Abstract
An extracellular protein of Streptococcus pyogenes, streptococcal inhibitor of complement (SIC), and its variant, called DRS (distantly related to SIC), are expressed by some S. pyogenes strains. SIC from type 1 (M1) isolates of S. pyogenes interferes with complement-mediated cell lysis, reportedly via its interaction with complement proteins. In this study we demonstrate that S. pyogenes strains carrying emm12 and emm55 (the genes for the M12 and M55 proteins, respectively) express and secrete DRS. This protein, like SIC, binds to the C6 and C7 complement proteins, and competition enzyme-linked immunosorbent assay experiments demonstrate that DRS competes with SIC for C6 and C7 binding. Similarly, SIC competes with DRS for binding to the complement proteins. Despite this, the recombinant DRS preparation showed no significant effect on complement function, as determined by lysis of sensitized sheep erythrocytes. Furthermore, the presence of DRS is not inhibitory to SIC activity.
Streptococcus pyogenes (group A streptococcus), a human pathogen responsible for a wide spectrum of diseases and sequelae such as rheumatic fever and poststreptococcal glomerulonephritis (PSGN), produces many extracellular surface and secretory products (14, 22) that may help overcome host responses. Such streptococcal proteins may interact with host cells or host proteins and could contribute to pathogenesis (6). Recently much attention has been drawn to a secretory protein, streptococcal inhibitor of complement function (SIC) (1). Despite much work, the role of SIC in pathogenesis is largely elusive. The gene for this protein (sic) is generally found in a restricted number of group A streptococcal strains (1), although in a recent study, Ma et al. (23) reported a greater distribution of this gene among group A streptococcal M serotypes in a Japanese population.
SIC is expressed by all S. pyogenes isolates of serotype 1 (M1), is highly immunogenic in natural infection, and shows extraordinarily high sequence divergence which could arise within an epidemic wave (19, 20, 24, 25, 31). It appears that there is no association between specific sic alleles and severity of streptococcal invasive diseases (4, 17). Although sic variants have been known to emerge rapidly on the mucosal surface (20), examination of consecutive pharyngeal isolates from persistently infected patients suggests that variations in sic are not required for persistence (3).
The sic gene in M1 strains is present within the mga regulon, which also harbors genes for M protein (a major surface antigen) and C5a peptidase (1, 25). Earlier studies in this laboratory showed that M57 strains also possess a gene closely related to sic (crs57). However, the crs57 gene is located elsewhere in the genome (16). In the same report, we also showed that strains containing emm12 and emm55 (the genes for the type M12 and M55 proteins, respectively) possess a gene for a protein that is Distantly Related to SIC (DRS) within the mga regulon. Furthermore, emm12 and emm55 strains do not possess the crs gene. The similarity between DRS and SIC from M1 is confined to the signal sequence and a proline-rich region within the C-proximal half of SIC (and DRS). Ubiquitous occurrence of the DRS gene (drs) in all emm12 and emm55 strains suggests that DRS may have an important role in increasing the fitness of these strains in the host.
SIC is a multifunctional protein able to interact with diverse host cell proteins such as clusterin, a histidine-rich glycoprotein, ezrin, and components of the innate immune system (1, 9, 12, 18). The significance of its inhibitory action on complement-mediated cell lysis in group A streptococcal pathogenesis or virulence is not understood. Fernie-King et al. (10) showed that SIC prevented the incorporation of the C6 and C7 complement proteins into the membrane attack complex (MAC). Consistent with this, SIC has been shown to bind the C6 and C7 proteins and the intermediate forms of terminal complement complex. In their study, SIC marginally inhibited the hemolytic activity of preformed MAC on guinea pig erythrocytes.
In this communication, we show that DRS does not appear to inhibit complement function, as judged by hemolysis of sensitized sheep erythrocytes, nor does it seem to modulate hemolytic activity of SIC. However, DRS does bind to the C6 and C7 complement proteins and partially competes with SIC for binding. These results suggest that binding of SIC to C6 or C7 in itself may be insufficient for the inhibition of complement function.
MATERIALS AND METHODS
Strains, DNA, and antibodies.
