Abstract
The physiological ramifications of oral tolerance remain poorly understood. We report here that mice fed ovalbumin (OVA) exhibit oral tolerance to subsequent systemic immunization with OVA in adjuvant, and yet they clear systemic infection with a recombinant OVA-expressing strain of Salmonella enterica serovar Typhimurium better than unfed mice do. Mice fed a sonicated extract of S. enterica serovar Typhimurium are also protected against systemic bacterial challenge, and the protection is Th1 mediated, as feeding enhances clearance in interleukin-4-null (IL-4−/−) and IL-10−/− mice but not in gamma interferon-null (IFN-γ−/−) mice. When T-cell priming in vivo is tracked temporally in T-cell receptor-transgenic mice fed a single low dose of OVA, CD4 T-cell activation and expansion are restricted largely to mucosal lymphoid organs. However, T cells from spleens and peripheral lymph nodes of fed mice proliferate and secrete IFN-γ when restimulated with OVA in vitro, indicating the presence of primed T cells in systemic tissues following oral exposure to antigen. Nonetheless, oral tolerance can be observed in the fed mice as reduced recall responses following subsequent systemic immunization with OVA in adjuvant. Soluble OVA administered systemically has similar effects in vivo, and the “tolerance” seen in both cases can be partially reversed if the initial priming is made more immunogenic. Together, the results indicate that antigen exposure under poor adjuvantic conditions, whether oral or systemic, may lead to T-cell commitment to effector rather than proliferative capabilities, necessitating a reassessment of therapeutic modalities for induction of oral tolerance in allergic or autoimmune states.
The epithelial surfaces of the gastrointestinal tract are exposed to the external environment and are the site of entry for many pathogenic microbes that often cause local or systemic infections and disease. The intestine is also home to a host of commensal microbes that constitute the normal flora of the gut, and some control of their number and diversity seems to prevent opportunistic infections (1, 32). Individuals appear to be immunologically tolerant to their own flora, and a break in tolerance to autologous flora can lead to inflammatory conditions such as colitis (5, 6). While the fine regulatory interactions between microbes and the mucosal immune system remain uncharacterized, it appears that microbial homeostasis in the intestine is the result of controlled immune responses against autologous flora (3, 22, 31, 36). The intestine is also the site where absorption of food occurs, and the poor immunogenicity of soluble antigens administered orally is considered to be due to local suppressive mechanisms that are in place to prevent potentially harmful immune responses against food antigens.
In the gut, protective immune responses are initiated in Peyer's patches (PPs), and many antigen-specific T cells primed here tend to selectively lodge in mucosal tissues (18). Although the systemic and mucosal immune systems are somewhat discrete, some antigens in the gut lumen can affect elements of both systems, and a striking example of gut antigens acting across the mucosal-systemic interface is oral tolerance, which manifests as systemic T-cell hyporesponsiveness of fed mice to a subsequent parenteral immunization (reviewed in reference 35). It is thought that oral tolerance might reflect the balance that is struck between desirable active immunity against microbial pathogens in the gut and undesirable responses against food antigens (14, 15, 23).
Simple nominal antigens such as ovalbumin (OVA) that are used for induction of experimental oral tolerance end up in the gut partly as a complex mixture of soluble material that includes microbial products and partly as particulate material adsorbed onto microbial surfaces, but the role of enteric flora and their products in oral tolerance induction remains unclear. On the one hand, it has been reported that oral tolerance cannot be induced in germfree mammals (41). On the other, sonicated extracts of normal gut commensal bacteria cannot induce oral tolerance, while similar extracts of nonenteric microbes can (9). Colonization of mice with bacteria expressing a tolerogenic dietary antigen abolishes induction of oral tolerance to the antigen, but adults that were colonized as neonates show tolerance to the dietary antigen as well as to microbial antigens (16). We have reported previously that mice fed a tolerogenic microbial sonicate are not significantly impaired in their ability to clear a challenge systemic infection with virulent bacteria and that mice fed a nontolerogenic sonicate show enhanced clearance of a homologous systemic challenge that is mediated by CD4 cells (9). Together, these results raised the possibility that soluble antigens encountered orally may induce an effector T-cell response that can protect mice against certain acute systemic bacterial infections.
In an effort to more clearly define the relationship between T-cell hyporesponsiveness and T-cell immunity that is induced by oral antigen, we generated a recombinant OVA-expressing strain of Salmonella enterica serovar Typhimurium so that tolerance to OVA and immunity to infection can be observed in mice fed the same antigen, OVA. Using transgenic mice, we also tracked the kinetics of T-cell activation in mucosal and systemic tissues following oral or systemic exposure to soluble antigen and subsequent systemic immunization with antigen in adjuvant. We report that soluble antigens may skew responding T cells towards subsets capable of effector capabilities rather than of proliferative capabilities upon antigen reencounter.
MATERIALS AND METHODS
Reagents.
OVA (Sigma), complete Freund's adjuvant (CFA) (Difco), the C-terminal (amino acids 323 to 339) peptide of OVA (Peptron; Yuseong-ku, Daejeon, South Korea), CyChrome-anti-CD4, fluorescein-anti-CD25, phycoerythrin (PE)-anti-CD69, fluorescein-anti-CD62L, PE/-CyChrome anti-CD44, and CyChrome-streptavidin (all from BD Biosciences); biotin KJ1-26 (12) (kindly provided by A. O'Garra, DNAX Research Institute); PE-streptavidin and fluorescein-streptavidin (Jackson ImmunoResearch); and streptavidin-horseradish peroxidase (Genzyme) were used.
Mice and immunizations.
Six- to 10-week-old BALB/c, C57BL/6, OT.II, and DO11.10 mice (The Jackson Laboratory), bred and maintained in the Small Animal Facility of the National Institute of Immunology, were used. DO11.10 × RAG−/− mice were a kind gift of J. Cebra, University of Pennsylvania, and DO11.10 mice, used in some experiments, were kindly provided by A. O'Garra, DNAX Research Institute. For priming with soluble antigen, mice were treated with 1 mg of OVA or maleylated OVA (maleyl-OVA) (33) in phosphate-buffered saline (PBS) intraperitoneally (i.p.) or with 1 to 10 mg OVA in 3.5% bicarbonate solution orally as indicated. For subcutaneous (s.c.) immunizations, 10 μg of OVA in CFA was injected into the hind footpad. Approval from the Institutional Animal Ethics Committee was obtained for all experimental procedures involving animals.
Bacteria and in vivo clearance assays.
