Starvation triggers relocation of membranes of the secretory compartments and endosomes to create a compartment called CUPS, which may be involved in processing and secretion of proteins that cannot enter the ER–Golgi pathway.
Abstract
Upon starvation, Grh1, a peripheral membrane protein located at endoplasmic reticulum (ER) exit sites and early Golgi in Saccharomyces cerevisiae under growth conditions, relocates to a compartment called compartment for unconventional protein secretion (CUPS). Here we report that CUPS lack Golgi enzymes, but contain the coat protein complex II (COPII) vesicle tethering protein Uso1 and the Golgi t-SNARE Sed5. Interestingly, CUPS biogenesis is independent of COPII- and COPI-mediated membrane transport. Pik1- and Sec7-mediated membrane export from the late Golgi is required for complete assembly of CUPS, and Vps34 is needed for their maintenance. CUPS formation is triggered by glucose, but not nitrogen starvation. Moreover, upon return to growth conditions, CUPS are absorbed into the ER, and not the vacuole. Altogether our findings indicate that CUPS are not specialized autophagosomes as suggested previously. We suggest that starvation triggers relocation of secretory and endosomal membranes, but not their enzymes, to generate CUPS to sort and secrete proteins that do not enter, or are not processed by enzymes of the ER–Golgi pathway of secretion.
Introduction
Secretory cargoes containing a signal sequence enter the ER, where they are processed, folded, and then packaged into coat protein complex II (COPII)-coated vesicles for trafficking to the Golgi. Within the Golgi, cargoes are further processed, sorted, and transported to different cellular destinations, including the extracellular space. However, there are many examples of secreted proteins that do not contain a signal sequence for translocation into the ER (Rabouille et al., 2012). This class of proteins lack any known, common structural feature or posttranslational modification. In Saccharomyces cerevisiae, Pichia pastoris, and Dictyostelium discoideum, the 87-aa signal sequence–lacking protein Acb1 (AcbA in D. discoideum) is secreted under conditions of nutrient deprivation (Kinseth et al., 2007; Duran et al., 2010; Manjithaya et al., 2010). In D. discoideum, secreted AcbA is proteolytically cleaved to generate a 34-aa peptide, SDF2, which triggers rapid encapsulation of pre-spore cells (Anjard and Loomis, 2005). How Acb1 is secreted, how the enzyme required for the production of SDF2 is expressed on the exoplasmic face of the plasma membrane, and whether Acb1 undergoes any modification before its release from the cells is not known (Malhotra, 2013). GrpA and Grh1, the respective D. discoideum and yeast homologues of the mammalian Golgi-associated proteins GRASP55 and GRASP65, respectively, are required for AcbA/Acb1 secretion and production of functionally active SDF2, but their exact role in this overall pathway is not known (Kinseth et al., 2007; Duran et al., 2010; Manjithaya et al., 2010). Interestingly, in S. cerevisiae, Grh1 localizes to ER exit sites and early Golgi membranes under growth conditions, and upon starvation, Grh1 redistributes to a large compartment called compartment for unconventional protein secretion (CUPS; Bruns et al., 2011). CUPS are present at 1–3 copies/cell, localized in the vicinity of the Sec13-containing ER exit sites; they lack Golgi processing enzymes and endocytic markers (Bruns et al., 2011). The function of CUPS in Acb1 secretion is not known, but it is has been suggested that CUPS collect Acb1 from the cytoplasm and then generate small Acb1-enriched vesicles for its further processing and secretion (Malhotra, 2013). What is the molecular composition of CUPS, how do they form, and what is their fate when starved cells return to nutrient-rich conditions?
We now report that formation of CUPS depends on COPII- and COPI-independent membrane recruitment from the Golgi. The formation and maintenance of CUPS also requires membrane export from late Golgi/endosomes. Upon return of cells to growth condition, CUPS are reabsorbed into the ER in a COPI-dependent process. The description of this hybrid membrane compartment and its dynamics follows.
Results and discussion
CUPS contain the vesicle tethering protein Uso1 and the Golgi t-SNARE Sed5
Grh1 is a peripheral membrane protein that has been reported to be associated with early Golgi membranes (Behnia et al., 2007) and ER exit sites in growing yeast cells (Levi et al., 2010). Our previous data showed that incubation of cells in 2% potassium acetate (starvation medium) caused relocation of Grh1 from ER exit sites/early Golgi to CUPS (Bruns et al., 2011). Our present experiments confirm the previous data and reveal that Grh1 redistribution to CUPS is much faster and can be detected as early as 30 min after incubation in starvation medium (Fig. 1, A and B).
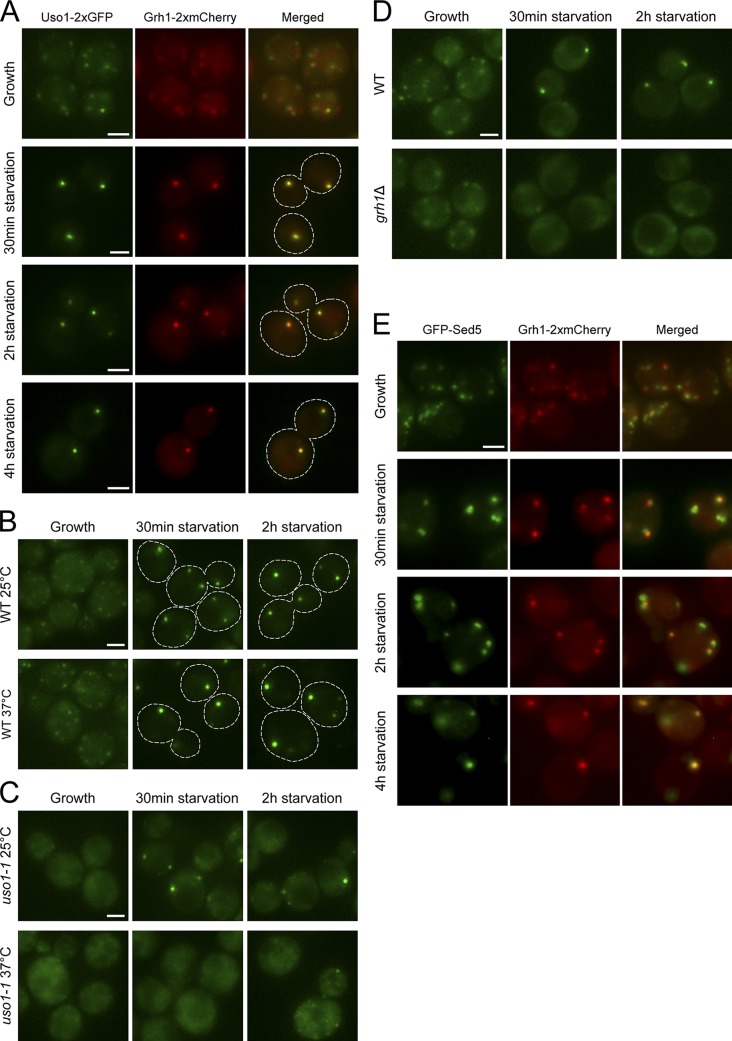
Figure 1.
