Abstract
Purpose
The burden of cancer in Africa is an enlarging public health challenge. Breast cancer in Ghana is the second most common cancer among Ghanaian women and the proportion of diagnosed patients who complete prescribed treatment is estimated to be very limited, thereby potentially adding to lower survival and poor quality of life after diagnosis. The objective of this study was to identify the patient and system factors related to incomplete treatment of breast cancer among patients.
Methods
This study was conducted at the Komfo Anokye Teaching Hospital in Kumasi, Ghana. We interviewed 117 breast cancer patients and next of kin of breast cancer patients diagnosed from 2008 to 2010.
Results
Islamic religion, seeking treatment with traditional healers, and lack of awareness about national health insurance coverage of breast cancer treatment were predictors of incomplete treatment.
Conclusions
The results of this study support that Ghanaian women with diagnosed breast cancer have multiple addressable and modifiable patient factors that may deter them from completing the prescribed treatment. The results highlight the need for developing and testing specific interventions about the importance of completing treatment with a special focus on addressing religious, cultural, and system navigation barriers in developing countries.
Keywords: Breast cancer, Ghana, Africa, Incomplete treatment factors
Introduction
Breast cancer is the most common cancer among women and is the leading cause of cancer-related mortality in women worldwide [1]. Breast cancer is the second most common cancer among African women, after cervical cancer, and is among the top two leading causes of cancer deaths among Ghanaian women [2–5]. Globocan estimated the annual incidence rate of breast cancer in Ghana as 25.8 cases per 100,000 women and the mortality rate at 15.2 deaths per 100,000 women, but these rates likely reflect underestimates[6, 7]. In Ghana, data on breast cancer cases are slowly becoming more available through better documentation of hospital admissions, suggesting that Ghana has a higher incidence of breast cancer than estimations from global statistical predictions [3]. Breast cancer incidence rates in Ghana are rising due to the aging population, demographic and reproductive changes, and improved access to diagnostic and treatment resources [8]. Despite multiple attempts at improving cancer control, late-stage diagnosis characterizes about 60% of the cases, which coupled to a preponderance of high-grade histological subtypes and high rate of triple negative tumors, all contribute to poor prognosis and short survival [3, 4, 9, 10]. From the Western literature, it has been established that prompt initiation of treatment and completion of evidence-based effective treatment are essential to ensure optimal survival, especially in more aggressive breast cancer subtypes, which appear to be more prevalent in African and in women of African extraction in the US, thus, understanding barriers to care and treatment completion within the country will be vital in improving access to care, increasing the utilization of diagnostic and treatment facilities, and facilitating early diagnosis and treatment of the increasing number of patients [11]. We characterized the breast cancer patients seen at Komfo Anokye Teaching Hospital (KATH) and recorded their disease management during the period of 2008–2010, and the current study builds upon the preliminary studies that explored reasons for lack of treatment completion for breast cancer [7, 12]. Because of limited information in some medical records about reasons for lack of completion of prescribed treatment in previous studies, the objective of this study was to identify the diverse salient factors specifically related to incomplete treatment of breast cancer among patients diagnosed at KATH through patient interviews.
Methods
This study was conducted in the Ashanti region of central Ghana, which is the most populous and fastest growing region, with a population of 4,780,380 in the 2010 census [13]. KATH in Kumasi currently serves as an epicenter for cancer treatment, and breast cancer is the most common cancer seen at KATH, making it an ideal location to study breast cancer treatment patterns in Ghana [12, 13].
In a previous study, we collected information on all cancer patients seen at the KATH’s departments of surgery, pathology, and medical oncology/radiology to estimate the cancer burden during the period of 2008–2010 [12]. We also delineated patterns of breast cancer treatment and established preliminary predictors of treatment completion. However, due to incomplete information in the medical records and the necessity for follow-up, previously, we were not able to identify reasons for 20% of the women who had not completed their treatment.