Group A streptococcal isolates NS25 (emm55), NS488 (emm12), DRV1 (emm55), NS27 (emm91), NS844 (emm1), and BSA5 (emm57) were from the Northern Territory, Australia, group A streptococcal collections. A reference strain for emm1 (2031) was from Prague. The bacteria were grown in Todd-Hewitt Broth (THB; Oxoid) supplemented with neopeptone (15). DNA manipulations, vir typing, and sequencing were done as described previously (13, 16). Antibodies to SIC or DRS were raised in rabbits by immunizing (IMVS, Gilles Plains, Australia) with recombinant M1 SIC from 2031 or recombinant DRS from NS488, respectively. The polyclonal antibodies reacted specifically with their target antigens, although some cross-reactivity with SIC antibodies and DRS and vice versa was noted.
Cloning, expression, and purification of recombinant DRS and SIC.
The DRS gene (drs) was amplified from NS488 with sicFall (CTACTAGGAGCTACACAACC) and sicRdrs (TTTAATACCTTCAAAATAACCTCT) as the forward and reverse primers, respectively, in a PCR, and the product was cloned into the pBAD-TOPO-thio vector (Invitrogen). Likewise, sic from BSA5 was cloned after amplification with sicRcrs (CGTTGCTGATGGTGTATATGG) as the reverse primer. The recombinant vectors were transformed into Escherichia coli BL21. The thioredoxin fusion proteins containing a His6 tag at the C terminus were purified on an Ni-nitrilotriacetic acid matrix under nondenaturing conditions as described by the manufacturer (Qiagen). Clones expressing only His-tagged thioredoxin were also obtained, and the recombinant protein was purified as above. Figure 1 shows the expression of the recombinant SIC-thioredoxin and DRS-thioredoxin fusion proteins. The anti-SIC and anti-DRS antibodies did not react with thioredoxin. The majority of the fusion proteins are intact, as seen on the Coomassie blue-stained gel.
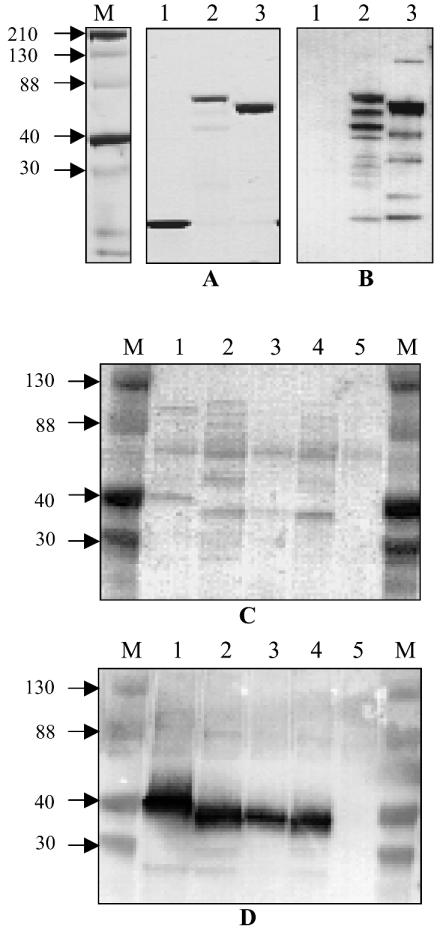
FIG. 1.
(A and B) Recombinant DRS expressed in E. coli as thioredoxin fusion proteins. The proteins were purified as described in the text. (A) Coomassie blue-stained SDS-polyacrylamide gels. (B) Western blot reacted with a mixture (1:1) of polyclonal antibodies against SIC and DRS. Lanes 1 to 3 show His-tagged thioredoxin, SIC (BSA5)-thioredoxin fusion protein, and DRS (NS488)-thioredoxin fusion protein, respectively. The antibodies did not react with thioredoxin. (C and D) Presence of DRS in culture supernatants of DRS-expressing strains. The culture supernatants were processed as described in the text. The SDS-polyacrylamide gels were transferred to a nitrocellulose membrane (Amersham) and either stained with Ponceau S (C) or reacted with a mixture of SIC and DRS antibodies (D) as described for panel B. Lanes 1 to 5 are culture supernatants from NS844, DRV1, NS488, NS25, and NS27, respectively. NS27 is a SIC- and DRS-negative control. Channels marked M contain Kaleidoscope (Bio-Rad) markers, and approximate sizes of the proteins are shown (in kilodaltons).
Detection of DRS12 and DRS55 in culture supernatants.