Uso1 and Sed5 colocalize with Grh1 in CUPS. (A) Cells coexpressing Uso1-2×GFP and Grh1-2×mCherry were grown in complete medium and imaged by fluorescence microscopy (Growth), or centrifuged and resuspended in 2% potassium acetate to induce starvation and visualized by fluorescence microscopy at 0.5, 2, and 4 h after incubation in starvation medium. (B and C) Wild-type (B) and uso1-1 (C) cultures expressing Grh1-2×GFP were grown at 25°C, then either held at 25°C or shifted for 45 min to 37°C, followed by incubation in starvation medium at 25°C or 37°C, respectively, for 2 h. Cells were observed by fluorescence microscopy just before starvation (Growth) and after 0.5 and 2 h of starvation. (D) Wild-type or grh1Δ cells expressing Uso1-GFP were cultured in growth medium or starved for 2 h, and the distribution of Uso1-GFP was monitored by fluorescence microscopy. (E) Cells expressing GFP-Sed5 and Grh1-2×mCherry were processed and analyzed as described in A. Broken lines indicate cell boundaries. Bars, 2 µm.
The attachment of Grh1 to Golgi membranes is mediated in part by a protein called Bug1 (Behnia et al., 2007); we have shown previously that binding of Grh1 to CUPS is also Bug1 dependent (Bruns et al., 2011). Bug1 and Grh1 interact genetically with Uso1 (Behnia et al., 2007), which is a long peripheral protein that tethers COPII vesicles with Golgi membranes before their fusion (Nakajima et al., 1991; Cao et al., 1998). We first tested whether Uso1 is associated with CUPS. Cells coexpressing endogenously tagged Grh1-2×mCherry and Uso1-2×GFP were visualized by fluorescence microscopy. In cells grown in complete synthetic medium to mid-log phase, Uso1 was located in a small number of elements that were scattered in the cytoplasm and frequently colocalized with (or were adjacent to) Grh1-positive structures (Fig. 1 A). Within 30 min of starvation, Uso1 and Grh1 colocalized in CUPS, and this colocalization was maintained for up to 4 h of starvation (Fig. 1 A).
We then asked whether Uso1 is required for the recruitment of Grh1 to CUPS by using a strain harboring the temperature-sensitive allele, uso1-1. First, we confirmed that the assembly of CUPS in wild-type cells expressing Grh1-2×GFP was not affected by incubation at the nonpermissive temperature of 37°C (Fig. 1 B). In uso1-1 cells starved at 25°C, Grh1 was located in CUPS-like structures, although at a lower frequency than in starved wild-type cells (Fig. 1, B and C). Moreover, the number of uso1-1 cells showing a diffuse distribution and/or multiple, small Grh1-2×GFP puncta was higher than that observed for the wild-type cells (Fig. 1, B and C). Inactivation of Uso1 function by incubation at 37°C prevented the association of Grh1-2×GFP with CUPS-like structures in starved uso1-1 cells, as revealed by an increase in the proportion of cells displaying a diffuse/small punctate distribution of Grh1, compared with wild-type starved cells (Fig. 1, B and C). These data reveal that Uso1 is required for Grh1 recruitment to CUPS.
To investigate whether Grh1 is necessary for the association of Uso1 with CUPS membranes, we examined the localization of Uso1 in a grh1Δ strain. When GRH1 was deleted, the distribution of Uso1-GFP in growing cells was more diffuse than that observed in wild-type cells (Fig. 1 D). This effect on the Uso1 localization was more pronounced in starved cells, where the localization of Uso1-GFP to CUPS was barely detectable (Fig. 1 D), indicating that Grh1 is required for Uso1 recruitment to CUPS.
Because Uso1 is required for the fusion of ER-derived COPII vesicles with Golgi membranes (Cao et al., 1998), we tested whether the Golgi-specific t-SNARE Sed5 (Hardwick and Pelham, 1992) was localized to CUPS. Fluorescence microscopy analysis of cells coexpressing Grh1-2×mCherry and GFP-Sed5 revealed that in growing cells the Grh1-labeled structures colocalized with or were in close proximity to Sed5 (Fig. 1 E). Under starvation conditions, Grh1 and Sed5 were found to colocalize in CUPS (Fig. 1 E). Sed5 was also detected in puncta devoid of Grh1 in starved cells (Fig. 1 E). These results reveal the presence of a pool of membrane-anchored Sed5 protein in CUPS, which suggests that Golgi membranes are used for the formation of CUPS.
To further test the localization of Uso1 and Sed5 in CUPS, we performed confocal microscopy followed by a quantitative colocalization analysis between Grh1-2×mCherry and Uso1-2×GFP or GFP-Sed5 in cells starved for 2 h. As controls, we used two early Golgi enzymes, Mnn2-GFP and Mnn5-GFP. This analysis revealed that the Manders’ colocalization coefficients between Grh1 and Uso1 or Sed5 were significantly higher than those obtained for the pairs Grh1/Mnn2 or Grh1/Mnn5 (Fig. S1), further indicating that CUPS contain Golgi proteins, such as Sed5, but are largely devoid of Golgi enzymes.
