Abstract
Chemical investigation of the EtOAc extract of the fungus Chaetomium aureum, an endophyte of the Moroccan medicinal plant Thymelaea lythroides, afforded one new resorcinol derivative named chaetorcinol, together with five known metabolites. The structures of the isolated compounds were determined on the basis of one- and two-dimensional NMR spectroscopy and high-resolution mass spectrometry as well as by comparison with the literature. All compounds were tested for their activity towards the Hsp90 chaperoning machine in vitro using the progesterone receptor (PR) and rabbit reticulocyte lysate (RRL). Among the isolated compounds, only sclerotiorin efficiently inhibited the Hsp90 machine chaperoning activity. However, sclerotiorin showed no cytotoxic effect on breast cancer Hs578T, MDA-MB-231 and prostate cancer LNCaP cell lines. Interestingly, deacetylation of sclerotiorin increased its cytotoxicity toward the tested cell lines over a period of 48h.
Keywords: Chaetomium aureum, Bioactive metabolites, Structure elucidation, Hsp90, Cytotoxicity
1. Introduction
In the last decades major efforts have been undertaken in bioprospecting miroorganisms and resulted in the discovery of several structurally diverse natural products and an array of bioactivities.1-4 Recently, however, drug discovery studies have shown a great interest in plant derived microorganisms, such as endophytic fungi, as sources of pharmacologically active molecules. Endophytic fungi inhabit internal tissues of plants without causing apparent diseases symptoms.5 The fungal endophytes produce promising lead compounds of their own, as well as plant associated products, for instence the famous antitumor agent taxol,6 isolated from Taxomyces andreanae, tetramic acid derivatives, which are produced by the fungal strain Myceliophthora thermophile,7 and chloropupukeanolides C–E, isolated from Pestalotiopsis fici.8
Literature surveys, however, indicated that only a fraction of hitherto described secondary metabolites have been investigated for their ability to modulate the chaperoning activity of the Hsp90 chaperoning machine in vitro. Because of its proteostatic maintenance of oncoproteins, the Hsp90 chaperone machine has become an exciting therapeutic target for cancer treatment.9 Hsp90 maintains the stability of activated metastable oncoproteins, such as protein kinases and transcription factors, and buffers the cellular stresses of the tumors environment. The use of geldamycin and its derivatives as chemical probes has been crucial in understanding the role of Hsp90 in stabilizing oncoproteins and how destabilizing Hsp90 client complexes leads to their cellular degradation mainly through the proteasome pathway and cancer cell death.10 Extensive research effort in the last two decades resulted in 17 Hsp90 inhibitors currently in clinical trials. The vast majority of which target the ATP binding pocket in the N-terminus of the chaperone, thus inhibiting its ATPase activity.11 Unfortunately, all these N terminal inhibitors induce activation of the heat shock factor 1 (HSF1) causing upregulation of the antiapoptotic proteins Hsp70 and Hsp27.12-15 This is thought, at least in part, to hamper the efficacy of these Hsp90 inhibitors in the clinic. Therefore, targeting HSF1 may enhance the cancer cell sensitivity to Hsp90 inhibitors.
Another target for Hsp90 inhibitors has been identified as a second ATP binding site in the C-terminus of Hsp90.16,17 Novobiocin, a coumarin antibiotic, interacts with this site to induce apoptotic cell death of cancer cells. Generally, C-terminal inhibitors were found to induce much less heat shock response than N-terminal inhibitors. Furthermore, chemical optimization of novobiocin led to a significant improvement in its affinity to Hsp90 and a superior efficacy in killing cancer cells. Other strategies to improve the clinical outcome of Hsp90 inhibitors are under investigation, for instance targeting the postranslational modifiers of Hsp90, such as HDAC6 and its cochaperones such p23, FKBP52 , Aha1 and Cdc37.18-22 During our ongoing search for new bioactive metabolites from terrestrial endophytes,2,4,23,24 we isolated the endophytic fungus Chaetomium aureum from healthy stem tissues of Thymelaea lythroides collected in the Maâmora forest in Rabat, Morocco. To