Study Population
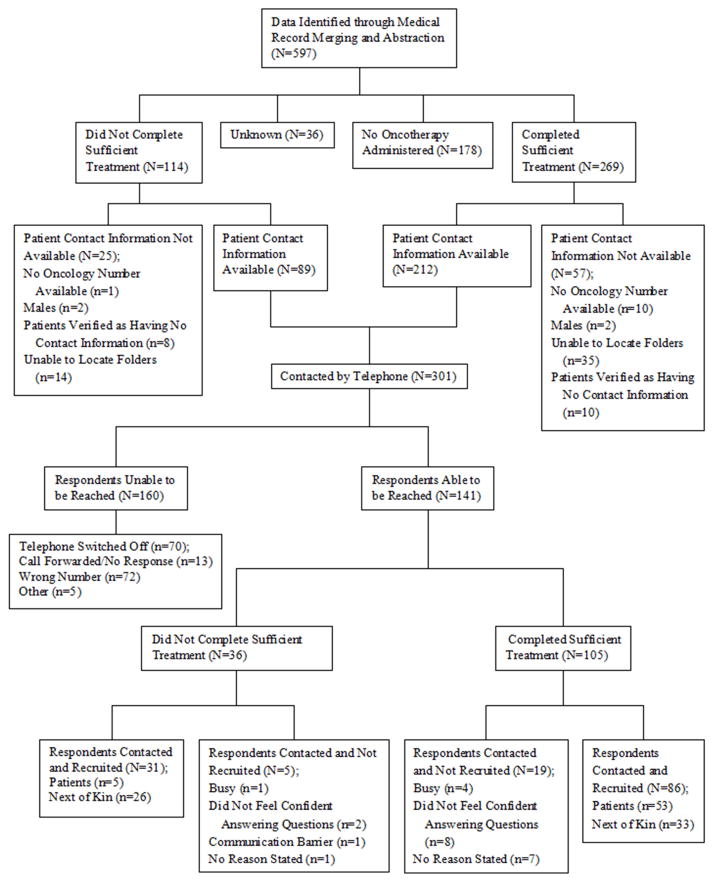
The target population for this study was Ghanaian women with diagnosed breast cancer at KATH. Our previous studies identified 597 breast cancer patients who had attended KATH at the Medical Oncology/Radiation Center, Surgical Center, or Pathology Department at KATH in 2008–2010 [7]. For this study, the inclusion criteria were Ghanaian women 19 years of age or older, who had a positive diagnosis for breast cancer, and those who were identified previously through medical record abstraction as having either completed treatment or not having completed treatment. The category of “patients who were identified as having completed treatment” applies only to patients who finished their prescribed combination of chemotherapy, hormone therapy, and/or radiotherapy, or those individuals who received a partial cycle of their last chemotherapy regimen, but had no written note from the doctor or nurse expecting them to return. Patients who did not complete therapy were classified into one of three categories: 1) those who completed a partial regimen of chemotherapy and had an explicitly written note in their medical charts expecting them to return for more cycles; 2) those who did not complete radiotherapy; and 3) patients who were prescribed hormone therapy, and who were explicitly expected in the medical records to return for further evaluation or a drug change but did not return. Exclusion criteria consisted of individuals who were seen at the center, but had no radiation, chemotherapy, or hormone therapy prescribed or had an unknown treatment completion status, individuals whose follow-up was impossible due to missing contact information, and patients who are deceased and do not have next of kin available for questionnaire administration. After inclusion and exclusion criteria were met, the eligible sample consisted of 301 females. All 301 eligible participants were called via a mobile phone by a KATH oncology nurse. All patients and next of kin successfully contacted were asked to consider the informed consent over the telephone prior to recruitment into the study. We were able to successfully speak with 141 patients or next of kin, and recruited 117 patients or next of kin to the study. Figure 1 illustrates the selection and recruitment process.
Figure 1.
Selection and Recruitment Process Diagram
Data Collection
Data were collected in two ways: obtaining information via a medical record database at KATH and using interviewer-administered questionnaires. Socio-demographic information was extracted from the medical records, and the questionnaires solely contained questions that were considered to be patient factors or systems factors. Patient factors included items such as family support structures, beliefs about treatment, response to treatment, and attitudes towards the disease and treatments. The systems factors focused on items such as access to care, follow-up appointments being clearly stated, and availability of chemotherapy drugs.
The questionnaire was developed based on information in the literature commonly associated with delayed or absconding from treatment, known issues involving scarcity of drugs in developing countries, and qualitative observations by University of Michigan students and Ghanaian health care providers from KATH regarding disparities in beliefs about insurance coverage among hospital employees and patients alike [4, 14–16]. The questionnaire was first written for patients, and was then adapted in form only as needed for administration to the next of kin, so that the slight differences in wording would reflect the opinions of the patient, transmitted through the recollection of the next of kin. In Ghanaian culture, family values and loyalty are strong; thus, most often, the patients are accompanied to appointments at the oncology center by next of kin, and close family members are often involved with the patient’s treatment. Each questionnaire was translated into the native language, Twi, by a Ghanaian community health expert. The questionnaires were reviewed by the head of the oncology directorate, and changes were made based on his experience with direct contact with breast cancer patients and their families. An experienced oncology nurse was selected for questionnaire administration, and was trained on the protocol, the questionnaire administration process, and ethical considerations. The nurse recruited and administered all the questionnaires in the native language as needed, between July and October of 2013. After interviewing a sample of 10 patients, it was determined that 4 questions were too specialized as was evident by the number of next of kin who did not know the answers and the number of patients who were able to recall the answers. These questions were removed from the analysis based on the preliminary findings after it was discovered that one patient subgroup contained significantly more next of kin. It was also determined that patient comments not included in the questionnaire would be written in the margin to maximize the insight provided by the patient or next of kin into their breast cancer treatment.
The study was approved by the Institutional Review Boards (IRBs) of the Kwame Nkrumah University of Science and Technology (KNUST)/KATH, University of Nebraska Medical Center (UNMC), the Eppley Cancer Center, and the University of Michigan.
Data Management and Statistical Analysis
All answers were cross-checked three times with each completed questionnaire and recruitment form to ensure quality assurance of data transfer into a database. Data analysis was carried out using the Statistical Analysis Software (SAS) version 9.3. Socio-demographic factors, patient factors, and systems factors were tabulated to provide the number and percentage for categorical variables, and the mean, median, standard deviation, and range for continuous variables for the two case groups of interest: those who did not complete treatment (DNC) and those who completed treatment (CT). Statistical differences were analyzed to determine significant differences between the DNC and CT groups using univariate t-tests for continuous variables, and univariate chi squared tests or Fisher’s Exact tests for categorical variables. A total of 11 patients received chemotherapy at locations outside of KATH, and they were included in the analysis because some portion of their breast cancer treatment occurred at KATH. Logistic models were constructed to predict the final outcome as defined by treatment completion, which was coded dichotomously. The logistic regression model was constructed stepwise, and adjusted for all socio-demographic variables throughout each course of model development. The first step in model development included socio-demographic variables and all significant univariate results from Table 2 and Table 3. The model continued with the least significant patient or system factors variables removed from the model until only the socio-demographic variables and the significant variables were left in the final model. Variables such as region of residence, occupation, and marital status were included in the multivariate model because of their importance even though they were not statistically significant in the univariate analysis. Any observation designated ‘unknown’ was eliminated prior to the final model construction. Variables in which ‘unknown’ was present in Table 1 included stage (N=8), marital status (N=2), and religion (N=4, ‘traditional/unknown’). Multiple questions about patient or systems factors had answers of ‘I don’t know’, and these answers were verified as including primarily next of kin respondents. Any next of kin respondent stating ‘I don’t know’ was eliminated from the final logistic regression model. Any cell containing 0 was eliminated from the final model to remove inaccuracy of the calculated odds ratio (OR) and 95% Confidence Interval (CI).