Overnight cultures (10 ml) of S. pyogenes (NS488, NS25, DRV1, NS844, and NS27) in THB were centrifuged at 10,000 × g for 10 min, and 1 ml of the culture supernatant was transferred to a new tube. The culture supernatants were concentrated by precipitation with trichloroacetic acid (10% final concentration) at −20°C for approximately 20 min. To retrieve the precipitated proteins the mixture was centrifuged at 16,000 × g for 20 min. The supernatant was discarded, and the pellet was resuspended in 100 μl of 0.1 M NaOH. Detection of SIC and DRS was achieved by fractionation of the sample on polyacrylamide gel electrophoresis (PAGE) (Gradipore Miniprotean II; Bio-Rad), followed by Western blotting. Anti-SIC and Anti-DRS primary antibodies at 1:1,000 in phosphate-buffered saline (PBS), and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin secondary antibodies (Sigma) were used for detection.
Binding of DRS to the complement proteins C6 and C7.
Assays for binding of DRS to the complement proteins C6 and C7 were performed by indirect enzyme-linked immunosorbent assay (ELISA). Ninety-six well plates (Titertek) were coated with 10 μg of recombinant proteins (DRS12 or thioredoxin) in PBS at 4°C overnight. After blocking with 5% skim milk in PBS, human sera (1:100; Sigma) or purified C6 and C7 (1 μg/ml final concentration; Sigma) was added and incubated for 1 h at 37°C in a final volume of 100 μl. The wells were then washed three times with PBS containing 0.5% Tween 20. Binding was detected with primary goat anti-C6 or anti-C7 antibody (1:1,000; ICN Biomedical) and horseradish peroxidase-conjugated rabbit anti-goat immunoglobulin (1:1,000; Sigma) secondary antibodies. The reaction was developed with o-phenylenediamine dihydrochloride (Sigma), and the absorbance was read at 450 nm in a Bio-Rad benchmark microplate reader. In competitive ELISAs, the complement proteins were incubated with the competing protein in PBS for 15 min at 37°C.
Complement-mediated hemolytic assay.
Sheep erythrocytes were activated with hemolysin (Virion, 1:500 dilution) in GVB++ (gelatin veronal buffer; Current Protocols in Immunology) buffer for 30 min at 37°C, followed by 30 min at 4°C. Human serum, used in this study as complement source, was usually titrated to 1:100 to 1:150 dilution in GVB++ buffer to give approximately 30 to 50% lysis. For the SIC assay, the serum was incubated with DRS (4 μg), SIC (4 μg), thioredoxin (control protein, 1.5 μg), or PBS for 30 min at 37°C. Osmolysis of erythrocytes with water was considered the 100% value. After removal of the unlysed erythrocytes by centrifugation at 1 700 × g, hemolysis was measured by reading absorbance at 415 nm in the Bio-Rad benchmark microplate reader.
RESULTS
Expression of DRS by emm12 and emm55 strains.
The genes for both DRS12 and DRS55 (drs12 and drs55, respectively) have signal sequences which are highly homologous to that of sic from M1 strains (16). This suggests that DRS, like SIC, is a secretory product. To test this, culture supernatant and whole-cell extracts from overnight cultures of NS844, DRV1, NS488, NS25, and NS27 were fractionated by sodium dodecyl sulfate (SDS) PAGE, and the Western blot was reacted with a mixture of SIC and DRS antibodies. In the representative experiment shown in Fig. 1, the antibodies reacted with 40- to 50-kDa proteins in culture supernatants of the emm12 and emm55 strains, and the intensity of the bands was not affected when the supernatants were passed through a 0.22-μm filter (data not shown). In general, both DRS and SIC showed anomalous mobilities on SDS-PAGE. DRS was detected in the whole-cell extracts of these strains, but in much lower quantities (data not shown). Similar results were obtained with SIC-expressing NS844 (an emm1 positive control). Strain NS27 (an emm91 strain), which does not possess sic or drs, did not show any detectable signal (negative control). Together, these observations provide further support that DRS, like SIC, is an extracellularly secreted protein.
DRS binds to C6 and C7.