the best of our knowledge, this is the first report on endophytes from this medicinal plant. Our interest in investigating the endophytes of T. lythroides was prompted by its use as anticancer in Moroccan traditional medicine.25 A literature survey showed that Chaetomium species are rich sources of a broad spectrum of bioactive secondary metabolites. These include cytoglobosins C and D showing a cytotoxic effect on A-549 cell line,26 chaetoviridin E with antimalarial activity,27 epipolythiodioxopiperazines as chaetocochins A and C with anticancer activity,28 antibacterial furanopolyene 3-epi-aureonitol,29 as well as chaetomanone with a slight antimycobacterial effect.30
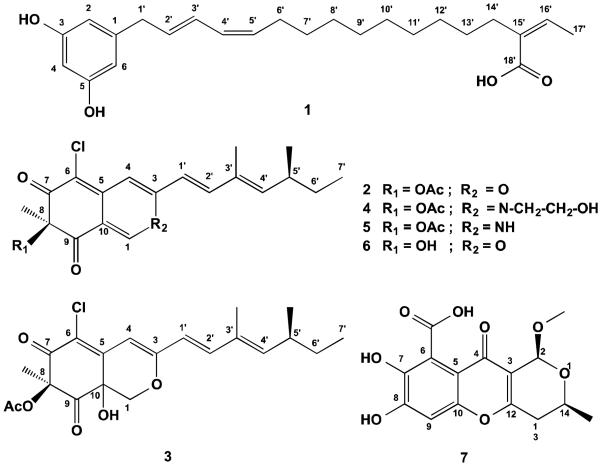
In this study, we report one new resorcinol derivative, named chaetorcinol (1), in addition to five known metabolites, including (+)-sclerotiorin (2),31,32 (+)-sclerotioramin (3),33 (+)-isochromophilone IV (4),34 (+)-isochromophilone VII (5)35 and SB 236050 (7).36
Furthermore, the isolated compounds were tested for their ability to inhibit the Hsp90 chaperoning machine including Hsp90 itself and/or its core components that are essential for folding the progesterone receptor (i.e Hsp70, hsp40, Hop and p23). We herewith present the first report on the investigation of compounds from Moroccan medicinal plants and their endophytes for their action on molecular chaperones for cancer treatment. Interestingly, sclerotiorin was found to inhibit the activity of the Hsp90 chaperoning machine in vitro. However, it had no significant cytotoxic effect on cancer cell lines from breast and prostate origins. Deacetylation reduced sclerotiorin inhibition of Hsp90 chaperoning machine in vitro but, at inhibitory concentration, deacetylsclerotiorin became cytotoxic to Hs578T, MDA-MB-231 and LNCaP cell lines over a period of 48h.
2. Experimental Section
2.1. General Experimental Procedures
1D and 2D NMR spectra were recorded on Bruker AVANCE III 600 NMR spectrometers. Optical rotations were measured out on a Jasco P-2000 polarimeter. ESIMS and HRESIMS with an Agilent 1100 series HPLC, respectively. Solvents were distilled prior to use, and spectral grade solvents were used for spectroscopic measurements. HPLC analysis was carried out on a Dionex UltiMate 3000 HPLC system coupled to a photodiode array detector (DAD-3000RS). Routine detection was performed at 235, 254, 280, and 340 nm. The separation column (125 × 4 mm, L × i.d.) was prefilled with Eurospher-10 C18 (Knauer, Germany), and the following gradient was used (MeOH/0.1% formic acid in water); 0 min, 10% MeOH; 5 min, 10% MeOH; 35 min, 100% MeOH; 45 min, 100% MeOH. Column chromatography was performed using Merck MN Silicagel 60 M (0.04–0.063 mm) or Sephadex LH20 (GE HealthCare) as stationary phases. For TLC analyses pre-coated Silica Gel 60 F254 plates (Merck) were used followed by detection under UV at 254 and 366 nm or after spraying with anisaldehyde reagent.
2.2. Plant Material
Fresh healthy plant material of Thymelaea lythroides was collected in the Maâmora forest, Rabat, Morocco. The whole plant was harvested and placed in plastic bags, after any excess moisture was removed. The plant material was stored at 4° C until isolation procedures could be instituted. Reference samples are deposited at the herbarium of the Scientific Institute of Rabat, and given the collection number (RAB 77777) has been assigned.
2.3. Fungal Material
The endophytic fungal strain was isolated from healthy stem tissues of Thymelaea lythroides (Thymelaeaceae). Stems were rinsed twice with sterilized distilled water. Surface sterilization was achieved by immersing the leaves in 70% ethanol for 2 min (twice) followed by rinsing in sterilized distilled water. Then, the leaves were cleaved aseptically into small segments (approx.1 cm in length). The material was placed on a Petri dish (malt agar medium) containing chloramphenicol and streptomycin to suppress bacterial growth (medium composition: 15 g/L malt extract, 15 g/L agar, 0.2 g/L chloramphenicol and 0.1g/L streptomycin in distilled water, pH 7.4-7.8) and incubated at room temperature (21 °C). After several days, hyphae growing from the plant material were transferred to fresh plates with the same medium, incubated again for 10 days, and periodically checked for culture purity.