Table 2.
Patient factors affecting treatment completion
| Variable (N1,N2) | Completed Treatment N (%1) |
Did Not Complete Treatment N (%1) |
p-value2 |
|---|---|---|---|
| Questionnaire Respondents (86, 31) | <0.0001 | ||
| Patients | 53 (61.63%) | 5 (16.13%) | |
| Next of Kin | 33 (38.37%) | 26 (83.87%) | |
| Patient Factors | |||
| Traditional Healer Sought for Breast Care (86, 31) | <0.0001 | ||
| Yes | 12 (13.95%) | 14 (45.16%) | |
| No | 74 (86.05%) | 14 (45.16%) | |
| I Don’t Know | 0 (0.00%) | 3 (9.68%) | |
| Knowledge of Insurance coverage (85, 31) | 0.0124 | ||
| Yes | 76 (89.41%) | 21 (67.74%) | |
| No | 8 (9.41%) | 7 (22.58%) | |
| I Don’t Know | 1 (3.45%) | 3 (9.68%) | |
| Fearful of Community Reaction (84, 31) | <0.0001 | ||
| Yes | 60 (71.43%) | 16 (51.61%) | |
| No | 23 (27.38%) | 8 (25.81%) | |
| I Don’t Know | 1 (1.19%) | 7 (22.58%) | |
| Knowledge of a Person Surviving Cancer (84, 30) | 0.0098 | ||
| Yes | 51 (60.71%) | 10 (33.33%) | |
| No | 33 (39.29%) | 20 (66.67%) | |
| Delayed Treatment after Self-Detected Symptom (81, 26) | 0.3455 | ||
| Less than 1 Month | 30 (37.04%) | 7 (26.92%) | |
| Greater than 1 Month | 51 (62.96%) | 19 (73.08%) | |
| Source of Knowledge About Traditional Healer (85, 31) | |||
| Internet | N/A | ||
| No | 85 (73.28%) | 31 (26.72%) | |
| Family, Friend, Coworker | 0.5630 | ||
| Yes | 28 (32.94%) | 12 (38.71%) | |
| No | 57 (67.06%) | 19 (61.29%) | |
| Street Advertising | 1.0000 | ||
| Yes | 1 (1.18%) | 0 (0.00%) | |
| No | 84 (98.82%) | 31 (100.00%) | |
| Radio | 0.3777 | ||
| Yes | 62 (72.94%) | 20 (64.52%) | |
| No | 23 (27.06%) | 11 (35.48%) | |
| Community Traditional Healer | 0.3890 | ||
| Yes | 2 (2.35%) | 0 (0.00%) | |
| No | 83 (97.65%) | 31 (100.00%) | |
| Patient was scared of treatment (85, 31) | 0.5122 | ||
| Yes | 30 (35.29%) | 13 (41.94%) | |
| No | 55 (64.71%) | 18 (58.06%) | |
| Patient believed cancer is incurable (85, 31) | 0.7072 | ||
| Yes | 4 (4.71%) | 2 (6.45%) | |
| No | 81 (95.29%) | 29 (93.55%) | |
| Patient believed traditional healer is better At managing cancer than the physician (82, 27) | 0.0859 | ||
| Yes | 4 (4.88%) | 4 (14.81%) | |
| No | 78 (95.12%) | 23 (85.19%) | |
Percents reflect that of each column
P-values were calculated using the Chi-squared test or the Fisher’s Exact test. The Fisher’s Exact test was employed if any variable had a cell count of less than 5
N1 The total number of respondents for a given variable within the ‘Completed Treatment’ group
N2 The total number of respondents for a given variable within the ‘Did Not Complete’ group
Table 3.