CUPS form independent of COPII- and COPI-mediated membrane transport
Are COPII components involved in the biogenesis of CUPS? In our previous work, we addressed this question by analyzing the redistribution of Grh1 to CUPS after 4 h of starvation in conditions where COPII vesicle production was blocked in a thermosensitive sec12 strain (Bruns et al., 2011). We decided to explore this finding further by testing the involvement of the COPII coat proteins Sec13 and Sec31, and to gauge the role of COPII-mediated transport at earlier time points of starvation. Mid-log phase cells expressing Grh1-2×GFP were preincubated at 37°C for 45 min prior to starvation to ensure that the tested Sec protein was inactivated at the beginning of starvation. The analysis by fluorescence microscopy revealed that the distribution of Grh1-2×GFP in growing sec13-1 and sec31-1 cells was similar to that observed in the control wild-type cells (Fig. 2, A and B). In wild-type cells starved at 37°C, Grh1-2×GFP was localized to one to three large dots in the majority of cells. Similarly, Grh1 was found to be distributed in CUPS in most of the sec13-1 and sec31-1 cells starved at 37°C (Fig. 2, A and B). Thus, conventional COPII vesicle–mediated transport of membranes is not required to form CUPS.
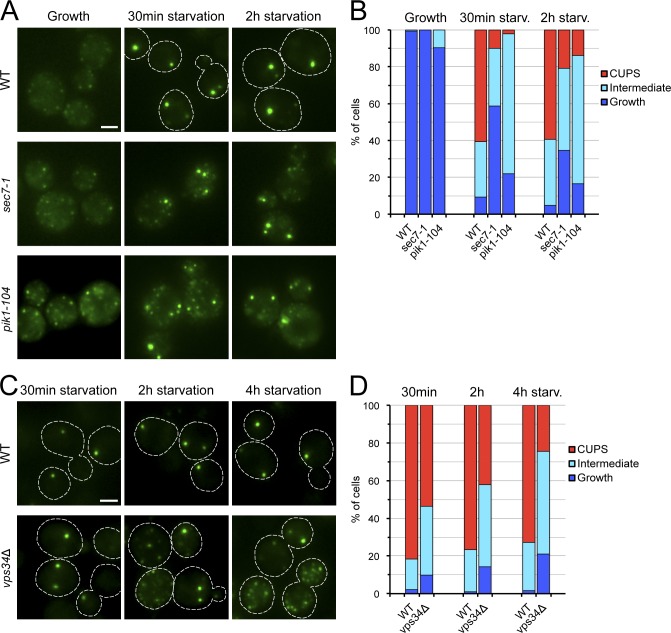
Figure 2.
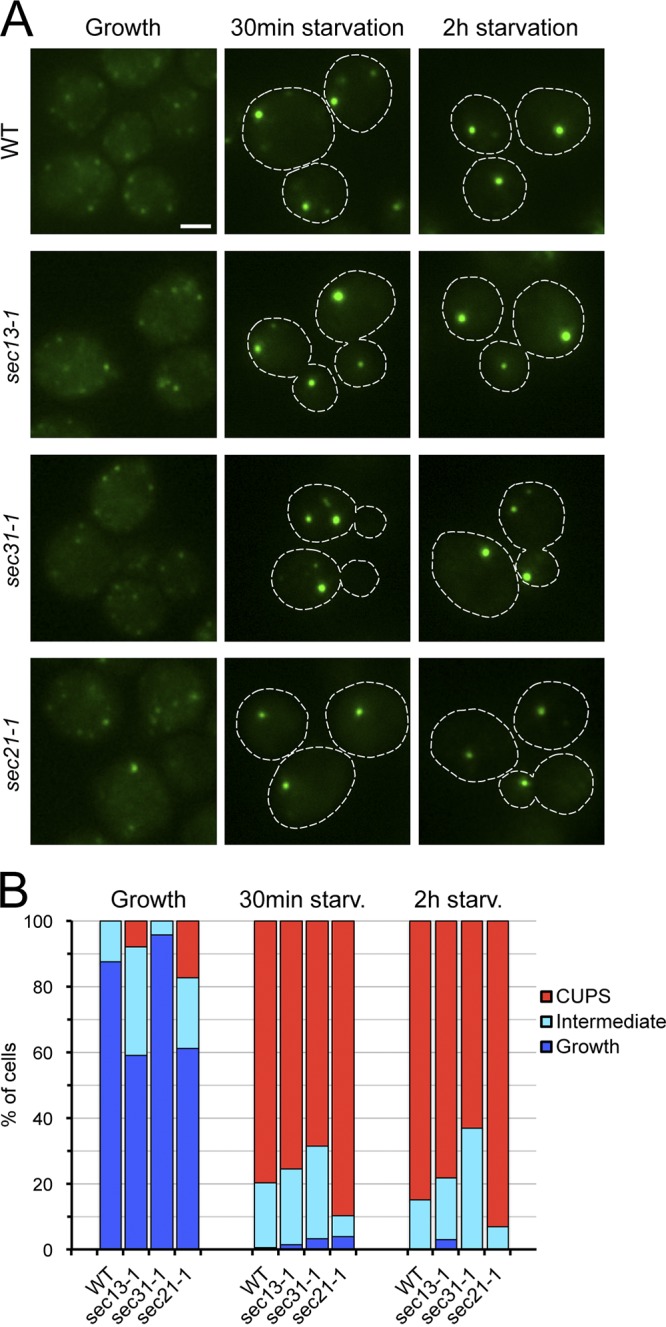
COPII- and COPI-mediated membrane transport is not necessary for CUPS formation. (A) Wild-type, sec13-1, sec31-1, and sec21-1 cells expressing Grh1-2×GFP were grown to mid-log phase at 25°C, then preincubated at 37°C for 45 min followed by washing of the cells and incubation in 2% potassium acetate at 37°C for 2 h. Cells were imaged by fluorescence microscopy just before starvation (Growth) and within 0.5 and 2 h of starvation. Broken lines indicate cell boundaries. Bar, 2 µm. (B) Quantification of the different Grh1-2×GFP distributions observed in the conditions tested in A. The data shown are from a single representative experiment out of two repeats. For each condition in the experiment shown, n ≥ 80 cells.