Clinical isolates of S. enterica serovar Typhimurium (BRD058 and 754) (37) are routinely maintained in the laboratory. Bacterial stocks were stored in glycerol broth at −70°C, and a fresh aliquot was plated out on salmonella-shigella agar (Difco) for all experiments. For preparation of bacterial sonicate, an overnight culture of bacteria in Luria-Bertani broth (Difco) was spun down, washed in PBS, and inactivated by heating the cell suspension in a boiling water bath for 45 min. The suspension was sonicated for 15 min in PBS containing 10 mM phenylmethylsulfonyl fluoride (Sigma) as a protease inhibitor. The sonicate was spun at 100,000 × g for 60 min, and the supernatant was filtered and used as soluble antigen. For bacterial challenge experiments, mice were challenged i.p. with 103 CFU of S. enterica serovar Typhimurium, and 6 to 8 days later their spleens were harvested and appropriate dilutions of the lysate were plated out on salmonella-shigella agar. The number of bacteria was enumerated as CFU per spleen. The limit of detection was 50 CFU/spleen.
Generation of recombinant Salmonella expressing OVA constitutively.
The OVA gene was amplified out of plasmid pAc-neo-OVA, which has the complete OVA cDNA cloned under control of the human β-actin promoter (26), by using the following primers: sense, 5′-GGG CTC GAG AAA GCT GTA TAC AAC TTC GCT ACT ATG ATG GGC TCC ATC GGT GCA-3′; antisense, 5′-GGG AAG CTT GCT TAA ACA TGT TTT TAT TAT GAT TAA AGG-3′. The primers include an XhoI site to facilitate further cloning and the lymphocytic choriomeningitis virus (LCMV) epitopic peptide KAVYNFATM upstream of the OVA gene, the nucleotide sequence for which was derived from the protein sequence. The 1,840-bp amplified fragment was cloned into the XhoI and HindIII sites of Escherichia coli expression vector pGEXTAG (28), which has the glutathione S-transferase gene and a C-terminal c-myc tag fusion placed under control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible Tac promoter. This construct (pSTLCOvi) expresses the glutathione S-transferase-c-myc-LCMV-OVA fusion protein (∼70 kDa) upon IPTG induction. From this construct, the appropriate 1.9-kb BamHI/HindIII fragment containing the c-myc-LCMV-OVA-coding sequence was cloned into the constitutive expression vector pQE-60 (Qiagen) to generate construct pSTLCOVc. The T5 promoter of pQE-60 is recognized efficiently by Escherichia and Salmonella RNA polymerases, leading to constitutive cytoplasmic expression of the fusion protein (∼45 kDa) in both. Following transformation of S. enterica serovar Typhimurium BRD058, expression of OVA by the recombinant strain was confirmed by enzyme-linked immunosorbent assay. Briefly, 96-well plates were coated with 2 μg of rabbit anti-OVA (Rockland) per ml. Serial dilutions of bacterial sonicates from the two strains were then added, followed by horseradish peroxidase-coupled anti-OVA (Rockland), o-phenylenediamine, and hydrogen peroxide (Sigma). Color development in the assay is proportional to the amount of OVA present in each sonicate. The concentration of OVA in the sonicates was calculated from a standard curve set up in parallel with OVA, and the data were expressed as amount of OVA per microgram of total protein in the sonicate, estimated with micro-bicinchoninic acid reagents (Pierce).
T-cell assays.
Single cell suspensions of spleen and peripheral lymph nodes (PLN) were cultured in triplicate at 3 × 105 cells/well with graded doses of antigen in 200 μl of Click's medium (Irvine) containing 10% fetal calf serum (HyClone), 100 μg of penicillin per ml, 100 U of streptomycin per ml, and 0.05 mM 2-mercaptoethanol (Gibco) in 96-well flat-bottom plates (Falcon). Proliferation was measured by pulsing the wells with 0.5 μCi of [3H]thymidine (NEN) 48 to 72 h after initiation of culture, as indicated, and harvesting them 12 to 16 h later onto glass fiber filters for scintillation spectroscopy (Betaplate; Wallac, Turku, Finland). The data are shown as actual counts per minute (mean ± standard error of triplicates) for each antigen dose used, as well as stimulation indices (SI) for antigen concentrations where the response is in the linear range. Cytokines in supernatants from replicate wells were estimated by enzyme-linked immunosorbent assay, using purified and biotinylated antibody pairs (BD Biosciences), according to the manufacturer's protocols.
Oral tolerance in DO11.10 chimeras.
BALB/c mice chimeric for DO11.10 transgenic cells were generated by transferring 40 million to 50 million pooled lymphocytes from the spleens, PLN, PP, and mesenteric lymph nodes (MLN) of DO11.10 transgenic mice intravenously into naive BALB/c mice (17). One day later, chimerism was tested by staining their peripheral blood lymphocytes with anti-CD4 and the clonotypic antibody KJ1-26 (which is specific for the DO11.10 transgenic T-cell receptor [TCR]). Groups of chimeric mice were treated with OVA or PBS orally, and donor DO11.10 cells in PP, MLN, PLN, and spleens were tracked at various times (1, 2, 4, and 7 days later). Each group was then immunized s.c. with OVA in CFA, and the tracking was continued for another week (days 2, 4, and 7 after the s.c. immunization). At the last time point (day 7 after s.c. immunization), draining PLN cells were plated out with OVA to estimate the T-cell recall responses in vitro. Proliferation data are plotted as actual counts per minute (mean ± standard error of triplicates) for each antigen dose used as well as SI, as described above.
Statistics.
Statistical significance was calculated by Student's t test and by analysis of variance. The P values for comparison of SI are included in the plots, and the P values for curve comparisons are included in the figure legends.
RESULTS
Concomitant generation of oral tolerance and enhanced antibacterial immunity by oral administration of soluble OVA.