Systems factors affecting treatment completion
| Variable (N1,N2) | Completed Treatment N (%1) |
Did Not Complete Treatment N (%1) |
p-value5 |
|---|---|---|---|
| Questionnaire Respondents (86, 31) | <0.0001 | ||
| Patients | 53 (61.63%) | 5 (16.13%) | |
| Next of Kin | 33 (38.37%) | 26 (83.87%) | |
| Systems Factors | |||
|
| |||
| Time to Travel to hospital (minutes) (85, 30) | 0.5297 | ||
| Mean | 105.65 | 107.16 | |
| Median | 60 | 45 | |
| Std. Dev. | 139.32 | 107.16 | |
| Range | 10–720 | 15–480 | |
|
| |||
| Breast cancer education materials Provided by hospital (85, 30) | 0.4144 | ||
| Yes | 7 (8.54%) | 4 (13.33%) | |
| No | 78 (91.76%) | 26 (86.67%) | |
| Follow-up appointments clearly stated by hospital staff (85, 30) | 0.0664 | ||
| Yes | 85 (100.00%) | 28 (93.33%) | |
| No | 0 (0.00%) | 2 (6.67%) | |
| Lengthy travel to hospital (84, 30) | 0.6197 | ||
| Yes | 38 (45.24%) | 12 (40.00%) | |
| No | 46 (54.76%) | 18 (60.00%) | |
| Lengthy oncology waiting periods (85, 29) | 0.7102 | ||
| Yes | 56 (65.88%) | 18 (62.07%) | |
| No | 29 (34.12%) | 11 (37.93%) | |
| Knowledge of cancer funding source in Ghana (not including the National Health Insurance Scheme) (84, 30) | 1.0000 | ||
| Yes | 5 (5.95%) | 2 (6.67%) | |
| No | 79 (94.05%) | 28 (93.33%) | |
| Hospital staff made patient aware of the importance of completing treatment (85, 30) | 0.3538 | ||
| Yes3 | 76 (89.41%) | 27 (90.00%) | |
| No | 7 (8.24%) | 1 (3.33%) | |
| I Don’t Know | 2 (2.35%) | 2 (6.67%) | |
| Chemotherapy administered (85, 29) | 0.7506 | ||
| Yes | 75 (88.24%) | 25 (86.21%) | |
| No | 10 (11.76%) | 4 (13.79%) | |
| Drug always available at KATH (75, 25) | 0.5636 | ||
| Yes | 38 (50.67%) | 11 (44.00%) | |
| No | 37 (49.33%) | 14 (56.00%) | |
| Traveled to outside pharmacy to pick up chemotherapy drugs (75, 25) | 0.1534 | ||
| Yes | 74 (98.67%) | 23 (92.00%) | |
| No | 1 (1.33%) | 2 (8.00%) | |
| Unavailability of chemotherapy drugs at hospital delayed treatment (75, 25) | 0.0329 | ||
| Yes | 1 (1.33%) | 3 (12.00%) | |
| No | 73 (97.33%) | 21 (84.00%) | |
| I Don’t Know | 1 (1.33%) | 1 (4.00%) | |
| Early detection (≤ Stage IIIa) (81, 28) | 0.0133 | ||
| Yes | 39 (48.15%) | 6 (21.43%) | |
| No | 42 (51.85%) | 22 (78.57%) | |
Percents reflect that of each column
P-values were calculated using the Chi-squared test or the Fisher’s Exact test. The Fisher’s Exact test was employed if any variable had a cell count of less than 5
Yes indicates the respondent stated a doctor, nurse, or other hospital staff member made them aware completing treatment was important
N1 The total number of respondents for a given variable within the ‘Completed Treatment’ group
N2 The total number of respondents for a given variable within the ‘Did Not Complete’ group
Table 1.
Socio-Demographic Characteristics of Breast Cancer Patients
| Variable | Completed Treatment N=86 |
Did Not Complete Treatment N=31 |
p-value5 |
|---|---|---|---|
| Age (years) | 0.9974 | ||
| Mean | 53.56 | 53.55 | |
| Median | 51 | 52 | |
| Std. Dev. | 15.16 | 11.11 | |
| Range | 24–99 | 31–82 | |
| Stage of Disease N (%1) | 0.2030 | ||
| I/II | 24 (27.91%) | 4 (12.90%) | |
| III/IV | 57 (66.28%) | 24 (77.42%) | |
| Unknown | 5 (5.81%) | 3 (9.68%) | |
| Region of Residence N (%1) | 0.2056 | ||
| Ashanti | 68 (79.07%) | 20 (64.52%) | |
| Greater Central And Northern Ghana2 | 14 (16.28%) | 9 (29.03%) | |
| Southern Ghana and External Areas3 | 4 (4.65%) | 2 (6.45%) | |
| Occupation N (%1) | 0.0647 | ||
| Business | 36 (41.86%) | 12 (38.71%) | |
| Farming/Agriculture | 11 (12.79%) | 9 (29.03%) | |
| Professional Occupations | 18 (20.93%) | 1 (3.23%) | |
| Service (Low-Income) | 7 (8.14%) | 3 (9.68%) | |
| Unemployed4 | 14 (16.28%) | 6 (19.35%) | |
| Marital Status N (%1) | 1.0000 | ||
| Married | 47 (54.65%) | 17 (54.84%) | |
| Other (Divorced/Single/Widowed) | 37 (43.02%) | 14 (45.16%) | |
| Unknown | 2 (2.33%) | 0 (0.00%) | |
| Religion N (%1) | 0.0248 | ||
| Christian | 76 (88.37%) | 21 (67.74%) | |
| Muslim/Islamic | 8 (9.30%) | 8 (25.81%) | |
| Traditional/Unknown | 2 (2.33%) | 2 (6.45%) |
Percents reflect that of each column
Includes Brong Ahofo, Eastern, Northern, Upper East, Upper West, and Volta Regions
Includes Central, Greater Accra, Western Regions, and areas outside of Ghana
Includes Students, Retired/Pensioners, and Unemployed
P-values were calculated using the t-test for continuous variables and the Chi-squared test or the Fisher’s Exact test for categorical variables. The Fisher’s Exact test was employed if any variable category had a cell count of less than 5.
Results
Out of 141 potential participants who were successfully contacted for the study, 117 completed the phone interviews, with 58 patients and 59 next of kin individuals providing evaluable data. About half (45.3%) of the respondents in the study population were determined to be deceased when the interviews were conducted.