Sed5 is required for COPI-mediated intra-Golgi transport (Banfield et al., 1995; Nichols and Pelham, 1998). We therefore tested the involvement of COPI-coated vesicles in CUPS formation using the conditional mutant strain, sec21-1 (Hosobuchi et al., 1992; Letourneur et al., 1994). When growing sec21-1 cells were incubated at 37°C, Grh1-2×GFP retained a wild-type distribution in most of the population, with a small percentage of cells displaying a localization of Grh1 in CUPS-like structures (Fig. 2, A and B). Nutrient starvation at 37°C did not affect the relocation of Grh1-2×GFP to CUPS in the sec21-1 strain (Fig. 2, A and B). This result indicates that COPI-dependent membrane transport is not necessary for CUPS biogenesis. Taking into account that COPI vesicles have been proposed to play a role in the localization of Golgi processing enzymes (Tu et al., 2008), the lack of involvement of COPI vesicle-mediated transport in CUPS biogenesis is consistent with the absence of Golgi enzymes in CUPS (Fig. S1; Bruns et al., 2011).
We then asked whether CUPS formed in wild-type cells were the same as in COPII or COPI mutants. We tested this hypothesis by monitoring the distribution of Anp1, an early Golgi marker. Anp1-RFP did not colocalize with Grh1-2×GFP–containing CUPS in wild-type cells under growth or starvation condition (Fig. S2). Similarly, in the COPII (sec31-1) or COPI (sec21-1) mutant cells under starvation, CUPS did not contain Anp1-RFP (Fig. S2). Therefore, a defect in COPII- or COPI-dependent transport does not create CUPS that are biochemically different from those that form in wild-type cells.
The biogenesis of CUPS requires membrane export from the late Golgi
To investigate whether membrane transport from the late Golgi is involved in the formation of CUPS, we used strains bearing thermo-sensitive mutations in the ARF-GEF Sec7 and in the phosphatidylinositol 4-kinase Pik1, two essential proteins localized primarily at the late Golgi. In these mutant strains (sec7-1 and pik1-104), the export of cargo from the late Golgi is blocked or delayed (Achstetter et al., 1988; Franzusoff et al., 1991; Walch-Solimena and Novick, 1999). The analysis by fluorescence microscopy of growing sec7-1 and pik1-104 cells incubated at 37°C revealed that the distribution of Grh1-2×GFP in these conditions was similar to that observed in the control wild-type strain (Fig. 3, A and B). In contrast, when both sec7-1 and pik1-104 cells were starved at 37°C, Grh1 failed to relocate completely to CUPS, and it was found in few larger CUPS-like structures and multiple small puncta dispersed throughout the cytoplasm (Fig. 3, A and B). This defect in Grh1 redistribution to CUPS was observed as early as 30 min after starvation and was also visible in cells starved for 2 h (Fig. 3, A and B). These results indicate the requirement of Sec7 and Pik1 for the complete assembly of CUPS and suggest that transport from the late Golgi is involved in CUPS biogenesis.
Figure 3.
Sec7, Pik1, and Vps34 are required for the formation and/or maintenance of CUPS. (A) Wild-type, sec7-1, and pik1-104 cells expressing Grh1-2×GFP were processed and analyzed as described in Fig. 2. (B) Quantification of the phenotypes observed for the localization of Grh1-2×GFP in A. The data shown are from a single representative experiment out of three repeats. For each condition in the experiment shown, n ≥ 50 cells. (C) Wild-type and vps34Δ cells expressing Grh1-2×GFP were grown to mid-log phase, then starved for 4 h and observed by fluorescence microscopy within 0.5, 2, and 4 h of starvation. (D) Quantification of the different Grh1-2×GFP distributions observed in the conditions tested in C. The data shown are from a single representative experiment out of three repeats. For each condition in the experiment shown, n ≥ 180 cells. Broken lines indicate cell boundaries. Bars, 2 µm.
We have shown previously that Vps34, a phosphatidylinositol 3-kinase, is required for CUPS formation (Bruns et al., 2011). Based on the new observation that the phosphatidylinositol 4-kinase Pik1 is required for early events in CUPS formation, we retested the dependence on Vps34 for this process. A VPS34 deletion strain was incubated in starvation medium, and the location of Grh1-2×GFP was examined at various time points after starvation. The loss of Vps34 did not strongly alter the formation of CUPS within 30 min of starvation (Fig. 3, C and D). However, at later time points and especially within 4 h of starvation, Grh1 distribution was affected in the vps34Δ cells as revealed by the significant percentage of cells showing numerous small Grh1-2×GFP–containing structures (Fig. 3, C and D; Bruns et al., 2011). Together, these results suggest that PI3P production by Vps34 is not necessary for CUPS biogenesis but is instead required for the maintenance of Grh1-containing CUPS during starvation.
Next, we tested whether CUPS contain PI4P and PI3P, the enzymatic products of Pik1 and Vps34, respectively. Cells coexpressing Grh1-2×GFP and the phosphoinositide-specific probes mCherry-Osh2-2×PH or mRFP-EEA1-FYVE were visualized by fluorescence microscopy at different time points after starvation. Our results revealed that CUPS do not contain the amount of PI4P and PI3P measured by this approach (Fig. S3, A and B). These findings suggest that Pik1 and Vps34 are required for the export of specific cargoes from Golgi and endosomes, respectively, and not for their activities at the CUPS.
The biogenesis of CUPS is induced by glucose starvation
Incubation of cells in 2% potassium acetate triggers the formation of CUPS. However, this treatment could potentially be signaling via glucose- and/or nitrogen-sensing pathways. We therefore tested specifically whether CUPS form upon nitrogen or glucose starvation. Mid-log phase cells expressing Grh1-2×GFP were washed, placed in medium lacking either glucose or nitrogen, and visualized by fluorescence microscopy after 30 min and 2 h of incubation. Grh1 relocated to CUPS upon glucose deprivation, whereas it displayed a diffuse distribution with some faint cytoplasmic puncta in nitrogen-starved cells (Fig. 4, A and B). Colocalization analysis in cells coexpressing Grh1-2×mCherry and either Uso1-2×GFP or GFP-Sed5 revealed that both Uso1 and Sed5 are found in CUPS at 30 min and 2 h after glucose deprivation (Fig. 4, C and D). However, Anp1-RFP and Sec7-2×mCherry, two Golgi markers excluded from CUPS in potassium acetate–starved cells (Bruns et al., 2011), did not colocalize with Grh1-2×GFP–containing CUPS in cells starved for glucose for 2 h (Fig. S3, C and D). These results highlight the involvement of signals that are triggered upon glucose starvation in the events leading to CUPS biogenesis. In addition, our data reveal that CUPS and the enlarged Sec7-containing Golgi membranes observed in glucose-deprived cells (Aoh et al., 2011) are different compartments.
