. Selection and evaluation of the immunogenicity of protective antigen mutants as anthrax vaccine candidates. Vaccine. 2008;26:947–955. doi: 10.1016/j.vaccine.2007.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer V, Hu L, Schante CE, Vance D, Chadwick C, Jain NK, et al. Biophysical characterization and immunization studies of dominant negative inhibitor (DNI), a candidate anthrax toxin subunit vaccine. Human vaccines & immunotherapeutics. 2013;9:2362–2370. doi: 10.4161/hv.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peek LJ, Brey RN, Middaugh CR. A rapid, three-step process for the preformulation of a recombinant ricin toxin A-chain vaccine. J Pharm Sci. 2007;96:44–60. doi: 10.1002/jps.20675. [DOI] [PubMed] [Google Scholar]
- 17.Neal LM, O'Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010;78:552–561. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly-Cirino CD, Mantis NJ. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect Immun. 2009;77:4859–4867. doi: 10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moayeri M, Wiggins JF, Leppla SH. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect Immun. 2007;75:5175–5184. doi: 10.1128/IAI.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 21.Denoel PA, Goldblatt D, de Vleeschauwer I, Jacquet JM, Pichichero ME, Poolman JT. Quality of the Haemophilus influenzae type b (Hib) antibody response induced by diphtheria-tetanus-acellular pertussis/Hib combination vaccines. Clin Vaccine Immunol. 2007;14:1362–1369. doi: 10.1128/CVI.00154-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskola J, Olander RM, Hovi T, Litmanen L, Peltola S, Kayhty H. Randomised trial of the effect of co-administration with acellular pertussis DTP vaccine on immunogenicity of Haemophilus influenzae type b conjugate vaccine. Lancet. 1996;348:1688–1692. doi: 10.1016/S0140-6736(96)04356-5. [DOI] [PubMed] [Google Scholar]
- 23.Reisler RB, Gibbs PH, Danner DK, Boudreau EF. Immune interference in the setting of same-day administration of two similar inactivated alphavirus vaccines: eastern equine and western equine encephalitis. Vaccine. 2012;30:7271–7277. doi: 10.1016/j.vaccine.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Compton JR, Legler PM, Clingan BV, Olson MA, Millard CB. Introduction of a disulfide bond leads to stabilization and crystallization of a ricin immunogen. Proteins. 2011;79:1048–1060. doi: 10.1002/prot.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Souza AJ, Mar KD, Huang J, Majumdar S, Ford BM, Dyas B, et al. Rapid deamidation of recombinant protective antigen when adsorbed on aluminum hydroxide gel correlates with reduced potency of vaccine. J Pharm Sci. 2013;102:454–461. doi: 10.1002/jps.23422. [DOI] [PubMed] [Google Scholar]
- 26.Verma A, McNichol B, Dominguez-Castillo RI, Amador-Molina JC, Arciniega JL, Reiter K, et al. Use of site-directed mutagenesis to model the effects of spontaneous deamidation on the immunogenicity of Bacillus anthracis protective antigen. Infect Immun. 2013;81:278–284. doi: 10.1128/IAI.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froude JW, 2nd, Thullier P, Pelat T. Antibodies against anthrax: mechanisms of action and clinical applications. Toxins (Basel) 2011;3:1433–1452. doi: 10.3390/toxins3111433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mechaly A, Levy H, Epstein E, Rosenfeld R, Marcus H, Ben-Arie E, et al. A novel mechanism for antibody-based anthrax toxin neutralization: inhibition of prepore-to-pore conversion. J Biol Chem. 2012;287:32665–32673. doi: 10.1074/jbc.M112.400473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Hara JM, Yermakova A, Mantis NJ. Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol. 2012;357:209–241. doi: 10.1007/82_2011_193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hara JM, Mantis NJ. Neutralizing Monoclonal Antibodies against Ricin's Enzymatic Subunit Interfere with Protein Disulfide Isomerase-Mediated Reduction of Ricin Holotoxin In Vitro. J Immunol Methods. 2013 doi: 10.1016/j.jim.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song K, Mize RR, Marrero L, Corti M, Kirk JM, Pincus SH. Antibody to ricin a chain hinders intracellular routing of toxin and protects cells even after toxin has been internalized. PLoS One. 2013;8:e62417. doi: 10.1371/journal.pone.0062417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA) Immunol Lett. 2014;158:7–13. doi: 10.1016/j.imlet.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dormitzer PR, Grandi G, Rappuoli R. Structural vaccinology starts to deliver. Nat Rev Microbiol. 2012;10:807–813. doi: 10.1038/nrmicro2893. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph MJ, Vance DJ, Cheung J, Franklin MC, Burshteyn F, Cassidy MS, et al. Crystal Structures of Ricin Toxin's Enzymatic Subunit (RTA) in Complex with Neutralizing and Non-Neutralizing Single-Chain Antibodies. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.