2.4. Identification of Fungal Cultures
The fungal strain (EMBL accession number HF546136) was identified based on analysis of the DNA sequences of internal transcribed spacer regions of its ribosomal RNA gene. The identification was performed according to the previously described a molecular biological protocol.37 The endophytic strain was identified as Chaetomium aureum. A voucher strain (strain designation MM10S2-1) is kept in the Institute of Pharmaceutical Biology and Biotechnology, Düsseldorf, Germany.
2.5. Cultivation
The fungus was cultivated on solid rice medium (to 100 g commercially available rice 110 mL of distilled water was added and autoclaved). Fungal strain cultures were grown at room temperature for 30 days.
2.6. Extraction and Fractionation
Each culture flask was extracted three times with ethyl acetate (500 mL). The crude ethyl acetate extract (12g) of C. aureum, was partitioned between n-hexane and 90% methanol. The 90% methanol fraction (3g) was chromatographed by size exclusion (Sephadex LH-20) followed by silica column chromatography. Final purification was achieved by semipreparative reversed-phase HPLC (Merck-Hitachi L-7100) using an Eurosphere 100–10 C18 column (300 × 8 mm, L × i.d.) with the following gradient (MeOH:H2O): 0 min, 10% MeOH; 5 min, 10% MeOH; 35 min 100% MeOH; 45 min, 100% MeOH. In total seven compounds were obtained, with following yields: 1 (5mg), 2 (150mg), 3 (1mg), 4 (4mg), 5 (3mg) and 6 (6mg).
Chaetorcinol (1): Red oil; 1H and 13C in CD3OD, see Table 1; UV λmax (PDA, MeOH) 220.1, 264.8 and 377.7 nm, HRESI-MS m/z 409.2351 [M+Na]+ (calcd for C24H34O4Na, 409.2355).
Table 1.
NMR data of 1 measured at 600 (1H) and 150 (13C) MHz (CD3OD).
| No. | 1 |
|
|---|---|---|
| δ C | δ H | |
| 1 | 144.1 | - |
| 2 | 108.0 | 6.13b |
| 3 | 161.7 | - |
| 4 | 101.1 | 6.08 |
| 5 | 159.7 | - |
| 6 | 108.0 | 6.14b |
| 1' | 34.7 | 3.35c |
| 2' | 131.05 | 5.54 |
| 3' | 125.3 | 6.35 |
| 4' | 124.7 | 6.38 |
| 5' | 133.8 | 5.52 |
| 6' | 28.6 | 2.20 |
| 7' | 30.34a | 1.44 |
| 8' | 30.38a | 1.33 |
| 9' | 30.44a | 1.33 |
| 10' | 30.63a | 1.33 |
| 11' | 30.69a | 1.25 |
| 12' | 30.76a | 1.25 |
| 13' | 30.86 | 1.37 |
| 14' | 35.9 | 2.22 |
| 15' | 135.2 | - |
| 16' | 136.5 | 5.97 (q, J=7.1 Hz) |
| 17' | 15.9 | 1.92 (d, J=7.1 Hz) |
| 18' | 172.0 | - |
may be exchangeable
may be exchangeable
overlapped with solvent peak
2.7. Deacetylation of 2
Deacetylsclerotiorin (6) was obtained by deacetylation of 2 according to the procedure described previously by Isak.38
2.8. Progesterone Receptor (PR) Reconstitution Assay
Purified PR was adsorbed onto PR22 antibody-protein A-sepharose resin beads and was assembled into complexes as described previously.39 Briefly, about 0.05 μM PR was incubated with RRL diluted twice in reaction buffer (20 mM Tris/HCl, pH 7.5, 10 mM MgCl2, 4 mM DTT, 0.02% NP-40, 100 mM KCl and 10 mM ATP). After incubation for 30 min at 30°C, 0.1 μM [3H]-progesterone (American Radiolabeled Chemicals, Inc #ART 0063) was added. Samples were incubated on ice for 3 h at 4°C. Complexes were then washed three times with 1ml of reaction buffer and assessed for bound progesterone by liquid scintillation using PerkinElmer Microbeta plate reader.