Socio-demographic characteristics of patient population
Table 1 shows the socio-demographic characteristics of study participants. Ages ranged from 24 to 99 years with a mean of 53.6 ± 14.2 years. Equal variances and normality were assumed for age and a t-test was performed, which indicated no significant difference between the mean age of those in the DNC and the CT groups. Over half of the patients were diagnosed with Stage III breast cancer, though a significant difference was not observed between the groups (p=0.2871). Most of the patients seen at KATH for breast cancer treatment were from the Ashanti region (75.21%), and approximately half of the individuals were married. There was no significant difference between case groups for region of residence or marital status. Numerous categories of occupation are represented within the total group of patients, and there were no occupation that was significantly more represented in either case group. Patients overwhelmingly stated their practiced religion as Christian (82.91%). Interestingly, the proportion of patients who practiced Islam was significantly higher in the DNC than in the CT group (p=0.0259).
Patient factors affecting treatment completion
Table 2 shows patient factors that were significantly associated with treatment completion. Patients who sought traditional healers after any visit to the KATH oncology center were significantly associated with treatment incompletion (45.2%) rather than treatment completion (14%) (p<0.0001). Furthermore, traditional healers were overwhelmingly identified or discovered through radio advertisements (70.7%).
Patients who completed treatment (89.4%) were significantly more likely to understand what their insurance covered regarding surgery, radiation, chemotherapy, and other medications than those who did not complete treatment (67.74%) (p=0.0124). The DNC group had a significantly larger proportion of women who did not know whether they were fearful of the community’s reaction (p<0.0001). After the removal of the ‘I don’t know’ category, there was no longer a significant difference between groups regarding being fearful of community reaction, though it is important to note that over 70% of the respondents said that the patient was fearful of the community reaction. Women who completed treatment (71.4%) were significantly more likely than women who did not complete treatment (33.3%) to know a person who is a cancer survivor (p=0.0098). Women who did not complete treatment (22.6%) were significantly more likely than women who completed treatment (4.7%) to believe that they would not respond to the prescribed breast cancer treatment (p=0.0079). Only 34.6% of women waited less than 1 month to visit KATH upon the first signs there was something wrong with her body after self-detection.
System factors affecting treatment completion
Table 3 shows only two systems factors associated with treatment completion. Women who did not complete treatment (12.0%) were significantly more likely than women who completed treatment (1.3%) to feel that unavailability of chemotherapy drugs at the hospital delayed their treatment (p=0.0329). Women who completed treatment (48.8%) were significantly more likely than women who did not complete treatment (21.4%) to be diagnosed under the NCI definition of early detection (p=0.0116). Unlike the patient factors where only a few factors were primarily one-sided, many systems factors totals contained heavily one-sided answers reflecting that regardless of treatment group, patients overwhelmingly share the same experience with the hospital system. Nearly all (96.6%) respondents stated that the patient was confident in the physician for disease management, and 98.3% stated that follow-up appointments were clearly stated by hospital staff, both very positive factors attributable to KATH staff. Two other large proportional differences existed among the total respondent population regarding education. Most women stated breast cancer education materials were not provided by the hospital (90.43%) and a vast majority of women were not aware that any cancer funding sources in Ghana existed aside from the National Health Insurance Scheme (93.9%).
Determinants of patients not completing treatment
Table 4 shows the results of the final unconditional logistic regression model. After adjusting for socio-demographic variables, it was discovered that women were more likely to not complete their breast cancer treatment if they practiced the Islamic faith compared with women of the Christian faith (OR=6.557; 95% CI = 1.151, 37.343). Women who sought treatment from a traditional healer after any visit to KATH for diagnosis or for treatment had a higher propensity to not complete their breast cancer treatment (OR = 10.732; 95% CI = 2.515, 45.802). Lastly, women who were unaware of the insurance policy coverage regarding their breast care had a higher odds than those who were aware of their insurance coverage to not complete their prescribed breast cancer treatment protocol (OR = 11.859; 95% CI = 1.936, 72.627).
Table 4.