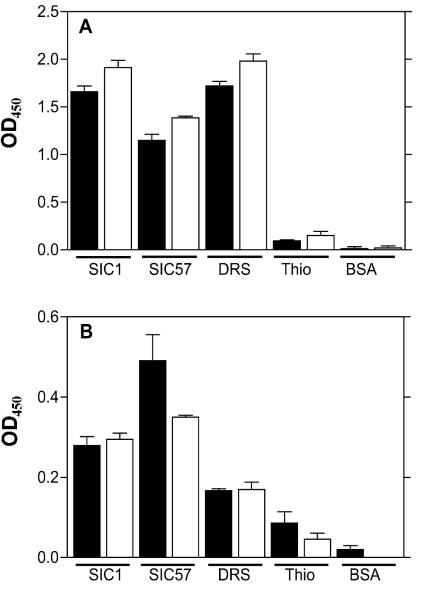
SIC from an M1 strain binds to intermediate terminal complement complexes (10). To determine whether the recombinant DRS protein also binds to the C6 and C7 complement proteins in serum, we purified the fusion protein containing thioredoxin (pBAD-TOPO-thio cloning system; Invitrogen). In our hands, expression of DRS in the pQE (Qiagen) system gave low yields when purified under nondenaturing conditions, and much of the recombinant protein accumulated in inclusion bodies. Furthermore, these recombinant proteins often degraded upon purification. The thioredoxin fusion has made a considerable improvement in the yield, solubility, and stability of the recombinant proteins (Fig. 1). Thioredoxin did not bind to the complement proteins and hence did not interfere with this assay (Fig. 2). Wells coated with the recombinant fusion proteins were reacted with human serum, and the binding of the C6 and C7 complement proteins was detected by appropriate antibodies. The results demonstrate that the complement components C6 and C7 in serum directly or indirectly bind to SIC and DRS (Fig. 2A). To confirm direct binding to the complement C6 and C7, we tested the binding using purified components in place of the human serum (Fig. 2B). It is noteworthy that the binding to C6 and C7 in the serum is far greater than to the individual complement component. The difference in binding may be due to conformational changes of C6 and C7 in the terminal complement complex or to the contribution of an indirect interaction with the complex. Taken together, our results demonstrate that DRS resembles SIC in the ability to bind to complement proteins.
FIG. 2.
Ability of DRS to bind to the C6 and C7 complement proteins. The microtiter wells were coated with proteins as indicated. The proteins were reacted with either (A) human serum or (B) complement protein C6 or C7. Binding was detected with anti-C6 (solid bars) or anti-C7 (open bars) primary antibodies.
SIC and DRS compete for complement binding.
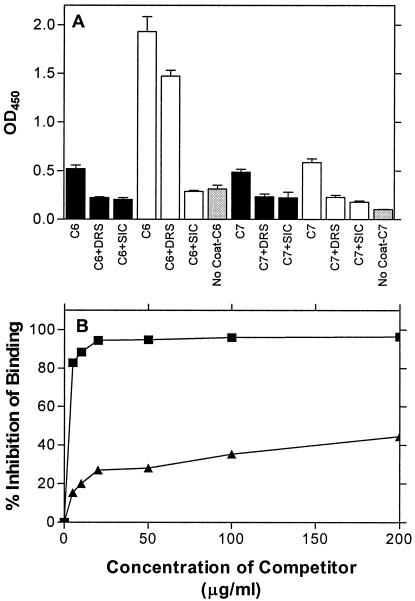
To test whether DRS inhibits binding of SIC to complement C6 and vice versa, competitive ELISA experiments were performed (Fig. 3). SIC-coated wells that were reacted with C6 that had been incubated with SIC or DRS (molar equivalent) showed significant inhibition of binding to C6. These experiments revealed that both SIC and DRS in solution competed against solid-surface-bound SIC for C6. Similarly, both SIC and DRS in solution competed against the bound DRS for C6 (Fig. 3A). Therefore, SIC and DRS mutually inhibited each other's binding to C6. The results of competition experiments were similar with C7. It is noteworthy that binding of C6 to SIC is greater than to DRS at the same molar concentrations. However, DRS competed only poorly with SIC for C6 (Fig. 3A). To further investigate this, we tested the effect of different concentrations of the competitors on C6 binding to SIC (Fig. 3B). Collectively, these results indicate that DRS has lower affinity than SIC for C6.
FIG. 3.