2.9. Cell Culture
All cell lines were purchased from American type culture collection (ATCC). The cell culture medium MEM and foetal bovine serum (FBS) were obtained from Gibco. Penicillin/streptomycin, and tissue culture grade trypsin were bought from Sigma Chemical Co. 3.103 cells of breast cancer triple negative cell lines Hs578T and MDA-MB-231 and prostate cancer cell line LNCaP were seeded in 6-well plates (Corning #3516). The next day, cells were treated with various concentrations of sclerotiorin and deacetylsclerotiorin for 48h. Every 24h, the old media was substituted with new one containing new drug at the same concentration. Cells were harvested at 48 h and cell lysate were made. 10 μg of protein lysate were analyzed by Western blotting using the following specific antibodies: anti-total GR (rabbit # 218) was a gift from Dr. Garabedian (New York University School of Medicine). Anti Hsp70 was from Enzo Life Sciences (C92F3A-5). Anti Hsp40 was from Neomarkers (KA2A5.6). Anti β-actin was from Santa Cruz (sc-47778).
3. Results and Discussion
3.1. Isolation and identification of 1
Compound 1 was obtained as a red oil and showed three UV maxima at λmax (MeOH) 220.1, 264.8 and 377.7 nm. ESIMS exhibited a prominent quasi-molecular base peak at m/z 385.3 [M-H]-, in the negative mode, indicating a molecular weight of 386 g/mol. The chemical formula of 1 was deduced as C24H33O4 from the prominent peak appearing at m/z 409.2351 [M+Na]+ in the HRESIMS (calcd for C24H34O4Na 409.2355). 13C NMR and DEPT-135 experiments (Fig. S3 in SI) exhibited 24 carbon atoms attributable to one olefinic methyl group, ten methylenes, eight olefinic methines, four quaternary carbons, and one carbonyl carbon. Thorough analysis of 2D NMR data indicated that 1 is a resorcinol derivative containing a side chain of 18 carbons. 1H-1H COSY (Fig. S4 in SI) confirmed the presence of three spin systems assigned for CH3(17')CH(16'), a continuous spin system CH2(1')→CH2(14'), and the resorcinol ring system (H-2, H-4 and H-6), whereas the connection of these spin systems was established by inspection of 2J and 3J HMBC correlations (Figure 1 and Fig. S6 in SI). CH2-14' showed a 2J correlation to the olefinic quaternary carbon C-15' (δC 135.2 ppm), and 3J correlations to C-16' and C-18', appearring at δC 136.5 and 172.0 ppm, respectively. A strong 3J correlation was also detected for the olefinic proton H-16' to C-14' (δC 35.9 ppm), the latter correlating also with the methyl group CH3-17' via a 4J correlation. On the other hand, the aliphatic methylene protons H2-1' correlated to two overlapping aromatic carbons, which were attributed to C-2 and C-6 (δC 108.0 ppm), as well as to an isolated aromatic carbon assigned to C-1 (δC 144.1 ppm), thus establishing the connection between both spin systems.
Figure 1.
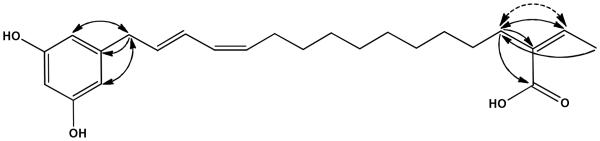
Key ROESY (↔) and HMBC correlations (→) of 1.
The configuration of the double bonds at C-2', C-4' and C-15' of the side chain was obtained by comparison of the observed chemical shifts of the respective carbons with 13C NMR data of 5-heptadeca-8'Z, 11'Z-dienylresorcinol,40 5-heptadeca-8'Z,11'Z,16-trienylresorcinol and 5-heptadeca-9'E,11'Z,16-trienylresorcinol.41 Accordingly, Δ2',3' and Δ15',16' were assigned to have E-configuration since the neighboring carbons C-1' and C-14' resonated more downfield at about δC 35 ppm. The allylic carbon of C-5' (C-6') appeared upfield (δC 28.6 ppm) in comparison to C-1' and C-14', and hence indicated a Z-configuration for the double bond Δ4',5'. This is in agreement with other known congeners42 where the allylic carbon of an E-configurated double bond resonates more downfield than that of a Z-configurated one. The configuration of the Δ15',16' double bond was further confirmed by a ROESY experiment (Fig. S7 in SI), where the olenific proton H-16' showed strong ROE correlation with CH2-14' (Figure 1). Accordingly, 1 was identified as a new natural product for which the name chaetorcinol is proposed.