Logistic regression model to predict patients not completing treatment
| Variable | Coefficient (β) | Standard Error | Wald χ2 | p-value | Odds Ratio1 | 95% CI1 |
|---|---|---|---|---|---|---|
| Intercept | −3.7166 | 1.5883 | 5.4754 | 0.0193 | ----- | ----- |
| Age | 0.0633 | 0.0273 | 0.0536 | 0.8169 | 1.006 | 0.954, 1.062 |
| Stage | ||||||
| I/II | Ref | Ref | Ref | Ref | Ref | Ref |
| III/IV | 0.2362 | 0.8709 | 0.0736 | 0.7862 | 1.266 | 0.230, 6.981 |
| Region | ||||||
| Ashanti | Ref | Ref | Ref | Ref | Ref | Ref |
| Greater Central and Northern Ghana | 0.4774 | 0.9687 | 0.2428 | 0.6222 | 1.612 | 0.241, 10.762 |
| Southern Ghana and External Areas | 1.4823 | 1.2246 | 1.4650 | 0.2261 | 1.4823 | 0.399, 48.546 |
| Occupation | ||||||
| Business | Ref | Ref | Ref | Ref | Ref | Ref |
| Farming/Agriculture | 0.5045 | 1.0477 | 0.2319 | 0.6301 | 1.656 | 0.212, 12.909 |
| Professional Occupation | −2.3649 | 1.3437 | 3.0974 | 0.0784 | 0.094 | 0.007, 1.308 |
| Service (Low-Income) | −0.1965 | 1.1173 | 0.0309 | 0.8604 | 0.822 | 0.092, 7.340 |
| Unemployed | 1.1710 | 0.9162 | 1.6337 | 0.2012 | 3.225 | 0.535, 19.428 |
| Marital Status | ||||||
| Married | Ref | Ref | Ref | Ref | Ref | Ref |
| Other (Divorced/Single/Widowed) | 0.2287 | 0.7221 | 0.1003 | 0.7515 | 1.257 | 0.305, 5.175 |
| Religion | ||||||
| Christian | Ref | Ref | Ref | Ref | Ref | Ref |
| Islamic | 1.8806 | 0.8876 | 4.4895 | 0.0341 | 6.557 | 1.151, 37.343 |
| Traditional Healer Sought for Breast Care | ||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 2.3732 | 0.7404 | 10.2746 | 0.0013 | 10.732 | 2.515, 45.802 |
| Knowledge of Insurance Coverage | ||||||
| Yes | Ref | Ref | Ref | Ref | Ref | Ref |
| No | 2.4731 | 0.9246 | 7.1541 | 0.0075 | 11.859 | 1.936, 72.627 |
Unconditional logistic regression adjusted for all variables in this model
Discussion
This study uncovered a number of relevant findings, but it is recognized that there are biases typical of studying cancer treatment completion patterns in developing countries. First, a fair number of individuals could not be recruited to the study because of wrong telephone numbers, which may not limit the generalizability of the study, unless the telephone number errors correlate with treatment completion. A feature of the study was that a little over half of the respondents in ‘Did Not Complete Treatment’ Group are next of kin, due to patient death (likely contributed to by non-completion of the treatment). It is possible that this could have contributed to less reliable recollections of information from this group, such as waiting time, confidence in the physician, perception of completion, family support, self-breast examinations, and some of the specific beliefs of the patient regarding treatment completion, but this would be the only way to access this information retrospectively if the patient is deceased, and was determined to be the best design as family members often accompany a breast cancer patient to appointments. However, when next of kin individuals were unable to answer multiple detailed questions, these questions were discarded and that reduced the sample size for some analyses. Approximate date of death was asked of the next of kin and recorded for each patient who had died, though we did not extract medical record information on the duration of time between the date of death and the last patient appointment at the hospital. Therefore, there is a possibility of incomplete treatment due to immediate death, as a cutoff point was not established for study inclusion based on duration of time between the last appointment at the hospital and patient date of death.
All biases considered and duly accounted for to the best of our ability through study design and data analysis, this study revealed the following interesting observations regarding patient and system factors related to incomplete breast cancer treatment. First, women of the Islamic religion were more likely to not complete treatment. Second, patients who sought traditional healers at any point after a breast cancer diagnosis were more likely to not complete treatment. Third, women who were not knowledgeable about breast cancer treatment insurance coverage were more likely to not complete treatment.
Overall, religion in Ghana is primarily Christian, and the Ashanti statistics reflects this distribution. The regional statistics of our study population show over 20% of the women were from Greater Central and Northern Ghana, which have higher rates of Islamic and traditionalist beliefs [17]. Islamic tradition may interfere with treatment completion if the men are not aware of the importance of cancer treatment because men typically exert a significant degree of control over their spouse’ and relatives’ medical actions and decisions [18]. Furthermore, the more traditional Islamic women’s beliefs are focused on fatalism not values of modern medicine. Further, women of Islamic faith may neglect seeking medical care if the health provider is a male, as the preponderance of physicians in Ghana are males [19].
Knowledge, attitudes, and beliefs about breast cancer are much different in developing countries than in developed countries. Although the physician serves as an authoritative figure in the medical system of developing countries, seeking other methods of treatment such as prayer camps, herbalists, and traditional healers are also common there [20, 21]. Due to cultural practices and beliefs in Ghana, most patients prefer to follow physicians’ prescribed treatment without discussions or decision making process because of complexities associated with comprehension of breast cancer treatment regimens [14]. The care-seeking behavior for breast cancer and a mindset incognizant of the importance of treatment completion causes frequent treatment delays and incomplete treatment in Ghana, which necessarily results in worse outcomes, controlling for stage [22, 23]. It is not uncommon for women in developing countries to visit traditional healers instead of their oncologist for breast cancer treatment, and traditional healers have been shown to serve various roles for the patients such as medicinal healer, emotional comforter, spiritual guide, and palliative caregiver [20, 21, 24]. Traditional healers are so embedded in cultures in developing countries and they are sometimes the first and only source of treatment visited by patients from all socio-economic and educational strata. Unfortunately, women may visit traditional healers prior to their official breast cancer diagnosis which was shown to significantly delay treatment [4, 25]. Our study showed that women continue to go to traditional healers even after their diagnosis and beginning treatment by an oncologist. Not only can this delay treatment, but we found that it leads to incomplete treatment. KATH educate and train traditional healers in the region to refer suspected cancer patients to the hospital [20]. It would be interesting to evaluate the referral patterns of the healers before and after the educational intervention.
Government insurance became widespread in Ghana since 2001, and the policy was implemented primarily to replace out of pocket fees at points of service, primarily to promote basic health services and prevention for all [26]. Less than 50% of the population are registered in the National Health Insurance Scheme (NHIS) [26], due to lack of understanding of benefits of health insurance. Since 2011, after recognizing the need for more allocation towards women’s health, the National Health Insurance Scheme of Ghana added treatment for cervical and breast cancers as part of the health insurance coverage. Despite these measures, our study showed that patients who did not complete treatment were less likely to have knowledge about what the insurance covered. This may be due to low literacy levels among women in Ghana and possibly lack of clarity of the regulations of the National Health Insurance plans for patients.