(A) Competition of SIC binding to complement proteins by DRS and vice versa. DRS (solid bars) and SIC (open bars) were used to coat microtiter wells and reacted with (a) C6, C6 and DRS, or C6 and SIC or (b) C7, C7 and DRS, or C7 and SIC, as indicated. Each set had a no-coat control (gray bars) in which the wells were not coated with either SIC or DRS. (B) Comparison of competitive inhibition by SIC and DRS of SIC binding to the C6 protein. Wells were coated with 10 μg of SIC and reacted with C6 that had been incubated with various concentrations of SIC (▪) or DRS (▴), as indicated. In the absence of the competitors, SIC and DRS bound to the C6 protein and had an optical density at 450 nm of 0.802 and 0.787, respectively, after subtraction of the respective backgrounds (0.126 and 0.154, respectively).
Recombinant DRS preparations did not inhibit complement-mediated hemolytic activity.
All SIC variants of M1 inhibit complement-mediated cell lysis. We recently demonstrated that SIC-like molecules (called CRS) from emm57 strains also inhibit complement function (2). Since DRS, like SIC, can bind to C6 and C7, we expected that DRS would also be endowed with such activity. To test this, sensitized sheep red blood cells were treated with human serum (complement source) with and without pretreatment with DRS. The results of three experiments are shown in Table 1. Human serum used as complement source lysed between 25 and 56% of the sensitized sheep erythrocytes, typifying the variability in these assays. Recombinant SIC from an emm57 strain at 25 μg/ml (4 μg/ml total) inhibited this complement-mediated hemolysis activity by 36 to 50%. Neither recombinant DRS nor thioredoxin (control protein) at comparable molar concentrations showed similar inhibitory activity.
TABLE 1.
DRS does not inhibit complement-mediated cell lysis
| Expt no. | Human serum preincubated with: | % hemolysisa | % inhibition |
|---|---|---|---|
| I | PBS | 55.5 | 0 |
| SIC | 35.2 | 36.6 | |
| DRS | 64.4 | 0 | |
| Thioredoxin | 67.6 | 0 | |
| II | PBS | 32.2 | 0 |
| SIC | 17.5 | 45.7 | |
| DRS | 35.3 | 0 | |
| Thioredoxin | 46.1 | 0 | |
| III | PBS | 25 | 0 |
| SIC | 12.6 | 49.6 | |
| SIC + DRS | 14.6 | 41.6 | |
| DRS | 27.1 | 0 | |
| Thioredoxin | 25.5 | 0 |
Percent hemolysis was determined for each sample in triplicate, and averages are shown. Osmolysis of erythrocytes with water was taken as 100% lysis.
Since DRS inhibited the binding of SIC to the C6 and C7 complement proteins, we questioned whether the addition of DRS, which on its own has no effect on complement function, would inhibit SIC by competing for binding sites on C6 and C7. For this study, human serum was incubated with a mixture of SIC and DRS (4 μg of each) before addition of the mixture to the hemolytic assay. The results (Table 1, experiment III) show that DRS did not have any significant effect on the hemolysis-inhibitory activity of SIC.
DISCUSSION
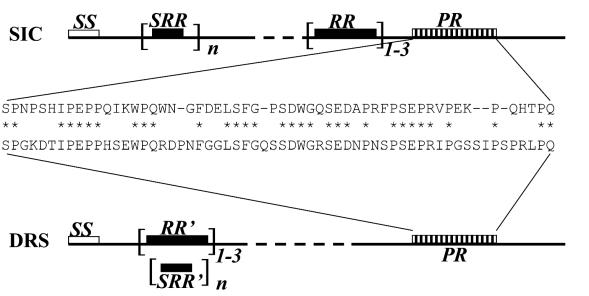
As mentioned earlier, SIC is likely to be a multifunctional protein able to partner with numerous host ligands. Mutations in SIC seem to occur throughout the molecule, and the number of repeat sequences varies. However, there are some common features of SIC that may account for the interactions mentioned above. The common features are a short consensus repeat structures containing tryptophan residues, one or more repeat regions, and a proline-rich region (Fig. 4). While all emm1 isolates express SIC, a gene for CRS was also found in emm57 strains. We recently demonstrated that CRS57, like SIC from M1 strains, exhibits inhibitory activity on complement-mediated lysis and binds to complement proteins (2). CRS57 has all the above features of SIC and has >80% amino acid sequence identity with SIC. On the other hand, emm12 and emm55 strains have DRS, which exhibits similarity only within the proline-rich region comprising approximately 55 residues. Although DRS molecules contain repeat regions and short repeats, their primary sequences are not similar to those of the SIC molecules. Therefore, it is possible that DRS may share only a limited repertoire of activities with SIC. In this report, we showed that DRS, like SIC, is capable of binding to the C6 and C7 complement proteins and competes with SIC for binding to these complement proteins. Since SIC and DRS share sequence similarity within the proline-rich region, we predict that it is this region that may be responsible for binding to complement proteins.