The known compounds 2-5 and 7 were identified based on the comparison of their spectroscopic data, including mass spectrometry as well as 1D and 2D NMR measurement (1H NMR spectra in SI) such as 1H, 13C, DEPT, COSY, HMBC, HMQC, ROESY, with the published data. The compounds were identified as (+)-sclerotiorin (2),31,32 (+)-sclerotioramin (3),33 (+)-isochromophilone IV (4),34 (+)-isochromophilone VII (5)35 and SB 236050 (7).36
3.2. Sclerotiorin inhibits the Hsp90 chaperoning of progesterone receptor (PR) in vitro
We used the in vitro PR reconstitution assay as a model system to identify novel compounds that may inhibit the Hsp90 chaperoning machine. This assay uses RRL as a source of molecular chaperones and it has been fundamental in furthering our understanding of how geldanamycin and related compounds such as 17-AAG inhibit Hsp90-dependent chaperoning.43 The assay directly measures the ability of molecular chaperones to refold the heat denatured PR to its hormone binding state. Hormone binding activity of the PR therefore reflects the functional integrity of molecular chaperones. The isolated compounds were tested for their activity to affect the recovery of hormone binding activity of PR after mild heat denaturation. Previous screening experiments for about 60 compounds of diverse chemical structures from medicinal plants and their endophytes did not result in interesting hits. As shown in (Figure 2), sclerotiorin efficiently inhibited the recovery of PR hormone binding activity using RRL. Interestingly, sclerotiorin showed similar efficiency to that of 17-AAG, the classical inhibitor of Hsp90. The oxygen atom of the heterocycle was found to be essential for this inhibitory activity, as sclerotioramine and isochromophilone VI containing a nitrogen atom instead of the oxygen atom proved inactive. This is in line with previous reports showing that sclerotioramine and isochromophilone VI were unable to inhibit the activity of cholesterol ester transfer protein (CETP).44 Chaetorcinol and isochromophilone, on the other hand, showed much less activity than sclerotiorin.
Figure 2.
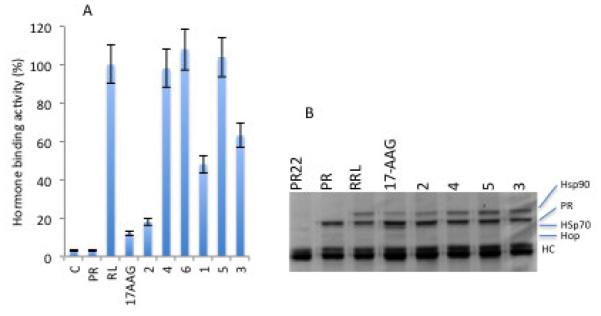
Effect of 1-6 on the Hsp90 chaperoning machine in vitro. A. PR hormone binding activity was reconstituted using rabbit reticulocyte lysate without (RL) or with the metabolites 1-6. 17-AAG is used as a positive control. B. Samples from (A) were used for analysis of protein complexes by SDS-PAGE and Coomassie blue staining. PR22 indicates the PR antibody incubated with RRL . PR indicates the PR alone with no RRL.
Cytotoxicity test results revealed that sclerotiorin was not active against the breast cancer cell lines Hs578T (Figure 4A), MCF7, MDA-MB-231 and MDA-MB-453, the prostate cancer cell line LNCaP, and the cervical cancer cell line HeLa (not shown). This is in contrast with a report showing that sclerotiorin induces apoptosis in colon cancer (HCT-116) cells through the activation of BAX and down-regulation of BCL-2.45
Figure 4.
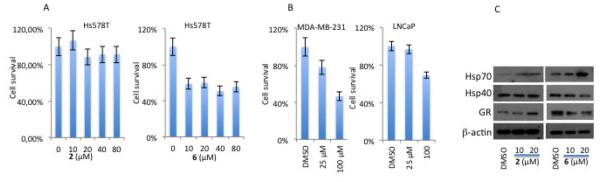
A. Comparison of the effect of 2 and 6 on cell survival of the breast cancer cell line Hs578Tusing MTS assay. B. 6 inhibits the growth of breast cancer cell line MDA-MB-231 and prostate cancer cell line LNCaP. C. Western blot analysis comparing the effect of 2 and 6 on the expression of Hs70, Hsp40 and GR. b-actin is used as a loading control.
Chemical modification of sclerotiorin by deacetylation resulted in 6, which exhibited relatively less efficiency in inhibiting the Hsp90 in vitro as shown in figure 3A. The mechanism of Hsp90 machine inactivation by sclerotiorin and deacetylsclerotiorin is not clear, but it seems to be different from that of the 17-AAG. As compared to 17-AAG, protein complexes analysis showed that sclerotiorin and deacetylsclerotiorin lower the level of Hsp90 without increase of Hop (Figure 3B). Furthermore, deacetylsclerotiorin does not increase Hsp70 level in PR complexes (Figure 3B).