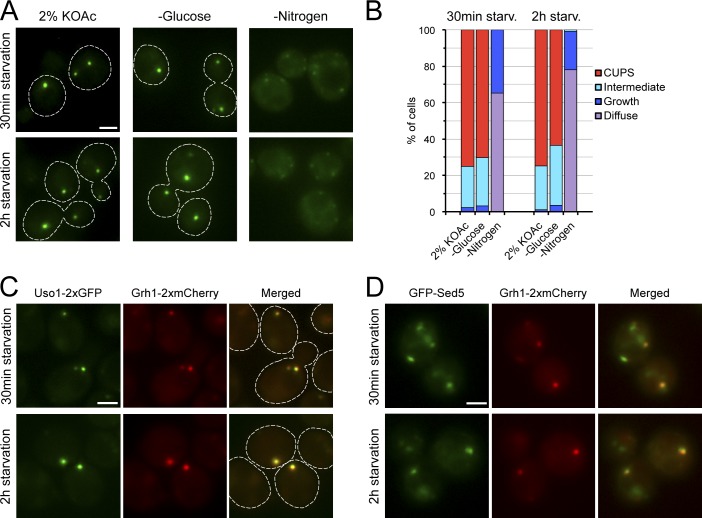
Figure 4.
CUPS formation is specifically triggered by glucose deprivation. (A) Grh1-2×GFP–expressing cells were grown in rich medium, washed, and cultured in 2% potassium acetate, SC-D (−glucose), or SD-N (−nitrogen) media for 0.5 or 2 h and visualized by fluorescence microscopy. (B) Quantification of the phenotypes observed for the distribution of Grh1-2×GFP in A. The data shown are from a single representative experiment out of two repeats. For each condition in the experiment shown, n ≥ 120 cells. (C and D) Cells coexpressing Uso1-2×GFP and Grh1-2×mCherry (C) or GFP-Sed5 and Grh1-2×mCherry (D) were grown in complete medium, then centrifuged and resuspended in SC-D medium to induce glucose deprivation and visualized by fluorescence microscopy at 0.5 and 2 h after incubation in the starvation medium. Broken lines indicate cell boundaries. Bars, 2 µm.
We previously reported the location of the pre-autophagosome markers Atg8 and Atg9 to CUPS (Bruns et al., 2011). However, our data indicate that COPII-mediated transport is not required for CUPS biogenesis, and that their formation is not activated by nitrogen starvation or rapamycin treatment but is rather induced by glucose deprivation. This contrasts with the assembly of pre-autophagosomal structures that requires COPII-dependent transport (Graef et al., 2013), and it is activated by rapamycin or nitrogen starvation. Thus, we reevaluated the localization of Atg8 and Atg9 in potassium acetate–starved cells. Our results revealed a lack of colocalization of mCherry-Atg8 and mRFP-Atg9 with Grh1-2×GFP-containing CUPS in a significant number of cells (Fig. S3 E). We cannot rule out the possibility that these components are recruited transiently and thus escape our detection, but they are clearly not a stable feature of CUPS.
CUPS are reabsorbed into the ER upon return to growing conditions
Grh1 relocates from CUPS to ER exit sites/early Golgi when starved cells are incubated in nutrient-rich medium (Fig. 5 A; Bruns et al., 2011). Grh1 is a peripheral membrane protein, and this finding therefore does not reveal whether CUPS are reabsorbed into the ER, the Golgi, or any other membrane compartment such as the vacuole. We first asked if Grh1 relocation was affected in a vam7Δ strain, which is defective in membrane fusion with the vacuole (Sato et al., 1998). Deletion of VAM7 did not alter the distribution of Grh1-2×GFP in starved cells or in cells returned to nutrient-rich conditions as compared with the wild-type strain (Fig. 5, A and B), which indicates that CUPS are not reabsorbed into the vacuole upon return to growth conditions.
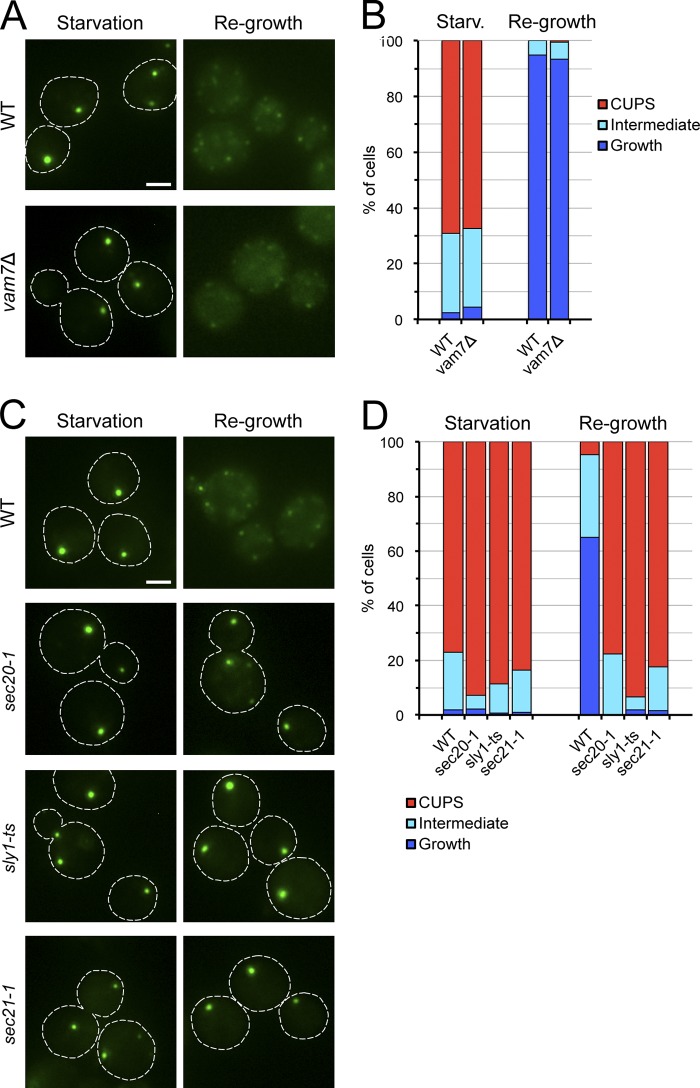
Figure 5.