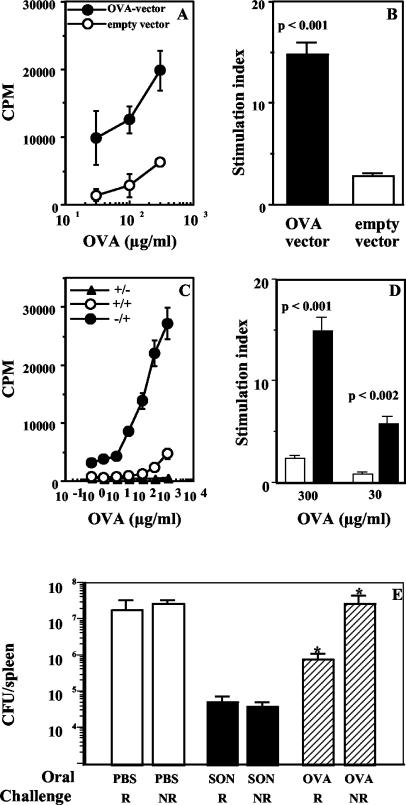
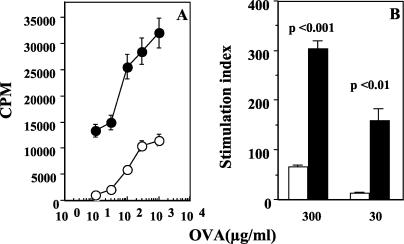
Oral administration of soluble antigens is known to induce systemic Τ-cell tolerance, raising the possibility that antigens in the intestinal lumen that cross-react with products of microbial pathogens may induce specific T-cell tolerance in systemic tissues and compromise T-cell-mediated immunity to the pathogen. In order to test this, we generated a strain of S. enterica serovar Typhimurium that expresses OVA cytosolically and assessed the ability of mice fed OVA to clear a challenge infection with the recombinant strain. Thus, the full-length OVA gene was cloned into a vector (as described in Materials and Methods) and used for transformation of a virulent S. enterica serovar Typhimurium strain. Constitutive, cytosolic expression of OVA in the recombinants was tested by OVA-specific enzyme-linked immunosorbent assay of bacterial sonicate. We found that sonicate from the nonrecombinant strain did not contain detectable OVA, while sonicate from the recombinant strain contained 37.25 ± 4.97 ng of OVA/μg of total protein (P < 0.001). Expression was also tested by Western blot analysis with an anti-c-myc antibody (data not shown). To ensure that the expressed OVA is sufficient to elicit an OVA-specific T-cell response in vivo, mice were immunized with heat-inactivated recombinant bacteria i.p., and 1 week later their spleen cells were plated out with titrating doses of OVA in vitro. As shown in Fig. 1A and B, cells from mice immunized with OVA-expressing recombinants proliferate well compared to cells from mice immunized with recombinants containing the empty vector.
FIG. 1.
Oral tolerance and systemic antibacterial immunity are induced by the same antigen. (A and B) Immunogenicity of OVA expressed by recombinant S. enterica serovar Typhimurium. In vitro recall responses of splenocytes from mice immunized i.p. with OVA-expressing and non-OVA-expressing S. enterica serovar Typhimurium 1 week earlier are represented as counts per minute in response to titrating doses of OVA in vitro (A) (P < 0.02) and as the SI at a representative (100-μg/ml) dose of OVA (B). (C and D) Oral tolerance to soluble OVA. In vitro recall responses of cells from draining PLN of mice fed 10 mg of OVA (+/+) or PBS (−/+) and immunized s.c with 10 μg of OVA in CFA are shown. Data are represented as counts per minute in response to titrating doses of OVA in vitro (C) (P < 0.02) and as the SI at two doses of OVA (D). The response of control mice fed OVA but immunized s.c. with PBS-CFA (−/−) is also shown. Cultures were set up 7 days after s.c. immunization, and the data are representative of those from six experiments. (E) Mice fed soluble OVA are protected against infection with recombinant OVA-expressing S. enterica serovar Typhimurium. Bacterial loads in spleens of mice fed PBS, 2 mg of bacterial sonicate (SON), or 10 mg of OVA and challenged i.p with 103 CFU of recombinant (R) or nonrecombinant (NR) S. enterica serovar Typhimurium BRD058 1 week later are shown. Data are representative of those from two experiments with five or six mice per group *, P < 0.05.
Next, mice were fed 10 mg of OVA and used for two different assays 1 week later: one group was immunized s.c. with OVA in CFA to assay oral tolerance to OVA, and the other was challenged with a recombinant or nonrecombinant S. enterica serovar Typhimurium strain to assay antibacterial protection. For the protection assay, mice fed PBS served as negative controls and mice fed 2 mg of bacterial sonicate served as positive controls. As expected, oral tolerance to OVA could be observed in vitro as lower proliferative responses of draining PLN cells from fed mice compared to cells from unfed mice (Fig. 1C and D). Interestingly, we found that the oral tolerance was accompanied by enhanced clearance of a challenge infection with OVA-expressing recombinant bacteria (Fig. 1E). PBS-fed control mice showed similar bacterial loads in their spleens when infected with the recombinant or nonrecombinant strain (1.4 ×107 and 2 × 107 CFU/spleen, respectively). Similar bacterial loads were also seen in OVA-fed mice when they were challenged with the nonrecombinant strain (2.1 × 107 CFU/spleen), indicating that OVA immunization does not generate nonspecific immunity to S. enterica serovar Typhimurium infection. However bacterial loads were lower in OVA-fed mice challenged with the recombinant strain (7 × 105 CFU/spleen; P < 0.05). Protection was better in mice fed whole sonicate (4 × 104 CFU/spleen; P < 0.008 versus the PBS-fed group), but it is striking that a single low dose of OVA given orally can protect mice against systemic infection with a virulent bacterium expressing OVA.
Generation of Th1-dependent systemic antibacterial immunity by oral administration of soluble antigens.
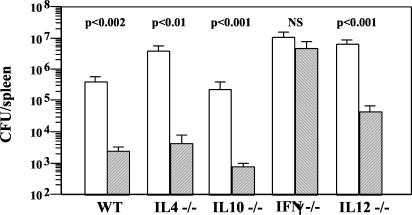
To examine the possible mechanism involved in antibacterial immunity to soluble antigens delivered orally, we next looked at the effect of oral administration of antigen on the ability of various cytokine-deficient mice to clear a subsequent systemic infection. Thus, interleukin-4-null (IL-4−/−), IL-10−/−, gamma interferon-null (IFN-γ−/−), IL-12−/−, and wild-type control C57BL/6 mice were fed S. enterica serovar Typhimurium sonicate and challenged 1 week later with virulent bacteria, and bacterial loads in spleens were compared with those in unfed mice at 6 to 8 days after infection. We found that feeding enhanced bacterial clearance in IL-4−/− mice and IL-10−/− mice, as well as in IL-12−/− mice, but not in IFN-γ−/−mice (Fig. 2). These data suggest that oral antigens can induce IFN-γ responses in vivo that can mediate antibacterial protection.
FIG. 2.
Oral antigen protects mice against systemic infection by an IFN-γ-dependent mechanism. Bacterial loads in spleens of C57BL/6 wild-type (WT), IL-4−/−, IL-10−/−, IFN-γ−/−, and IL-12−/− mice fed PBS (open bars) or 2 mg of S. enterica serovar Typhimurium sonicate (hatched bars) and challenged i.p. with 103 CFU of S. enterica serovar Typhimurium 754 1 week later are shown. Data are representative of those from four experiments with five mice per group. Statistical significance (P values for fed versus unfed groups for each strain) is indicated above the bars. NS, not significant.