Figure 3.
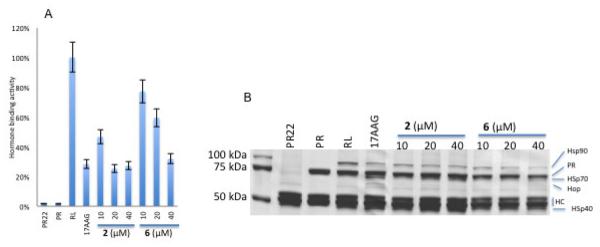
Comparison of 2 and 6. A. Effect increasing concentration of 2 and 6 on PR hormone binding activity. B. Effect of 2 and 6 on PR protein complexes. Molecular chaperones are indicated on the right. HC represents the heavy chains of the PR antibody PR22.
Surprisingly, at inhibitory concentrations, deactylsclerotiorin becomes cytotoxic to Hs578T, MDA-MB-231 and LNCaP cell lines over a period of 48h (Figure 4A, B). Western blot analysis (Figure 4C) showed that sclerotiorin induces overexpression of Hsp70 and degradation of Hsp90 client proteins, such as glucocorticoid receptor, in Hs578T cells, indicating that the Hsp90 machine may be inhibited in vivo. It is worth noting however, that sclerotiorin also induces degradation of the heat shock protein 40 (Hsp40) (Figure 4C), which may contribute to some of its cytotoxicity.
Sclerotiorin has been reported to have antibacterial activity against Bacillus spp46 and antifungal activity.47 At the molecular level, sclerotiorin inhibits aldose reductase activity,46 soybean lipoxygenase-1,48 Grb2-Shc interaction and antagonizes endothelin receptors.49 Sclerotirin and several isochromophilone analogues synthetized from it were also shown to inhibit gp120-CD4 binding50 and the activity of cholesterol ester transfer protein (CETP).44 From these studies, it could be concluded that the electrophilic enone CH(1)C(10)CO(9) and the oxygen atom of the heterocycle, as found in 2, are essential for the inhibitory activity. In contrast, compounds lacking these structural features (3-5) proved inactive. Furthermore, presence of an acetyl residue at C-8, as in 6, seems to reduce the activity. In 1957, sclerotiorin was reported to react with primary amines51 and this was proposed as the mechanism for its inactivation of CETP. This implied that sclerotiorin is able to modify primary amines, such as lysine residues or the N-terminal amino acid in the CETP.44 Hence, the inhibitory activity of sclerotiorin toward the Hsp90 machine may be due to reacting with the amine groups of components, such as Hsp90, Hsp70, Hsp40, Hop and p23, leading to their inactivation. Moreover, the loss of Hsp40 upon cell treatment with sclerotiorin could be the consequence of such covalent modifications.
Supplementary Material
Chart 1.
Structures of the isolated compounds 1-7.
Acknowledgements
This study was supported by grants of the BMBF (FKZ 01DH12030) awarded to A. Debbab. This work was supported by NIH grant R01 grant GM102443-01 to A. Chadli. We would like to thank Prof. FZ. ALAOUI Department of Botany, Faculty of Sciences, Mohamed V University, and Prof. Dr. M. IBN TATOU, Scientific Institute of Rabat, Morocco, for the plant identification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aly AH, Debbab A, Kjer J, Proksch P. Fung. Diver. 2010;41:1–16. [Google Scholar]
- 2.Aly AH, Debbab A, Clements C, Edrada-Ebel R, Orlikova B, Diederich M, Wray V, Lin WH, Proksch P. Bioorg. Med. Chem. 2011;19:414–421. doi: 10.1016/j.bmc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Debbab A, Aly AH, Proksch P. Fung. Diver. 2011;49:1–12. [Google Scholar]
- 4.Debbab A, Aly AH, Edrada-Ebel R, Wray V, Pretsch A, Pescitelli G, Kurtan T, Proksch P. Eur. J. Org. Chem. 2012;7:1351–1359. [Google Scholar]