Retrograde transport to the ER is required for Grh1 relocalization upon return to growth conditions. (A) Wild-type and vam7Δ strains expressing Grh1-2×GFP were starved in 2% potassium acetate for 3 h at 25°C and observed by fluorescence microscopy, or washed to remove the starvation medium and cultured in complete medium for 1 h before examination by fluorescence microscopy. (B) Quantification of the Grh1-2×GFP distribution observed in A. The data shown are from a single representative experiment out of two repeats. For each condition in the experiment shown, n ≥ 130 cells. (C) Wild-type, sec20-1, sly1-ts, and sec21-1 cells expressing Grh1-2×GFP were starved for 2 h at 25°C, then shifted to 37°C for 1 h followed by incubation in nutrient-rich medium for 1 h at 37°C. Cells were observed by fluorescence microscopy just before incubation in rich medium and within 1 h of incubation in rich medium. (D) Quantification of the phenotypes observed for the distribution of Grh1 in C. The data shown are from a single representative experiment out of two repeats. For each condition in the experiment shown, n ≥ 90 cells. Broken lines indicate cell boundaries. Bars, 2 µm.
Next, we investigated whether fusion with ER membranes is required for the relocation of Grh1 after return to growth medium. To test this, we used a strain harboring a temperature-sensitive mutation in Sec20, an ER-localized SNARE-like protein involved in ER membrane fusion (Cosson et al., 1997; Lewis et al., 1997). Wild-type and sec20-1 cells expressing Grh1-2×GFP were starved for 2 h at 25°C to allow CUPS formation, then shifted to 37°C and further incubated for 1 h followed by washing to remove the starvation medium and incubation in nutrient-rich medium for 1 h at 37°C. Upon return to growth conditions at 37°C, Grh1-2×GFP relocated from CUPS to multiple small puncta in wild-type cells, whereas it remained in a CUPS-like distribution in sec20-1 cells (Fig. 5, C and D). To ascertain further the role of ER membrane fusion by a Sec20-mediated reaction, we tested the requirement for Sly1, a protein of the Sec1/Munc-18 family that is needed for this membrane fusion event (Li et al., 2005). Inactivation of Sly1 by incubation of starved sly1-ts cells at 37°C prevented the redistribution of Grh1-2×GFP after returning to nutrient-replete medium (Fig. 5, C and D). These results indicate that upon return to normal growth conditions, CUPS are absorbed into the ER by a Sec20- and Sly1-dependent membrane fusion process.
Does ER reabsorption of CUPS membranes require COPI vesicle–mediated membrane transport? To address this question, Grh1-2×GFP distribution was analyzed in sec21-1 cells, which exhibit defects in membrane transport to the ER at nonpermissive temperatures (Letourneur et al., 1994). Sec21-1 cells failed to relocate Grh1 from CUPS when starved cells were incubated in nutrient-rich medium at 37°C (Fig. 5, C and D). Thus, CUPS membranes are consumed by COPI vesicle–dependent traffic.
In summation, our data suggest that CUPS form by the fusion of at least two classes of membranes. One is derived from early Golgi that contain Grh1, Bug1, the membrane tethering factor Uso1, and the t-SNARE Sed5. It is important to note that not all Sed5 relocated to CUPS in starved cells, which displayed some Sed5-containing elements lacking Grh1. The second are vesicles produced from the late Golgi in a Sec7- and Pik1-dependent manner. The fusion of these two sets of membranes is likely mediated by Sed5 and Uso1, and this structure is the fundamental unit of CUPS. CUPS then mature by addition of membranes from the endosomes by a Vps34-dependent process. We expect that many other components are added to CUPS to obtain a fully functional compartment. Upon returning cells to growth conditions, CUPS fuse by a COPI-, and Sly1-dependent process with Sec20-containing membranes of the ER.
CUPS are devoid of the glycosylating enzymes contained in the Golgi. This is reasonable given that these modifications are not required for the sorting, trafficking, and function of signal sequence–lacking proteins such as Acb1 that cannot enter the ER. This also explains the lack of COPI involvement in CUPS biogenesis. However, proteins such as Acb1 still have to be sorted from the total pool of cytosolic proteins and released in a signal-dependent manner. CUPS, a hybrid of the secretory and endosomal compartments, likely provide this sorting function by recruiting Acb1, or a processing activity, for its export from cells without involving the functions of ER–Golgi–endosomes. In other words, cells use portions of compartments of the conventional secretory pathway and endosomes to create a new compartment, CUPS, for the export of signal sequence-lacking proteins that are functional in the extracellular space. In higher eukaryotes, many cytokines, galectins, and insulin-degrading enzymes are secreted without the involvement of ER–Golgi (Malhotra, 2013). Do higher eukaryotes also make CUPS in a signal-dependent manner? The organization of yeast and mammalian Golgi is very different. Does the morphology and composition of CUPS vary accordingly? The identification of CUPS in other eukaryotes will help us understand this fundamentally important process.
Materials and methods
Strains
All yeast strains used in this study are listed in Table S1. The parental thermosensitive strains were provided by C. Boone (University of Toronto, Ontario, Canada; Li et al., 2011). Strains expressing C-terminally 2xyeGFP- and/or 2xyomCherry-tagged proteins were constructed by a PCR-based targeted homologous recombination using the pYM-2xyeGFP-HIS3MX6 and pFA6a-link-2xyomCherry-CaURA3 plasmids (Janke et al., 2004). Deletion of GRH1 or VPS34 was performed by replacing the open reading frame of interest with a KanMX4 cassette using PCR-based targeted homologous recombination. Strains coexpressing Grh1-2×mCherry and Mnn2-GFP or Mnn5-GFP, and those coexpressing Grh1-2×GFP and Anp1-RFP were made by mating the pertinent haploids, followed by sporulation and selection of the appropriate spore-derived clones.