Priming of systemic T cells for an IFN-γ response by oral administration of OVA.
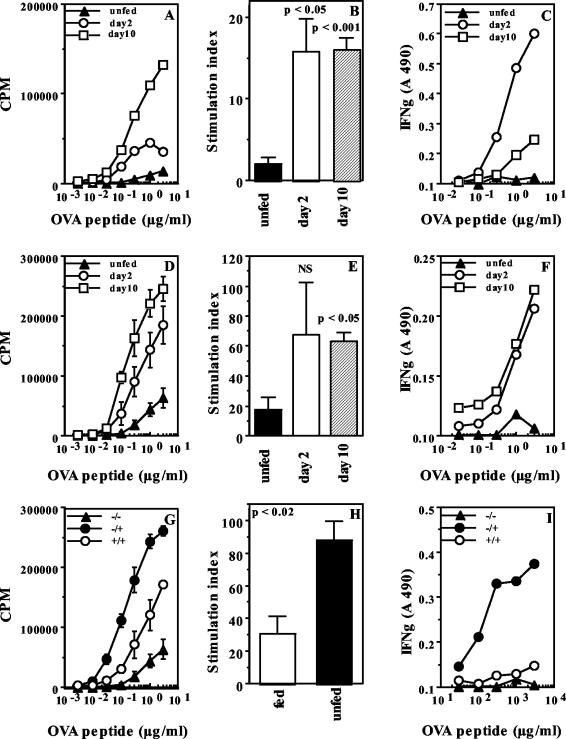
So far, our data indicate that oral exposure to soluble antigens can result in systemic T-cell tolerance, leading to poor proliferative responses in vitro, and in systemic T-cell priming, leading to enhanced antibacterial immunity by a mechanism that seems to involve IFN-γ. Since microbial sonicates are complex mixtures of antigens which can induce multiple T-cell responses in vivo, we used OT.II transgenic mice, which express a TCR specific for the peptide from amino acid 323 to 339 of OVA (30), to determine whether soluble oral OVA can induce peptide-specific T-cell priming for IFN-γ responses in systemic tissues. Transgenic mice were fed 1 mg of OVA, and T-cell proliferation and IFN-γ secretion in response to recall antigen in vitro were assessed 2 and 10 days later. We found that T cells from spleens and PLN of fed mice proliferated more extensively than cells from unfed mice at both time points (Fig. 3A, B, D, and E). Further, significant levels of IFN-γ were seen in supernatants of splenic cultures recalled early after feeding (day 2) (Fig. 3C) and in PLN cultures at the later time point (day 10) (Fig. 3F).
FIG. 3.
Oral OVA induces systemic Th1 responses and oral tolerance in transgenic mice. (A to F) Proliferation and IFN-γ secretion by T cells from spleens (A to C) and PLN (D to F) of unfed OT.II mice and mice fed 1 mg of OVA 2 or 10 days earlier. Proliferation data are represented as counts per minute in response to titrating doses of OVA in vitro (A and D) and as SI (B and E) at a representative dose of OVA. The proliferation curves of cells from fed and unfed mice were significantly different for both tissues at both time points (P < 0.04). IFN-γ curves were significantly different for splenic cultures at day 2 (C) (P < 0.04) but not at day 10 and were significantly different for PLN cultures at day 10 (F) (P < 0.02) but not at day 2. A 490, absorbance at 490 nm; NS, not significant. (G to I) Oral tolerance in fed OT.II mice. (G) Proliferation of draining PLN cells of OT.II mice fed 1 mg of OVA (+/+) or PBS (−/+) and immunized s.c. 10 days later with 10 μg of OVA (P < 0.004). The response of PLN cells from unmanipulated OT.II mice (−/−) is also shown. (H) Proliferation data are also shown as SI for a representative dose of OVA. (I) IFN-γ in culture supernatants from replicate cultures (P < 0.03 for fed versus unfed groups). Cultures were set up 7 days after s.c. immunization. Data are representative of those from two experiments.
This systemic priming notwithstanding, when fed mice were immunized s.c. with OVA in CFA on day 10, oral tolerance could be observed as diminished T-cell proliferation and cytokine secretion of cells from the draining PLN 1 week later compared to cells from unfed mice (Fig. 3G to I). Similar results were seen when mice were fed 10 mg of OVA (data not shown).
Kinetics of T-cell priming in vivo following oral administration of antigen.
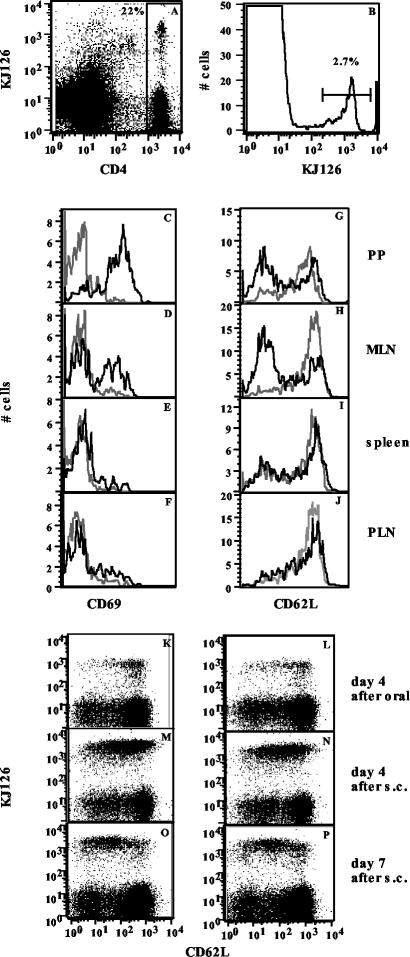
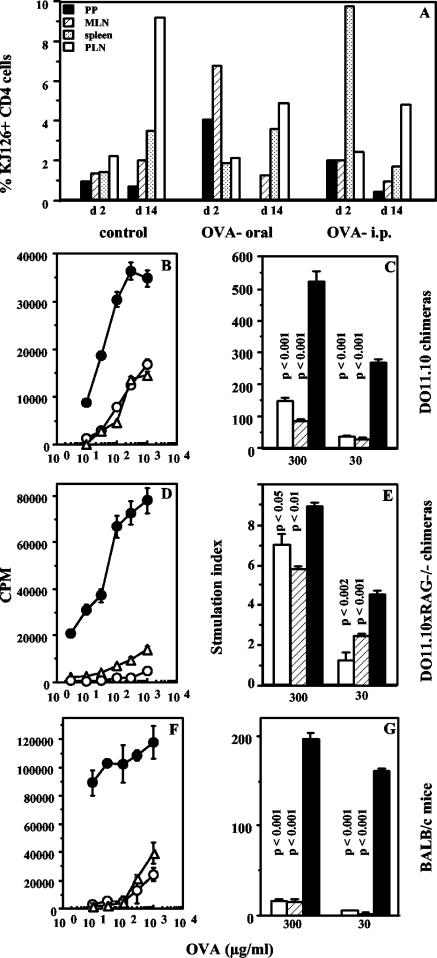
The above data indicate that transgenic systems can be used for further exploration of the paradoxical finding that soluble antigens given orally can immunize and induce tolerance concomitantly. Since the mucosal-systemic interface is involved, we decided to track T-cell responses at both sites over time in vivo following feeding and subsequent s.c. immunization. To make the experiments more physiological, we transferred small numbers of transgenic cells into normal BALB/c mice and used the stable chimeras rather than the transgenics themselves. DO11.10 transgenic mice, also bearing a TCR specific for the peptide from amino acid 323 to 339 of OVA (30), were used as donors for these experiments, as the availability of the clonotypic antibody KJ1-26 allows easy identification of donor transgenic cells in the recipient BALB/c mice (17). Chimeric mice, in which 1 to 4% of CD4 cells in various tissues bear the clonotypic TCR, were fed 10 mg of OVA, and donor KJ1-26+ CD4 T cells were identified in vivo as shown in Fig. 4A and B and over the course of oral tolerance induction as described in Materials and Methods.