FIG. 4.
Common features between SIC and DRS. Signal sequence (SS), short repeat regions (SRR), repeat regions (RR), and proline-rich regions (PR) are shown. The DRS molecule has different repeat regions (RR′) which contain short repeat region (SRR′). The similarity of the proline-rich region is indicated.
Despite its ability to compete with SIC for binding to complement proteins C6 and C7, the recombinant DRS preparation had little effect on the complement function, nor did it interfere with SIC's inhibition of complement function. Possible explanations for this observation are that binding to C6 or C7 is not necessary for the inhibitory activity of SIC on complement-mediated hemolysis; that subtle differences in the binding characteristics of SIC and DRS to the complement proteins (see Fig. 3) alter their effect on complement activity; and that greater affinity of SIC for the complement protein outcompetes DRS binding. The first possibility is consistent with the interpretation of an earlier study (10) that binding of SIC to the terminal complement complex or to the purified complement components is unlikely to have any functional implications.
DRS expressing M types M12 and M55 are historically associated with post-streptococcal glomerulonephritis (PSGN), a major sequela of S. pyogenes infection (8, 11, 21). PSGN is an immune complex-mediated disease (26, 32) wherein the glomerular injury is probably initiated by the activity of terminal complement complex (27) and in which circulating streptococcal antigen and complement complexes are codeposited on the glomerular basement membrane early in the natural history of the disease. Antibodies to the streptococcal antigen and decreased levels of complement have also been consistently noted in PSGN patients (7). The identity of the streptococcal nephritogenic antigens is still speculative, and there is no reason to believe that a single antigen is the cause of this disease (5, 26, 28, 33-35). However, Rodriquez-Iturbe (29) described possible criteria for a PSGN-associated streptococcal antigen. They are that the antigen is likely to be a secretory product of a nephritis-associated strain, that it is found in the glomerulus of PSGN patients early in natural history of the disease, and that convalescing patients show antibodies to the antigen. The biochemical and antigenic properties of DRS potentially fulfill these criteria for a PSGN-associated antigen.
DRS is a secretory product of M12 and M55 strains, historically PSGN-associated serotypes. The protein is capable of binding to the complement proteins C6 and C7 and thus has the potential to colocalize with the MAC in the glomeruli of PSGN patients. Yet DRS does not appear to interfere with MAC function, which could promote initiation of glomerular injury, and once planted on the glomerular basement membrane, DRS may form an antigen-antibody complex with circulating DRS antibodies. Consistent with this, we recently observed (30) that DRS is highly immunogenic, and antibodies are present in a significantly greater number of subjects who have a history of PSGN than in subjects without such a history in a PSGN-endemic population.
In summary, the ability to incorporate into MAC through interaction with C6 and C7 without affecting MAC function may endow DRS with the potential to cause renal tissue damage in PSGN. Further work is in progress to test this hypothesis.
Acknowledgments
We thank the Australian National Health and Medical Research Council for financial support.