Fear of death after diagnosis is common among Ghanaian women, and it has been suggested that exposing newly diagnosed women to healthy breast cancer survivors would reduce treatment delays [4]. Women will opt for alternative medicine if a family member did not survive cancer because of their expectation of similar non-response [27]. Moreover, patients often perceive cancer treatment as highly toxic and do more harm than benefit [27–29].
It is not uncommon for breast cancer patients at KATH to be asked to purchase and pay for chemotherapy which likely to lead to incomplete treatment due to non-availability of the drugs at the local pharmacy and the time needed for obtaining the chemotherapy and returning back for hospital appointments.
No significant difference existed in treatment completion between early and late stage diagnosis, yet there was a significant difference at the univariate level in treatment completion between those identified by the National Cancer Institute’s guidelines for early detected and late detected breast cancers. According to the American Cancer Society, following treatment yields a 67% – 93% 5-year survival rate for those cancers detected early, while those detected as stage IIIb and IIIc yield a survival rate between 41% and 49%. Stage IV cancers present a much lower 5-year survival rate of 15% [30]. It is not uncommon for those with stage IV breast cancer to choose to receive palliative care and forego conventional treatment altogether [31]. Those with stage IV breast cancer in Ghana may not have completed treatment as they believed they would not respond to treatment, did not want their family to take on the financial burden for a very low survival probability, or fatalistic attitude.
Beliefs and taboos that may interfere with completion of treatment tend to be more common in countries in which resources are scarce to promote awareness and education surrounding breast cancer. In some developing countries’ cultures, women keep the disease hidden to avoid social rejection and isolation, not only from their community but also their families. In these cultures, women believe that “bad genes” are in their family, which may hinder the affected women’s daughters from getting married, and further, there are beliefs that genes may be transmitted from daily activities such as sharing eating utensils [32, 33]. Gender roles are strongly defined in the Ghanaian culture, with women being very feminine and taking pride in their body image. Studies have shown that mastectomy may cause body image problems among women, as well as sexual self-confidence problems which can create fear of embarrassment and general anxiety [34, 35]. Interestingly, in the United States, it was shown that adverse reactions from others, were among the least intense of concerns reported by newly diagnosed breast cancer patients of multiple ethnicities [36]. Making the situation more complex, according to the head of the cancer directorate at KATH, in Ghana, patients believe that every disease must have a cure.
There were a few important strengths to this study. Foremost, we contacted all available breast cancer patients seen at KATH during the study period, collecting the largest population of breast cancer cases at KATH. The nurse administering the questionnaires received training about the importance of gathering complete data, and her fluency in both Twi and English were key factors for the success of the project. Ghanaian culture instills strong family bonds, therefore discussions between next of kin and the patient made for quite accurate recall of the patients’ perceptions. Because an accurate breast cancer database existed through our previous research, we utilized baseline data resources to facilitate efficient and complete follow-up and to double check the quality of the next-of-kin recollections.
Our data suggest that there is a need to disseminate the information to Ghanaians that breast cancer is curable, that Westernized medication currently provides the best opportunity for long term survival, and that understanding insurance coverage is important to understand in dealing with a chronic disease. Although a few questions were non-significant between groups, there was a striking proportion of individuals who were fearful of the community reaction to a breast cancer diagnosis, and large proportions of women who were unaware of additional funding for breast cancer in Ghana and most were not provided with any educational materials on breast cancer. Targeted education for breast cancer patients is paramount in this society and discussing factors associated with treatment completion is important for those individuals at any level of prevention. Further, there is a need to identify the most financially feasible route of follow-up that can be sustained leveraging the full repertoire of financial sources available. Future studies regarding interventions tailored for patient education through a behavioral health model, more studies and interventions to improve cancer health literacy, access to care and navigation of health systems, and more consistent patient follow-up are warranted.