FIG. 4.
Oral OVA induces activation of DO11.10 cells in PP and MLN of chimeric mice. (A and B) Gating of KJ1-26+ CD4 cells in chimeric mice. (C to J) Expression of CD69 (C to F) and CD62L (G to J) on gated KJ1-26+ CD4 cells in PP (C and G), MLN (D and H), spleens (E and I), and PLN (F and J) of mice fed PBS (dotted lines) or 10 mg of OVA (solid lines). A 24-h profile for CD69 and a 4-day profile for CD62L are shown. (K to P) Downregulation of CD62L in PLN after s.c. immunization. Two-color analysis for KJ1-26 and CD62L on gated CD4 cells in PLN of unfed (K, M, and O) and fed (L, N, and P) mice 4 days after oral OVA (K and L) and 4 days (M and N) or 7 days (O and P) after s.c. immunization in an oral tolerance experiment is shown. Data are representative of those from three experiments with two mice per group per time point.
We found rapid T-cell activation following oral administration of OVA at mucosal sites, with CD69 being upregulated on donor cells in the PP and MLN by 24 h (Fig. 4C and D) and downregulation of CD62L seen by day 4 (Fig. 4G and H). No significant changes were seen in the spleens and PLN of fed mice (Fig. 4E, F, I, and J) at this time. However, subsequent s.c. immunization led to activation of transgenic T cells in the PLN, with upregulation of CD69 (data not shown) and downregulation of CD62L (Fig. 4M to P), and the activation profiles were not significantly different for fed and unfed mice.
T-cell expansion in vivo mirrored the activation events and was most apparent at mucosal sites. Dramatically increased numbers of KJ1-26+ cells were seen in PP at 24 and 48 h after oral administration of OVA, more moderate increases were seen in the MLN by day 4, and some increase was seen in the spleen by day 7 (Table 1). When mice were immunized s.c. with OVA in CFA on day 7, prolific expansion of KJ1-26+ cells was seen in the draining PLN of fed mice 4 days later, albeit less than in unfed controls (for day 11, 22 versus 36%), and fed mice typically contained approximately half as many transgenic T cells in draining PLN as unfed mice 7days after s.c. immunization (for day 14, 6.1 versus 9.8%). Further, when stimulated with OVA in vitro at this time, cells from fed mice proliferated less well than cells from unfed mice (Fig. 5). Similar results were seen when mice were fed 1 mg of OVA and when DO11.10 × RAG−/− mice were used as transgenic cell donors (data not shown).
TABLE 1.
Kinetics of expansion of transgenic T cells in DO11.10 × BALB/c chimeric mice during induction of oral tolerance
| Day after oral OVA | % of CD4+ KJ1-26+ cells ina:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PP
|
MLN
|
Spleens
|
PLN
|
|||||
| Unfed mice | Fed mice | Unfed mice | Fed mice | Unfed mice | Fed mice | Unfed mice | Fed mice | |
| 1 | 2.3 | 6.0 | 3.7 | 4.6 | 3.8 | 3.3 | 4.9 | 4.4 |
| 2 | 2.6 | 11.3 | 4.4 | 4.4 | 3.9 | 2.7 | 5.6 | 3.0 |
| 4 | 2.0 | 4.7 | 4.1 | 8.8 | 3.8 | 5.0 | 5.5 | 4.3 |
| 7b | 2.1 | 3.2 | 3.3 | 5.5 | 4.2 | 5.3 | 4.0 | 4.1 |
| 11 | 2.0 | 2.2 | 4.1 | 4.3 | 9.9 | 7.0 | 36.3 | 22.0 |
| 14 | 1.1 | 1.3 | 2.3 | 3.2 | 3.4 | 3.7 | 9.8 | 6.1 |
Data are representative of tissues from two or three mice per group and of three independent experiments. Cell yields for each tissue were within 10% of each other in the fed and unfed groups. Boldface indicates T-cell expansion.
Mice were immunized with 10 μg of OVA in CFA on day 7.
FIG. 5.
Oral tolerance to OVA is induced in DO11.10 chimeras. In vitro recall responses of T cells from draining PLN of mice fed 10 mg of OVA (open circles and bars) and unfed mice immunized s.c with 10 μg of OVA in CFA (filled circles and bars) are shown. Data are represented as counts per minute in response to titrating doses of OVA in vitro (A) (P < 0.001) and as the SI at two doses of OVA (B). Cultures were set up 7 days after s.c. immunization. Data are representative of those from three experiments.
These data indicate that oral OVA primes T cells largely at mucosal sites. More interestingly, while fed and unfed mice contain equivalent numbers of antigen-specific T cells in the draining PLN at the time of s.c. immunization, cells in fed mice expand less well in vivo than cells in unfed mice. A more exaggerated difference in proliferation is seen when cells from the two groups are recalled in vitro with OVA, suggesting that repeated antigen exposures may lead to a progressive loss in the proliferation capacity of primed T cells.
Induction of T-cell hyporesponsiveness by systemic administration of soluble OVA.