Editor: V. J. DiRita
REFERENCES
- 1.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. [DOI] [PubMed] [Google Scholar]
- 2.Binks, M., D. McMillan, and K. S. Sriprakash. 2003. Genomic location and variation of the gene for CRS, a complement binding protein in the M57 strains of Streptococcus pyogenes. Infect. Immun. 71:6701-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt, C. M., F. Allerberger, B. Spellerberg, R. Holland, R. Lutticken, and G. Haase. 2001. Characterization of consecutive Streptococcus pyogenes isolates from patients with pharyngitis and bacteriological treatment failure: special reference to prtF1 and sic/drs. J. Infect. Dis. 183:670-674. [DOI] [PubMed] [Google Scholar]
- 4.Chatellier, S., N. Ihendyane, R. G. Kansal, F. Khambaty, H. Basma, A. Norrby-Teglund, D. E. Low, A. McGeer, and M. Kotb. 2000. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68:3523-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cu, G. A., S. Mezzano, J. D. Bannan, and J. B. Zabriskie. 1998. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 54:819-826. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dedeoglu, I. O., J. E. Springate, W. R. Waz, F. B. Stapleton, and L. G. Feld. 1996. Prolonged hypocomplementemia in poststreptococcal acute glomerulonephritis. Clin. Nephrol. 46:302-305. [PubMed] [Google Scholar]
- 8.Dillon, H. C., Jr. 1979. Post-streptococcal glomerulonephritis following pyoderma. Rev. Infect. Dis. 1:935-945. [DOI] [PubMed] [Google Scholar]
- 9.Fernie-King, B. A., D. J. Seilly, A. Davies, and P. J. Lachmann. 2002. Streptococcal inhibitor of complement inhibits two additional components of the mucosal innate immune system: secretory leukocyte proteinase inhibitor and lysozyme. Infect. Immun. 70:4908-4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernie-King, B. A., D. J. Seilly, C. Willers, R. Wurzner, A. Davies, and P. J. Lachmann. 2001. Streptococcal inhibitor of complement (SIC) inhibits the membrane attack complex by preventing uptake of C567 onto cell membranes. Immunology 103:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrieri, P., A. S. Dajani, S. S. Chapman, J. B. Jensen, and L. W. Wannamaker. 1970. Appearance of nephritis associated with type 57 streptococcal impetigo in North America. N. Engl. J. Med. 283:832-836. [DOI] [PubMed] [Google Scholar]
- 12.Frick, I. M., P. Akesson, M. Rasmussen, A. Schmidtchen, and L. Bjorck. 2003. SIC—a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J. Biol. Chem. 5:5. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner, D., J. Hartas, B. Currie, J. D. Mathews, D. J. Kemp, and K. S. Sriprakash. 1995. Vir typing: a long-PCR typing method for group A streptococci. PCR Methods Appl. 4:288-293. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, C. M., and M. G. Caparon. 2002. Alkaline phosphatase reporter transposon for identification of genes encoding secreted proteins in gram-positive microorganisms. Appl. Environ. Microbiol. 68:928-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillen, C. M., R. J. Towers, D. J. McMillan, A. Delvecchio, K. S. Sriprakash, B. Currie, B. Kreikemeyer, G. S. Chhatwal, and M. J. Walker. 2002. Immunological response mounted by Aboriginal Australians living in the Northern Territory of Australia against Streptococcus pyogenes serum opacity factor. Microbiology 148:169-178. [DOI] [PubMed] [Google Scholar]
- 16.Hartas, J., and K. S. Sriprakash. 1999. Streptococcus pyogenes strains containing emm12 and emm55 possess a novel gene coding for distantly related SIC protein. Microb. Pathog. 26:25-33. [DOI] [PubMed] [Google Scholar]
- 17.Haukness, H. A., R. R. Tanz, R. B. Thomson, Jr., D. K. Pierry, E. L. Kaplan, B. Beall, D. Johnson, N. P. Hoe, J. M. Musser, and S. T. Shulman. 2002. The heterogeneity of endemic community pediatric group a streptococcal pharyngeal isolates and their relationship to invasive isolates. J. Infect. Dis. 185:915-920. [DOI] [PubMed] [Google Scholar]
- 18.Hoe, N. P., R. M. Ireland, F. R. DeLeo, B. B. Gowen, D. W. Dorward, J. M. Voyich, M. Liu, E. H. Burns, Jr., D. M. Culnan, A. Bretscher, and J. M. Musser. 2002. Insight into the molecular basis of pathogen abundance: group A Streptococcus inhibitor of complement inhibits bacterial adherence and internalization into human cells. Proc. Natl. Acad. Sci. USA 99:7646-7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoe, N. P., P. Kordari, R. Cole, M. Liu, T. Palzkill, W. Huang, D. McLellan, G. J. Adams, M. Hu, J. Vuopio-Varkila, T. R. Cate, M. E. Pichichero, K. M. Edwards, J. Eskola, D. E. Low, and J. M. Musser. 2000. Human immune response to streptococcal inhibitor of complement, a serotype M1 group A Streptococcus extracellular protein involved in epidemics. J. Infect. Dis. 182:1425-1436. [DOI] [PubMed] [Google Scholar]