- 5.Strobel G, Daisy B. Microbiol. Mol. Biol. Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bills GF, Dombrowski A, Pelaez F, Polishook J, Watling RI, Frankland JC, Ainsworth AM, Isaac S, Robinson CH. Recent and future discoveries of pharmacologically active metabolites from tropical fungi. In: Watling R, Frankland JC, Ainsworth AM, Issac S, Robinson CH, editors. Tropical Mycology. Vol. 2. 2002. pp. 165–194. A. N, Z. [Google Scholar]
- 7.Yang YL, Lu CP, Chen MY, Chen KY, Wu YC, Wu SH. Eur. J. Chem. 2007;13:6985–6991. doi: 10.1002/chem.200700038. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Bruhn T, Guo LD, Götz DCG, Brun R, Stich A, Che YS, Bringmann G. Chemistry. 2011;17:2604–2613. doi: 10.1002/chem.201003129. [DOI] [PubMed] [Google Scholar]
- 9.Whitesell L, Lindquist SL. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 10.Neckers L, Workman P. Clin. Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trepel J, Mollapour M, Giaccone G, Neckers L. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davenport EL, Zeisig A, Aronson LI, Moore HE, Hockley S, Gonzalez D, Smith EM, Powers MV, Sharp SY, Workman P, Morgan GJ, Davies FE. Leukemia. 2010;24:1804–1807. doi: 10.1038/leu.2010.168. [DOI] [PubMed] [Google Scholar]
- 13.McCollum AK, TenEyck CJ, Stensgard B, Morlan BW, Ballman KV, Jenkins RB, Toft DO, Erlichman C. Cancer Res. 2008;68:7419–7427. doi: 10.1158/0008-5472.CAN-07-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagatell R, Paine-Murrieta GD, Taylor CW, Pulcini EJ, Akinaga S, Benjamin IJ, Whitesell L. Clin. Cancer Res. 2000;6:3312–3318. [PubMed] [Google Scholar]
- 15.Powers MV, Clarke PA, Workman P. Cancer Cell. 2008;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly AC, Mays JR, Burlison JA, Nelson JT, Vielhauer G, Holzbeierlein J, Blagg BS. J. of Org. Chem. 2008;73:8901–8920. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadli A, Felts SJ, Wang Q, Sullivan WP, Botuyan MV, Fauq A, Ramirez-Alvarado M, Mer G. J. Biol. Chem. 2010;285:4224–4231. doi: 10.1074/jbc.M109.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BS, Chadli A. J. Biol. Chem. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM, Cox MB. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearl LH. Curr. Opin. Genet. Dev. 2005;15:55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Rao R, Fiskus W, Yang Y, Lee P, Joshi R, Fernandez P, Mandawat A, Atadja P, Bradner JE, Bhalla K. Blood. 2008;112:1886–1893. doi: 10.1182/blood-2008-03-143644. [DOI] [PubMed] [Google Scholar]
- 23.Ebrahim W, Aly AH, Mandi A, Totzke F, Kubbutat MHG, Wray V, Lin W-H, Dai H, Proksch P, Kurtan T, Debbab A. J. of Org. Chem. 2012;18:3476–3484. [Google Scholar]
- 24.Debbab A, Aly AH, Edrada-Ebel R, Wray V, Muller WE, Totzke F, Zirrgiebel U, Schachtele C, Kubbutat MH, Lin WH, Mosaddak M, Hakiki A, Proksch P, Ebel R. J. Nat. Prod. 2009;72:626–631. doi: 10.1021/np8004997. [DOI] [PubMed] [Google Scholar]
- 25.Kabbaj FZ, Meddah B, Cherrah Y, Faouzi M-A. Phytopharm. 2012;2:243–256. [Google Scholar]
- 26.Cui CM, Li XM, Li CS, Proksch P, Wang BG. J. Nat. Prod. 2010;73:729–733. doi: 10.1021/np900569t. [DOI] [PubMed] [Google Scholar]
- 27.Phonkerd N, Kanokmedhakul S, Kanokmedhakul K, Soytong K, Prabpai S, Kongsearee P. Tetrahedron. 2008;64:9636–9645. [Google Scholar]
- 28.Li GY, Li BG, Yang T, Yan JF, Liu GY, Zhang GL. J. Nat. Prod. 2006;69:1374–1376. doi: 10.1021/np0602970. [DOI] [PubMed] [Google Scholar]
- 29.Marwah S, Khan MMR, Chaudhary A, Gupta S, Negi SS, Soin A, Nundy S. Two hundred and forty-one consecutive liver resections: an experience from India. HPB (Oxford) 2007;9:29–36. doi: 10.1080/13651820600985259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanokmedhakul S, Kanokmedhakul K, Phonkerd N, Soytong K, Kongsaeree P, Suksamrarn A. Planta Med. 2002;9:834–836. doi: 10.1055/s-2002-34415. [DOI] [PubMed] [Google Scholar]