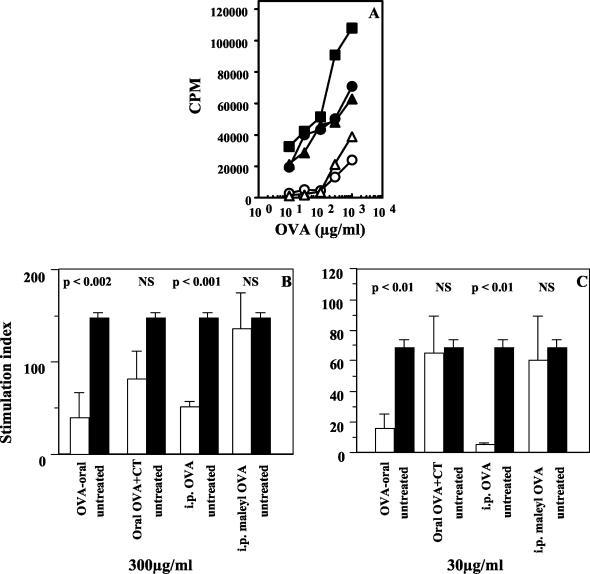
We next compared the courses of T-cell priming in vivo following oral and i.p. administration of soluble OVA to look for possible differences that might explain oral tolerance induction. Thus, DO11.10 chimeric mice were treated with a single dose of OVA either orally or i.p., and T-cell priming in various tissues was monitored over time as described above. We found that the kinetics of transgenic T-cell activation and expansion were similar whether antigen was given orally or parenterally. However, i.p. administration of OVA led to an early expansion of KJ1-26+ cells in the spleen, as opposed to the expansion in PP and MLN seen when OVA was given orally (Fig. 6A, day 2), and this was followed by increased numbers of transgenic cells in the MLN by day 4 (data not shown). When the mice were immunized s.c. as described above, tremendous expansion of KJ1-26+ cells was seen in the draining PLN of untreated mice, as seen earlier. The expansion was less in both treated groups by day 4 after s.c. immunization (data not shown), and by day 7 after s.c. immunization, lower frequencies of KJ1-26+ cells were seen in both treated groups (Fig. 6A, day 14). Further, T-cell hyporesponsiveness could be observed at this time as diminished proliferation of cells from the draining PLN from both i.p. and orally treated groups to OVA recall in vitro (Fig. 6B and C). Similar i.p. T-cell tolerance could be induced in BALB/c mice chimeric for DO11.10 × RAG−/− cells as well as in normal BALB/c mice (Fig. 6D to G). Notably, when the mucosal adjuvant cholera toxin was coadministered with oral OVA (7) or when soluble maleyl-OVA, which is known to be more immunogenic than OVA for systemic T-cell priming (33), was used for i.p. priming instead of OVA, partial reversal of in vitro T-cell hyporesponsiveness was seen (Fig. 7). Partial reversal of oral or i.p. tolerance was also seen in DO11.10 chimeras when cholera toxin and maleyl-OVA were used for primary antigen exposure (data not shown).
FIG. 6.
Soluble OVA given orally or systemically has similar effects in vivo. (A) Expansion of antigen-specific cells in vivo following oral or i.p. OVA. Proportions of KJ1-26+ CD4 cells in PP, MLN, spleens, and PLN of BALB/c mice chimeric for DO11.10 cells given 5 mg of OVA orally or 1 mg or OVA i.p. or left untreated (control), 2 days after oral or i.p treatment (d2) or 1 week after s.c. immunization of treated and untreated mice (d14), in an oral tolerance experiment are shown. Data are representative of those from two experiments with two mice per group per time point. (B to G) Induction of systemic T-cell hyporesponsiveness by oral or i.p. administration of OVA. Proliferation of PLN cells from BALB/c mice chimeric for DO11.10 cells (B and C), from mice chimeric for DO11.10 × RAG−/− cells (D and E), and from normal BALB/c mice (F and G) 1 week after s.c. immunization with OVA in CFA is shown. Filled circles and bars, mice untreated before s.c immunization; open circles and bars, mice given 5 mg of OVA orally 6 days earlier; triangles and hatched bars, mice given 1 mg of OVA i.p. 6 days earlier. Data are represented as counts per minute in response to titrating doses of OVA in vitro (P < 0.05 [B], P < 0.004 [D], and P < 0.005 [F]) and as the SI at two doses of OVA Data are representative of those from at least two experiments each, and P values for treated versus untreated groups are indicated above each bar.
FIG. 7.
Partial reversal of systemic T-cell hyporesponsiveness by enhancing the immunogenicity of the primary treatment. (A) Proliferation of draining PLN cells 1 week after s.c. immunization with OVA-CFA of untreated mice (squares) and mice treated orally with 10 mg of OVA (open circles), i.p. with 1 mg of OVA (open triangles), orally with 10 mg of OVA plus 10 μg of cholera toxin (filled circles), or i.p. with 100 μg of maleyl-OVA (filled triangles). Data are represented as counts per minute in response to titrating doses of OVA in vitro (P < 0.01 for oral OVA versus untreated group and P < 0.009 for i.p. OVA versus untreated group; the other two groups, i.e., oral OVA plus cholera toxin and i.p. maleyl-OVA, are not significantly different from the untreated group). (B and C) SI at two doses of OVA. Open bars, mice treated orally or i.p before s.c. immunization as indicated; filled bars, mice untreated before s.c. immunization. Data are representative of those from three experiments. Statistical significance for each group is indicated above the bars. NS, not significant.
Together, these results indicate that soluble antigens given orally and i.p. have similar effects in vivo. First, rapid activation and expansion of antigen-specific T cells are seen in vivo in both cases, and these events are restricted largely to lymphoid organs draining the site of immunization. Second, systemic hyporesponsiveness to a second immunization develops in both cases, and it appears to be related to poor immunogenicity. Third, effector T cells that can afford antibacterial protection are present in systemic tissues in both cases (9) (Fig. 2 and 3). Thus, it appears that soluble antigens given orally or i.p. may lead to T-cell commitment to effector rather than proliferative capabilities.
DISCUSSION
Our data show that oral administration of soluble antigen leads to the presence of primed T cells in systemic tissues that are capable of secreting secondary cytokines, notably IFN-γ, upon restimulation in vitro. These results significantly extend previous reports showing that oral antigens induce IFN-γ in PP and MLN (20). Significantly, the primed cells present in systemic tissues can also clear an i.p. infection with virulent Salmonella, an intracellular organism that requires a Th1-dominant immune response for effective clearance in vivo (37). Further, systemic antibacterial immunity to recombinant OVA-expressing Salmonella can be generated by oral administration of OVA, and it occurs in the face of demonstrable oral tolerance to OVA. Thus, the widely reported in vitro observation of systemic hyporesponsiveness to fed antigen that is characteristic of oral tolerance induction (35) may be accompanied in vivo by a significant IFN-γ-secreting effector T-cell population in systemic tissues.