Plasmids
pYM-2xyeGFP-HIS3MX6 was constructed by inserting a linker-yeGFP fragment (linker sequence: GAGAIL) in frame with the C terminus of the yeGFP in the pYM44 plasmid (Janke et al., 2004) by Gibson assembly (Gibson et al., 2009) with two PCRs using the following primers: 5′-TGGTATGGATGAATTGTACAAAGGTGCTGGAGCAATTCTGTCT-3′ and 5′-AGTGGCGCGCAGTTATTTGTACAATTC-3′ to amplify the linker-yeGFP fragment from the pYM44 plasmid, and 5′-TAACTGCGCGCCACTTCTAAATAAG-3′ and 5′-TTTGTACAATTCATCCATACCATGGGTAATACCA-3′ to amplify the linearized pYM44 plasmid. To generate the pFA6a-link-2xyomCherry-CaURA3 plasmid, a linker-yomCherry fragment (linker sequence: GSGAGAGAGAGA) was amplified by PCR using the pFA6a-link-yomCherry-CaURA3 plasmid (Sheff and Thorn, 2004) as a template and the primers 5′-ATAGTCGACGGATCCGGAGCAGGTGCTGGTGCTGGTGCTGGAGCAGTTAGCAAAGGCGAGGAAGATAACATG-3′ and 5′-ACATTAATTAATGCTCCAGCACCAGCACCCTTGTACAGTTCATCCATACCACCTG-3′, and cloned in frame with the N terminus of the yomCherry in the pFA6a-link-yomCherry-CaURA3 plasmid using the SalI and PacI restriction sites. SnapGene software (GSL Biotech; available at www.snapgene.com) was used for molecular cloning procedures. The centromeric plasmid for expression of GFP-Sed5 under the control of the ADH promoter (pRS315-prADH-GFP-Sed5) was provided by J. Gerst (Weizmann Institute of Science, Rehovot, Israel; Weinberger et al., 2005). The episomal plasmid coding for two tandem copies of the Osh2 PH domain fused to mCherry (pRS425-prGDP-mCherry-2xPHOsh2) was provided by S. Emr (Cornell University, Ithaca, NY). The plasmids for the expression of mRFP-EEA1-FYVE (pRS416-prADH-mRFP-FYVEEEA1), mCherry-Atg8 (pRS316-prATG8-mCherry-Atg8), and mRFP-Atg9 (pRS416-prCUP1-mRFP-Atg9) have been described previously (Bruns et al., 2011).
Media and medium shift procedures
Yeast cells were grown in synthetic complete (SC) media (0.67% yeast nitrogen base without amino acids, 2% glucose supplemented with amino acid drop-out mix; Sigma-Aldrich). Glucose starvation medium (SC-D) consisted of SC medium that lacked glucose. The nitrogen starvation medium (SD-N) consisted of 0.17% yeast nitrogen base lacking amino acids and ammonium sulfate, and 2% glucose. To induce nutrient starvation, mid-log phase cells were harvested by centrifugation at 3,000 g, washed twice in 2% potassium acetate, SC-D, or SD-N, and subsequently incubated in 2% potassium acetate, SC-D, or SD-N at 1 OD600nm/ml for the indicated time intervals. For re-feeding experiments, starved cells were collected by centrifugation, resuspended in SC medium at 0.3 OD600nm/ml, and incubated for 1 h. Unless otherwise indicated, cells were grown at 25°C.
Epifluorescence microscopy
After incubation in the appropriate medium, ∼1.5 OD600nm of cells were harvested by centrifugation at 3,000 g for 3 min, resuspended in a small volume of the corresponding medium, spotted on a microscopy slide, and subsequently live-imaged at 25°C with a DMI6000 B epifluorescence microscope (Leica) equipped with a DFC 360FX camera (Leica) using an HCX Plan Apochromat 100× 1.4 NA objective lens. Images were acquired using LAS AF software (Leica) with 0.5–2 s exposure times. For the quantification of the localization phenotypes of Grh1-2×GFP, whole cell z stacks with a step size of 0.4 µm were captured as described above. The corresponding maximum-projection images were created with ImageJ 1.45r and the number of cells showing the following phenotypes for the distribution of Grh1-2×GFP was manually counted: diffuse, growth (>5 small punctate structures), CUPS (1–3 large dots), or intermediate (1–3 large dots and several small punctate structures). At least three microscopic fields (≥50 cells) were examined for each condition. Results represent the percentage of cells with each distribution phenotype and are representative of at least two experiments.
Confocal fluorescence microscopy
After incubation in starvation medium for 1.5 h, ∼0.05 OD600nm of cells were plated in starvation medium on Concanavalin A–coated (Sigma-Aldrich) Lab-Tek chambers (Thermo Fisher Scientific) and were allowed to settle for 30 min at 25°C. Then, whole cell z stacks with a step size of 0.3 µm were acquired using a spinning-disk confocal microscope (Revolution XD; Andor Technology) with a Plan Apochromat 100× 1.45 NA objective lens equipped with a dual-mode electron-modifying charge-coupled device camera (iXon 897 E; Andor Technology) and controlled by the iQ Live Cell Imaging software (Andor Technology). Colocalization coefficients were calculated with ImageJ 1.45r and the JACoP plugin (Bolte and Cordelières, 2006).
Online supplemental material
Fig. S1 shows the high degree of colocalization between Grh1 and Uso1 or Sed5 and the low colocalization between Grh1 and the early Golgi enzymes Mnn2 or Mnn5 in starved cells. Fig. S2 shows that the early Golgi marker Anp1-RFP does not accumulate in CUPS formed in the COPII or COPI mutant cells. Fig. S3 shows the lack of localization of mCherry-Osh2-2×PH, mRFP-EEA1-FYVE, Anp1-RFP, Sec7-2×mCherry, mCherry-Atg8, and mRFP-Atg9 in Grh1-2×GFP-labeled CUPS. Table S1 lists the yeast strains used in this study. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201407119/DC1.
Supplementary Material
Acknowledgments
We thank members of the Malhotra Lab for helpful discussion. We thank Drs. Charlie Boone, Jeff Gerst, and Scott Emr for kindly sharing reagents. Confocal imaging was performed at the Center for Genomic Regulation Advanced Light Microscopy Unit.
We acknowledge support from the Spanish Ministry of Economy and Competitiveness, Centro de Excelencia Severo Ochoa 2013-2017, SEV-2012-0208. V. Malhotra is an Institució Catalana de Recerca i Estudis Avançats (ICREA) professor at the Center for Genomic Regulation and the work in his laboratory is funded by grants from Plan Nacional (BFU2008-00414), Consolider (CSD2009-00016), and the European Research Council (268692). The project has received research funding from the European Union (EU). This paper reflects only the authors’ views. The EU is not liable for any use that may be made of the information contained therein.