Acknowledgments
Mark Obrist was supported by the Cancer Epidemiology Education in Special Populations (CEESP) Program of the University of Nebraska, Grant R25 CA112383 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Sofia D. Merajver was supported in part by the Avon Foundation, the National Institutes of Health, and the Breast Cancer Research Foundation.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ly D, Forman D, Ferlay J, et al. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132:1918–1926. doi: 10.1002/ijc.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohene-Yeboah M, Adjei E. Breast cancer in Kumasi, Ghana. Ghana Med J. 2012;46:8–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A pilot study. Ghana Med J. 2009;43:127–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Bewtra C. Clinicopathologic Features of Female Breast Cancer in Kumasi, Ghana. International Journal of Cancer Research. 2010;6:154–160. [Google Scholar]
- 6.International Agency for Research on Cancer (IARC) GLOBOCAN 2008: Fast Stats. Ghana: 2010. [Accessed 2013]. http://globocan.iarc.fr/factsheet.asp. [Google Scholar]
- 7.Scherber S, Soliman AS, Awuah B, et al. Characterizing Breast Cancer Treatment Pathways in Kumasi, Ghana from Onset of Symptoms to Final Outcome: Outlook toward Cancer Control. Breast disease. doi: 10.3233/BD-140372. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braithwaite D, Boffetta P, Rebbeck TR, et al. Cancer prevention for global health: A report from the ASPO International Cancer Prevention Interest Group. Cancer Epidemiol Biomarkers Prev. 2012;21:1606–1610. doi: 10.1158/1055-9965.EPI-12-0848. [DOI] [PubMed] [Google Scholar]
- 9.Stark A, Kleer CG, Martin I, et al. African ancestry and higher prevalence of triple-negative breast cancer: Findings from an international study. Cancer. 2010;116:4926–4932. doi: 10.1002/cncr.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Health. 2012;17:1031–1043. doi: 10.1111/j.1365-3156.2012.03021.x. [DOI] [PubMed] [Google Scholar]
- 11.de-Graft Aikins A, Unwin N, Agyemang C, et al. Tackling Africa’s chronic disease burden: From the local to the global. Global Health. 2010;6:5–8603-6-5. doi: 10.1186/1744-8603-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien KS, Soliman AS, Awuah B, et al. Establishing effective registration systems in resource-limited settings: Cancer registration in Kumasi, Ghana. J Registry Manag. 2013;40:70–77. [PMC free article] [PubMed] [Google Scholar]
- 13.Ghana Statistical Service. 2010 Population and Housing Census: Provisional Results: Summary of Findings. Ghana Statistical Service; 2011. [Google Scholar]
- 14.Clegg-Lamptey JN, Dakubo JC, Attobra YN. Psychosocial aspects of breast cancer treatment in Accra, Ghana. East Afr Med J. 2009;86:348–353. doi: 10.4314/eamj.v86i7.54152. [DOI] [PubMed] [Google Scholar]
- 15.Eniu A, Carlson RW, Aziz Z, et al. Breast cancer in limited-resource countries: Treatment and allocation of resources. Breast J. 2006;12(Suppl 1):S38–53. doi: 10.1111/j.1075-122X.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 16.Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: A call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 17.Government of Ghana. [Accessed 2013];About Ghana: Northern. 2013 http://www.ghana.gov.gh/index.php/about-ghana/regions/northern.
- 18.Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(Suppl 1):S103–10. doi: 10.1111/j.1075-122X.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 19.Abuidris DO, Elsheikh A, Ali M, et al. Breast-cancer screening with trained volunteers in a rural area of Sudan: a pilot study. Lancet Oncol. 2013;14:363–370. doi: 10.1016/S1470-2045(12)70583-1. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien KS, Soliman AS, Annan K, et al. Traditional herbalists and cancer management in Kumasi, Ghana. J Cancer Educ. 2012;27:573–579. doi: 10.1007/s13187-012-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhamad M, Merriam S, Suhami N. Why breast cancer patients seek traditional healers. Int J Breast Cancer. 2012;2012:689168. doi: 10.1155/2012/689168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Story HL, Love RR, Salim R, et al. Improving outcomes from breast cancer in a low-income country: Lessons from Bangladesh. Int J Breast Cancer. 2012;2012:423562. doi: 10.1155/2012/423562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdulrahman GO, Jr, Rahman GA. Epidemiology of breast cancer in Europe and Africa. J Cancer Epidemiol. 2012;2012:915610. doi: 10.1155/2012/915610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merriam S, Muhamad M. Roles traditional healers play in cancer treatment in Malaysia: Implications for health promotion and education. Asian Pac J Cancer Prev. 2013;14:3593– 3601. doi: 10.7314/apjcp.2013.14.6.3593. [DOI] [PubMed] [Google Scholar]
- 25.Ross E. Traditional healing in South Africa: Ethical implications for social work. Soc Work Health Care. 2008;46:15–33. doi: 10.1300/j010v46n02_02. [DOI] [PubMed] [Google Scholar]
- 26.Agyepong IA, Adjei S. Public social policy development and implementation: A case study of the Ghana National Health Insurance scheme. Health Policy Plan. 2008;23:150–160. doi: 10.1093/heapol/czn002. [DOI] [PubMed] [Google Scholar]
- 27.Citrin DL, Bloom DL, Grutsch JF, et al. Beliefs and perceptions of women with newly diagnosed breast cancer who refused conventional treatment in favor of alternative therapies. Oncologist. 2012;17:607–612. doi: 10.1634/theoncologist.2011-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shumay DM, Maskarinec G, Kakai H, et al. Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J Fam Pract. 2001;50:1067. [PubMed] [Google Scholar]
- 29.Verhoef MJ, Rose MS, White M, et al. Declining conventional cancer treatment and using complementary and alternative medicine: A problem or a challenge? Curr Oncol. 2008;15(Suppl 2):s101–6. doi: 10.3747/co.v15i0.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Cancer Society. [Accessed 2013];Breast cancer survival rates by stage. 2014 http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stage.
- 31.Irvin W, Jr, Muss HB, Mayer DK. Symptom management in metastatic breast cancer. Oncologist. 2011;16:1203–1214. doi: 10.1634/theoncologist.2011-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Errico KM, Rowden D. Experiences of breast cancer survivor-advocates and advocates in countries with limited resources: a shared journey in breast cancer advocacy. Breast J. 2006;12(Suppl 1):S111–6. doi: 10.1111/j.1075-122X.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 33.Dow Meneses K, Yarbro CH. Cultural perspectives of international breast health and breast cancer education. J Nurs Scholarsh. 2007;39:105–112. doi: 10.1111/j.1547-5069.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- 34.Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 35.Consedine NS, Magai C, Krivoshekova YS, et al. Fear, anxiety, worry, and breast cancer screening behavior: A critical review. Cancer Epidemiol Biomarkers Prev. 2004;13:501–510. [PubMed] [Google Scholar]
- 36.Spencer SM, Lehman JM, Wynings C, et al. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol. 1999;18:159–168. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]