Our findings have potential relevance for the clinical use of oral tolerance induction in the control of systemic inflammatory responses in autoimmune conditions. Oral administration of antigen has been shown to be effective in suppressing some models of experimental autoimmune diseases, such as uveitis, arthritis, encephalomyelitis, and diabetes (reviewed in references 8 and 27). However, it has also been shown to actively induce a cytotoxic T-lymphocyte response and autoimmune diabetes in mice (2), and the tolerogenic properties of various antigens have been shown to differ in different strains of autoimmune-prone mice (25). Even with tolerogenic antigens, repeated oral exposure does not increase the systemic T-cell hyporesponsiveness above that seen with a single dose (9), suggesting that any amelioration of autoimmune conditions obtained by oral tolerance induction may be temporary. Further, a kinetic analysis of oral tolerance induction at various stages of an ongoing systemic immune response has shown that tolerance cannot be induced once memory T cells have been generated in vivo (4). The presence of Th1-effector T cells in systemic tissues, shown here to accompany the oral administration of antigen, raises concerns about undesirable inflammatory situations arising in vivo in response to antigens cross-reactive with soluble or particulate material in the intestinal lumen.
Orally applied antigens have been shown to be present in systemic tissues relatively soon after feeding (11), and depending on the dose used, antigen presentation has been shown either to occur exclusively on dendritic cells of PP and MLN or to extend to systemic tissues (19). In TCR-transgenic mice and in mice chimeric for transgenic T cells, rapid activation of antigen-responsive T cells occurs in PP, and this is often followed by some activation at systemic sites (11, 19, 34, 42). In order to correlate the observed T-cell priming seen after oral antigen administration with the systemic hyporesponsiveness seen after a subsequent parenteral immunization of fed mice, we tracked antigen-specific T cells in mucosal and systemic tissues at various times all the way through in oral tolerance experiments, using a single oral dose of antigen to avoid the overlapping kinetics that may result with multiple oral applications. Our results indicate that primed cells capable of secreting IFN-γ are present in systemic tissues at early (2 days) and late (10 days) times after feeding (Fig. 3 and 4). However, when fed mice are immunized s.c. subsequently, cells in the draining PLN of fed mice expand approximately twice as poorly in vivo as those in unfed controls, and recall responses in vitro a week later are dramatically reduced (Table 1; Fig. 5). As suggested earlier (24), these results indicate that T cells may proliferate less well in response to a secondary exposure in vivo than in response to a primary exposure, and they raise the possibility that the proliferative capacity of T cells may be lost with each subsequent antigen encounter. Together, our antibacterial protection data and the T-cell tracking data indicate that systemic T cells in orally primed mice can make IFN-γ upon antigen reencounter, which it is needed for protection against systemic infection with S. enterica serovar Typhimurium, and that the commitment to IFN-γ in this context appears to be relatively independent of IL-12.
We have shown previously that protective systemic antibacterial immunity is induced by systemic as well as by oral treatment of mice with S. enterica serovar Typhimurium sonicate (10). Using a transgenic system, we demonstrate here that both exposures induce activation and proliferation of responding cells in draining lymphoid tissues. Further, both exposures induce systemic hyporesponsiveness to subsequent systemic immunization in adjuvant (Fig. 6). Our results are in keeping with the reported ability of OVA administered parenterally in incomplete Freund’s adjuvant to be tolerogenic (40) and suggest that systemic T-cell hyporesponsiveness associated with experimental oral tolerance may be related to antigen exposure under poor adjuvantic conditions. Our results confirm that cholera toxin can partially reverse oral tolerance to soluble antigens (7) (Fig. 7) and also show that a similar reversal to parenteral tolerance can be achieved if soluble maleyl-OVA, a more immunogenic form of OVA (33), is used for the initial i.p. exposure. Thus, oral exposure to soluble antigens is a poorly immunogenic type of immunization, leading to the generation of primed T cells that can make IFN-γ in the short term but lose proliferative capabilities to subsequent challenges. The results are not incompatible with the generation of T-cell anergy or of regulatory T cells that suppress proliferation, as has been reported previously (13, 21, 29, 38, 39).
Acknowledgments
This work was supported in part by grants from the Departments of Science and Technology and Biotechnology, Government of India (to A.G., V.B., and S.R.); a short-term Overseas Associateship from the Department of Biotechnology, Government of India (to A.G.); the Indian Council of Medical Research, Government of India (to S.R.); and the Wellcome Trust, United Kingdom (to V.B.). The National Institute of Immunology is funded by the Department of Biotechnology, Government of India.
We thank John Cebra, University of Pennsylvania, and Leslie McEvoy, DNAX Research Institute, for extensive and varied support.