- 20.Hoe, N. P., K. Nakashima, S. Lukomski, D. Grigsby, M. Liu, P. Kordari, S. J. Dou, X. Pan, J. Vuopio-Varkila, S. Salmelinna, A. McGeer, D. E. Low, B. Schwartz, A. Schuchat, S. Naidich, D. De Lorenzo, Y. X. Fu, and J. M. Musser. 1999. Rapid selection of complement-inhibiting protein variants in group A Streptococcus epidemic waves. Nat. Med. 5:924-929. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, E. L., B. F. Anthony, S. S. Chapman, and L. W. Wannamaker. 1970. Epidemic acute glomerulonephritis associated with type 49 streptococcal pyoderma. I. Clinical and laboratory findings. Am. J. Med. 48:9-27. [DOI] [PubMed] [Google Scholar]
- 22.Lei, B., S. Mackie, S. Lukomski, and J. M. Musser. 2000. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect. Immun. 68:6807-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, X., H. Kikuta, N. Ishiguro, M. Yoshioka, T. Ebihara, T. Murai, I. Kobayashi, and K. Kobayashi. 2002. Association of the prtF1 gene (encoding fibronectin-binding protein F1) and the sic gene (encoding the streptococcal inhibitor of complement) with emm types of group A streptococci isolated from Japanese children with pharyngitis. J. Clin. Microbiol. 40:3835-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mejia, L. M., K. E. Stockbauer, X. Pan, A. Cravioto, and J. M. Musser. 1997. Characterization of group A streptococcus strains recovered from Mexican children with pharyngitis by automated DNA sequencing of virulence-related genes: unexpectedly large variation in the gene (sic) encoding a complement-inhibiting protein. J. Clin. Microbiol. 35:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mylvaganam, H., B. Bjorvatn, and A. Osland. 2001. Polymorphism of the virulence regulon and allelic variations of the sic gene among the emm1 isolates of group A Streptococcus from western Norway. Microb. Pathog. 30:71-79. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira, D. B. 1997. Poststreptococcal glomerulonephritis: getting to know an old enemy. Clin. Exp. Immunol. 107:8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parra, G., J. L. Platt, R. J. Falk, B. Rodriguez-Iturbe, and A. F. Michael. 1984. Cell populations and membrane attack complex in glomeruli of patients with post-streptococcal glomerulonephritis: identification using monoclonal antibodies by indirect immunofluorescence. Clin. Immunol. Immunopathol. 33:324-332. [DOI] [PubMed] [Google Scholar]
- 28.Parra, G., B. Rodriguez-Iturbe, S. Batsford, A. Vogt, S. Mezzano, F. Olavarria, R. Exeni, M. Laso, and N. Orta. 1998. Antibody to streptococcal zymogen in the serum of patients with acute glomerulonephritis: a multicentric study. Kidney Int. 54:509-517. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Iturbe, B. 1984. Epidemic poststreptococcal glomerulonephritis. Kidney Int. 25:129-136. [DOI] [PubMed] [Google Scholar]
- 30.Sriprakash, K. S., J. Hartas, and A. White. 2002. Antibodies to streptococcal inhibitor of complement function and M peptides in a post-streptococcal glomerulonephritis endemic region of Australia. J. Med. Microbiol. 51:589-594. [DOI] [PubMed] [Google Scholar]
- 31.Stockbauer, K. E., D. Grigsby, X. Pan, Y. X. Fu, L. M. Mejia, A. Cravioto, and J. M. Musser. 1998. Hypervariability generated by natural selection in an extracellular complement-inhibiting protein of serotype M1 strains of group A Streptococcus. Proc. Natl. Acad. Sci. USA 95:3128-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treser, G., M. Semar, M. McVicar, M. Franklin, A. Ty, I. Sagel, and K. Lange. 1969. Antigenic streptococcal components in acute glomerulonephritis. Science 163:676-677. [DOI] [PubMed] [Google Scholar]
- 33.Villarreal, H., Jr., V. A. Fischetti, I. van de Rijn, and J. B. Zabriskie. 1979. The occurrence of a protein in the extracellular products of streptococci isolated from patients with acute glomerulonephritis. J. Exp. Med. 149:459-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamakami, K., N. Yoshizawa, K. Wakabayashi, A. Takeuchi, T. Tadakuma, and M. D. Boyle. 2000. The potential role for nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Methods 21:185-197. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizawa, N., S. Oshima, A. Takeuchi, S. Kondo, T. Oda, J. Shimizu, J. Nishiyama, A. Ishida, I. Nakabayashi, K. Tazawa, and Y. Sakurai. 1997. Experimental acute glomerulonephritis induced in the rabbit with a specific streptococcal antigen. Clin. Exp. Immunol. 107:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]