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- COPI
- coat protein complex I
- COPII
- coat protein complex II
- CUPS
- compartment for unconventional protein secretion
- PI3P
- phosphatidylinositol 3-phosphate
- PI4P
- phosphatidylinositol 4-phosphate
References
- Achstetter T., Franzusoff A., Field C., and Schekman R.. 1988. SEC7 encodes an unusual, high molecular weight protein required for membrane traffic from the yeast Golgi apparatus. J. Biol. Chem. 263:11711–11717. [PubMed] [Google Scholar]
- Anjard C., and Loomis W.F.. 2005. Peptide signaling during terminal differentiation of Dictyostelium. Proc. Natl. Acad. Sci. USA. 102:7607–7611 10.1073/pnas.0501820102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoh Q.L., Graves L.M., and Duncan M.C.. 2011. Glucose regulates clathrin adaptors at the trans-Golgi network and endosomes. Mol. Biol. Cell. 22:3671–3683 10.1091/mbc.E11-04-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield D.K., Lewis M.J., and Pelham H.R.. 1995. A SNARE-like protein required for traffic through the Golgi complex. Nature. 375:806–809 10.1038/375806a0 [DOI] [PubMed] [Google Scholar]
- Behnia R., Barr F.A., Flanagan J.J., Barlowe C., and Munro S.. 2007. The yeast orthologue of GRASP65 forms a complex with a coiled-coil protein that contributes to ER to Golgi traffic. J. Cell Biol. 176:255–261 10.1083/jcb.200607151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S., and Cordelières F.P.. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224:213–232 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- Bruns C., McCaffery J.M., Curwin A.J., Duran J.M., and Malhotra V.. 2011. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 195:979–992 10.1083/jcb.201106098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew N., and Barlowe C.. 1998. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17:2156–2165 10.1093/emboj/17.8.2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P., Schröder-Köhne S., Sweet D.S., Démollière C., Hennecke S., Frigerio G., and Letourneur F.. 1997. The Sec20/Tip20p complex is involved in ER retrieval of dilysine-tagged proteins. Eur. J. Cell Biol. 73:93–97. [PubMed] [Google Scholar]
- Duran J.M., Anjard C., Stefan C., Loomis W.F., and Malhotra V.. 2010. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188:527–536 10.1083/jcb.200911154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A., Redding K., Crosby J., Fuller R.S., and Schekman R.. 1991. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J. Cell Biol. 112:27–37 10.1083/jcb.112.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A. III, and Smith H.O.. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 6:343–345 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Graef M., Friedman J.R., Graham C., Babu M., and Nunnari J.. 2013. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell. 24:2918–2931 10.1091/mbc.E13-07-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K.G., and Pelham H.R.. 1992. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J. Cell Biol. 119:513–521 10.1083/jcb.119.3.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M., Kreis T., and Schekman R.. 1992. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 360:603–605 10.1038/360603a0 [DOI] [PubMed] [Google Scholar]
- Janke C., Magiera M.M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E., and Knop M.. 2004. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 21:947–962 10.1002/yea.1142 [DOI] [PubMed] [Google Scholar]
- Kinseth M.A., Anjard C., Fuller D., Guizzunti G., Loomis W.F., and Malhotra V.. 2007. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell. 130:524–534 10.1016/j.cell.2007.06.029 [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E.C., Hennecke S., Démollière C., Duden R., Emr S.D., Riezman H., and Cosson P.. 1994. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 79:1199–1207 10.1016/0092-8674(94)90011-6 [DOI] [PubMed] [Google Scholar]
- Levi S.K., Bhattacharyya D., Strack R.L., Austin J.R. II, and Glick B.S.. 2010. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 11:1168–1179 10.1111/j.1600-0854.2010.01089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J., Rayner J.C., and Pelham H.R.. 1997. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 16:3017–3024 10.1093/emboj/16.11.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gallwitz D., and Peng R.. 2005. Structure-based functional analysis reveals a role for the SM protein Sly1p in retrograde transport to the endoplasmic reticulum. Mol. Biol. Cell. 16:3951–3962 10.1091/mbc.E05-02-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Vizeacoumar F.J., Bahr S., Li J., Warringer J., Vizeacoumar F.S., Min R., Vandersluis B., Bellay J., Devit M., et al. 2011. Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol. 29:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V.2013. Unconventional protein secretion: an evolving mechanism. EMBO J. 32:1660–1664 10.1038/emboj.2013.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjithaya R., Anjard C., Loomis W.F., and Subramani S.. 2010. Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 188:537–546 10.1083/jcb.200911149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Hirata A., Ogawa Y., Yonehara T., Yoda K., and Yamasaki M.. 1991. A cytoskeleton-related gene, uso1, is required for intracellular protein transport in Saccharomyces cerevisiae. J. Cell Biol. 113:245–260 10.1083/jcb.113.2.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B.J., and Pelham H.R.. 1998. SNAREs and membrane fusion in the Golgi apparatus. Biochim. Biophys. Acta. 1404:9–31 10.1016/S0167-4889(98)00044-5 [DOI] [PubMed] [Google Scholar]
- Rabouille C., Malhotra V., and Nickel W.. 2012. Diversity in unconventional protein secretion. J. Cell Sci. 125:5251–5255 10.1242/jcs.103630 [DOI] [PubMed] [Google Scholar]
- Sato T.K., Darsow T., and Emr S.D.. 1998. Vam7p, a SNAP-25-like molecule, and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol. Cell. Biol. 18:5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff M.A., and Thorn K.S.. 2004. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 21:661–670 10.1002/yea.1130 [DOI] [PubMed] [Google Scholar]
- Tu L., Tai W.C., Chen L., and Banfield D.K.. 2008. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 321:404–407 10.1126/science.1159411 [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C., and Novick P.. 1999. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1:523–525 10.1038/70319 [DOI] [PubMed] [Google Scholar]
- Weinberger A., Kamena F., Kama R., Spang A., and Gerst J.E.. 2005. Control of Golgi morphology and function by Sed5 t-SNARE phosphorylation. Mol. Biol. Cell. 16:4918–4930 10.1091/mbc.E05-02-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.