Editor: A. D. O'Brien
REFERENCES
- 1.Berg, R. D., and D. C. Savage. 1975. Immune responses of specific-pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect. Immun. 11:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanas, E., F. R. Carbone, J. Allison, J. F. A. P. Miller, and W. R. Heath. 1996. Induction of autoimmune diabetes by oral administration of autoantigen. Science 274:1707-1709. [DOI] [PubMed] [Google Scholar]
- 3.Bos, N. A., H.-Q. Jiang, and J. J. Cebra. 2001. T cell control of the gut IgA response against commensal bacteria. Gut 48:762-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung, Y., S. Y. Chang, and C. Y. Kang. 1999. Kinetic analysis of oral tolerance: memory lymphocytes are refractory to oral tolerance. J. Immunol. 163:3692-3698. [PubMed] [Google Scholar]
- 5.Duchmann, R., I. Kaiser, E. Hermann, W. Mayet, K. Ewe, and K. H. Meyer zum Buschenfelde. 1995. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 102:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchmann, R., E. Schmitt, P. Knolle, K. H. Meyer zum Buschenfelde, and M. Neurath. 1996. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur. J. Immunol. 26:934-938. [DOI] [PubMed] [Google Scholar]
- 7.Elson, C. O., and W. Ealding. 1984. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J. Immunol. 133:2892-2897. [PubMed] [Google Scholar]
- 8.Faria, A. M., and H. L. Weiner. 1999. Oral tolerance: mechanisms and therapeutic applications. Adv. Immunol. 73:153-264. [DOI] [PubMed] [Google Scholar]
- 9.Garg, S., V. Bal, S. Rath, and A. George. 1999. Effect of multiple antigenic exposures in the gut on oral tolerance and induction of antibacterial systemic immunity. Infect. Immun. 67:5917-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George, A. 1996. Generation of gamma interferon responses in murine Peyer's patches following oral immunization. Infect. Immun. 64:4606-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutgemann, I., A. M. Fahrer, J. D. Altman, M. M. Davis, and Y. H. Chien. 1998. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity 8:667-673. [DOI] [PubMed] [Google Scholar]
- 12.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauet-Broere, F., W. W. Unger, J. Garssen, M. A. Hoijer, G. Kraal, and J. N. Samsom. 2003. Functional CD25− and CD25+ mucosal regulatory cells are induced in gut-draining lymphoid tissue within 48 h after oral antigen application. Eur. J. Immunol. 33:2801-2810. [DOI] [PubMed] [Google Scholar]
- 14.Hoyne, G. F., and W. R. Thomas. 1995. T-cell responses to orally administered antigens. Study of the kinetics of lymphokine production after single and multiple feeding. Immunology 84:304-309. [PMC free article] [PubMed] [Google Scholar]
- 15.Kagnoff, M. F. 1978. Effects of antigen-feeding on intestinal and systemic immune responses. II. Suppression of delayed type hypersensitivity reactions. J. Immunol. 120:1509-1513. [PubMed] [Google Scholar]
- 16.Karlsson, M. R., H. Kahu, L. A. Hanson, E. Telemo, and U. I. Dahlgren. 1999. Neonatal colonization of rats induces immunological tolerance to bacterial antigens. Eur. J. Immunol. 29:109-118. [DOI] [PubMed] [Google Scholar]
- 17.Kearney, E. R., K. A. Pape, D. Y. Loh, and M. K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327-339. [DOI] [PubMed] [Google Scholar]
- 18.Kraal, G., I. L. Weissman, and E. C. Butcher. 1983. Differences in in vivo distribution and homing of T cell subsets to mucosal vs nonmucosal lymphoid organs. J. Immunol. 130:1097-1102. [PubMed] [Google Scholar]
- 19.Kunkel, D., D. Kirchhoff, S. Nishikawa, A. Radbruch, and A. Scheffold. 2003. Visualization of peptide presentation following oral application of antigen in normal and Peyer's patches-deficient mice. Eur. J. Immunol. 33:1292-1301. [DOI] [PubMed] [Google Scholar]
- 20.Lee, H. O., S. D. Miller, S. D. Hurst, L. J. Tan, C. J. Cooper, and T. A. Barrett. 2000. Interferon gamma induction during oral tolerance reduces T-cell migration to sites of inflammation. Gastroenterology 119:129-138. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald, T. T. 1998. T cell immunity to oral allergens. Curr. Opin. Immunol. 10:620-627. [DOI] [PubMed] [Google Scholar]
- 22.Manohar, M., D. O. Baumann, N. A. Bos, and J. J. Cebra. 2001. Gut colonization of mice with actA-negative mutant of Listeria monocytogenes can stimulate a humoral mucosal immune response. Infect. Immun. 69:3542-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattingly, J. A., and B. Waksman. 1978. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J. Immunol. 121:1878-1883. [PubMed] [Google Scholar]
- 24.Merica, R., A. Khoruts, K. A. Pape, R. L. Reinhardt, and M. K. Jenkins. 2000. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J. Immunol. 164:4551-4557. [DOI] [PubMed] [Google Scholar]
- 25.Miller, M. L., J. S. Cowdery, C. A. Laskin, M. F. Cutrin, Jr., and A. D. Steinberg. 1984. Heterogeneity of oral tolerance defects in autoimmune mice. Clin. Immunol. Immunopathol. 31:231-240. [DOI] [PubMed] [Google Scholar]
- 26.Moore, M. W., F. R. Carbone, and M. J. Bevan. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777-785. [DOI] [PubMed] [Google Scholar]
- 27.Mowat, A. M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331-341. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa, T. Y., H. Von Grafenstein, J. E. Sears, J. Williams, C. A. Janeway, and R. A. Flavell. 1991. The use of the polymerase chain reaction to map CD4+ T cell epitopes. Eur. J. Immunol. 21:2851-2855. [DOI] [PubMed] [Google Scholar]
- 29.Read, S., and F. Powrie. 2001. CD4+ regulatory T cells. Curr. Opin. Immunol. 13:644-649. [DOI] [PubMed] [Google Scholar]
- 30.Robertson, J. M., P. E. Jensen, and B. D. Evavold. 2000. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J. Immunol. 164:4706-4712. [DOI] [PubMed] [Google Scholar]
- 31.Shroff, K. E., K. Meslin, and J. J. Cebra. 1995. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 63:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon, G. L., and S. L. Gorbach. 1984. Intestinal flora in health and disease. Gastroenterology 86:174-193. [PubMed] [Google Scholar]
- 33.Singh, N., S. Bhatia, R. Abraham, S. K. Basu, A. George, V. Bal, and S. Rath. 1998. Modulation of T cell cytokine profiles and peptide-MHC complex availability in vivo by delivery to scavenger receptors via antigen maleylation. J. Immunol. 160:4869-4880. [PubMed] [Google Scholar]
- 34.Smith, K. M., J. M. Davidson, and P. Garside. 2002. T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology 106:144-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strobel, S., and A. M. Mowat. 1998. Immune responses to dietary antigens: oral tolerance. Immunol. Today 19:173-181. [DOI] [PubMed] [Google Scholar]
- 36.Talham, G. L., H.-Q. Jiang, N. A. Bos, and J. J. Cebra. 1999. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 67:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thatte, J., S. Rath, and V. Bal. 1993. Immunization with live versus killed Salmonella typhimurium leads to the generation of an IFN-gamma dominant versus an IL-4 dominant immune response. Int. Immunol. 5:1431-1436. [DOI] [PubMed] [Google Scholar]
- 38.Thorstenson, K. M., and A. Khoruts. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188-195. [DOI] [PubMed] [Google Scholar]
- 39.Van Houten, N., and S. F. Blake. 1996. Direct measurement of anergy of antigen-specific T cells following oral tolerance induction. J. Immunol. 157:1337-1341. [PubMed] [Google Scholar]
- 40.Vidard, L., L. J. Colarusso, and B. Benacerraf. 1994. Specific T-cell tolerance may be preceded by a primary response. Proc. Natl. Acad. Sci. USA 91:5627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wannemuehler, M. J., H. Kiyono, J. L. Babb, S. M. Michalek, and J. R. McGhee. 1982. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J. Immunol. 129:959-965. [PubMed] [Google Scholar]
- 42.Williamson, E., J. M. O'Malley, and J. L. Viney. 1999. Visualizing the T-cell response elicited by oral administration of soluble protein antigen. Immunology 97:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]