- 31.Whalley WB, Ferguson G, Marsh WC, Restivo RJ. J. Chem. Soc. Perkin Trans. 1976;1:1366–1369. [Google Scholar]
- 32.Pairet L, Wrigley SK, Chetland I, Reynolds EE, Hayes MA, Holloway J, Ainsworth AM, Katzer W, Cheng XM, Hupe DJ, Charlton P, Doherty AM. J. Antibiot. 1995;48:913–923. doi: 10.7164/antibiotics.48.913. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Sena, Filho JG, Hoover AR, King JB, Ellis TK, Powell DR, Cichewicz RH. J. Nat. Prod. 2010;73:942–948. doi: 10.1021/np100142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arai N, Shiomi K, Tomoda H, Tabata N, Yang DJ, Masuma R, Kawakuboand T, Omura S. J. Antibio. 1995;48:696–702. doi: 10.7164/antibiotics.48.696. [DOI] [PubMed] [Google Scholar]
- 35.Yang DJ, Tomoda H, Tabata N, Masuma R, Omura S. J. Antibio. 1995;49:223–229. doi: 10.7164/antibiotics.49.223. [DOI] [PubMed] [Google Scholar]
- 36.Payne DJ, Hueso-Rodríguez JA, Boyd H, Concha NO, Janson CA, Gilpin M, Bateson JH, Cheever C, Niconovich NL, Pearson S, Rittenhouse S, Tew D, Diez E, Pérez P, Fuente J, Rees M, Rivera-Sagredo A. Antimicrob. Agents Chemother. 2002;6:1880–1886. doi: 10.1128/AAC.46.6.1880-1886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kjer J, Debbab A, Aly AH, Proksch P. Nat. Protoc. 2010;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- 38.Isaka M, Jaturapat A, Rukseree K, Danwisetkanja K, Tanticharoen M, Thebtaranonth Y. J. Nat. Prod. 2001;64:1015–1018. doi: 10.1021/np010006h. [DOI] [PubMed] [Google Scholar]
- 39.Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. J. Biol. Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- 40.Barrow RA, Capon R. J. Aust. J. Chem. 1991;44:1393–1405. [Google Scholar]
- 41.Jin W, Zjawiony JK. J. Nat. Prod. 2006;69:704–706. doi: 10.1021/np050520d. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Esumi Y, Hyakutake H, Kono Y, Sakurai A. Phytochem. 1996;41:1485–1489. [Google Scholar]
- 43.Pratt WB, Toft DO. Exp. Med. Biol. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 44.Tomoda H, Matsushima C, Tabata N, Namatame I, Tanaka H, Bamberger MJ, Arai H, Fukazawa M, Inoue K, Omura S. J. Antibiot. 1999;52:160–170. doi: 10.7164/antibiotics.52.160. [DOI] [PubMed] [Google Scholar]
- 45.Giridharan P, Verekar SA, Khanna A, Mishra PD, Deshmukh SK. Indian J. Exp. Biol. 2012;50:464–468. [PubMed] [Google Scholar]
- 46.Chidananda C, Rao LJ, Sattur AP. Biotech. lett. 2006;28:1633–1636. doi: 10.1007/s10529-006-9133-4. [DOI] [PubMed] [Google Scholar]
- 47.Lin L, Mulholland N, Huang SW, Beattie D, Irwin D, Gu YC, Clough J, Wu QY, Yang GF. Chem. Biol. Drug. Des. 2012;80:682–692. doi: 10.1111/cbdd.12005. [DOI] [PubMed] [Google Scholar]
- 48.Chidananda C, Sattur AP. J. Agric. Food Chem. 2007;55:2879–2883. doi: 10.1021/jf062032x. [DOI] [PubMed] [Google Scholar]
- 49.Pairet L, Wrigley SK, Chetland I, Reynolds EE, Hayes MA, Holloway J, Ainsworth AM, Katzer W, Cheng XM, Hupe DJ, Charlton P, Doherty AM. J. Antibiot. 1995;48:913–923. doi: 10.7164/antibiotics.48.913. [DOI] [PubMed] [Google Scholar]
- 50.Sun XL, Takayanagi H, Matsuzaki K, Tanaka H, Furuhata K, Omura S. J. Antibiot. 1996;49:689–692. doi: 10.7164/antibiotics.49.689. [DOI] [PubMed] [Google Scholar]
- 51.Eade RA, Page H, Alexander A, Turner K, Whalley WB. J. Chem. Soc. 1957:4913–4924. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.