Abstract
Polymorphonuclear neutrophils are critical for resolution of bacterial infections. In tissues, most of the neutrophils quickly die through apoptosis. Using propidium iodide DNA staining and DNA gel electrophoresis, we found that spontaneous apoptosis of neutrophils from patients suffering osteomyelitis (n = 52) was significantly decreased in relation to control neutrophils (n = 20) (40.2% ± 25.2% versus 54.5% ± 23.5%; P < 0.03). Incubation of neutrophils from normal volunteers with sera from patients with osteomyelitis reduced apoptosis from 79.1% ± 14.8% in control sera to 62.2% ± 18.7% in osteomyelitis sera. A significant increase of serum interleukin-6 (IL-6) and IL-1α was found in osteomyelitis (IL-6, 8.8 ± 11.9 pg/ml versus 1.8 ± 1.2 pg/ml in controls [P < 0.004]; IL-1α, 3.8 ± 6.4 pg/ml versus 1.0 ± 2.2 pg/ml in controls [P < 0.02]). No differences in the levels of other cytokines, such as tumor necrosis factor alpha, were found. There was an inverse correlation between IL-6 levels and neutrophil apoptosis (r = −0.855; P < 0.007), but this was not the case for other cytokines. The antiapoptotic effect of the osteomyelitis sera was reversed with anti-IL-6 antibodies (P < 0.03) and was reproduced with recombinant human IL-6 (P < 0.001). The longer life span of neutrophils in osteomyelitis induced by IL-6 could contribute to the tissue damage that occurs in these chronic bone infections.
Polymorphonuclear leukocytes (polymorphonuclear neutrophils) (PMN) are potent phagocytes that are the first line of the host immune defense against many infections. Clearance of neutrophils during resolution of acute inflammation occurs by apoptosis (27), which is a crucial step in the mechanisms that govern the resolution of neutrophil inflammation (36). This process is controlled by down- or upregulation of genes of the bcl-2 family, which express the antiapoptotic proteins Mcl-1, A1, and Bcl-X, and the proapoptotic proteins Bax-α, Βid, Bak, and Bad (2, 13, 19, 38). In the normal resolution of inflammation, neutrophil apoptosis and subsequent ingestion of dead PMN by macrophages play an important role in limiting the destructive potential of these cells (35). Inappropriate or exaggerated neutrophil activation can cause severe tissue damage during several diseases characterized by neutrophilic inflammation (17). Histotoxic compounds, such as oxidants and primary granule constituents, are secreted by activated neutrophils in the extracellular milieu, leading to the development of tissue injury. Delayed neutrophil apoptosis is associated with various proinflammatatory diseases, including systemic inflammatory response syndrome (20), ischemia-reperfusion injury (18), and adult respiratory distress syndrome (29).
At the inflammatory loci, a number of cytokines and growth factors are produced, such as interleukin-1β (IL-1β), IL-2, IL-15, interferon gamma (IFN-γ), granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (25), that inhibit in vitro neutrophil apoptosis (2, 33). Others, such as tumor necrosis factor alpha (TNF-α), induce or delay apoptosis (30, 32, 33, 37). The reported effects of IL-6 on neutrophil apoptosis are variable (1, 6, 7, 8, 16, 21, 23, 39).
Osteomyelitis is a difficult-to-treat bone infection that is characterized by progressive inflammatory destruction of the infected bone and new apposition of bone at the site of infection. In adults, osteomyelitis is usually a complication of open wounds involving the bone, from either fractures, surgery, or both. The risk and severity of infection can be enhanced by the presence of a foreign body (metallic or prosthetic devices). It is reported that 0.4 to 7% of trauma and orthopedic operations are complicated by osteomyelitis (10, 26, 34). This infection can develop as well in a noninjured bone after bacteremia, mostly in prepubertal children and in elderly patients, when the infection involves the axial skeleton. Staphylococcus aureus is the microorganism most frequently isolated in both posttraumatic and hematogenous osteomyelitis. Despite appropriate combined medical and surgical therapies, up to 30% of osteomyelitis infections become chronic, causing major economic losses and personal morbidity and mortality (26). Much attention has been dedicated to improving the surgical and medical treatment of osteomyelitis, but little progress has been made toward understanding its pathogenesis. It is clear that it is multifactorial and influenced mainly by local factors related to the bone lesion and microorganisms inoculated into the bone, but inherited factors and cell immunity dysfunctions could play some role as well (3, 41).
Increased levels of inflammatory cytokines have been reported in osteomyelitis patients (14, 22). Furthermore, mice with experimental posttraumatic osteomyelitis have elevated levels of IL-1 and IL-6 in serum, and IL-1, IL-6, and TNF-α are synthesized at the site of infection for at least 2 weeks after infection (9, 41). Klosterhalfen et al. (22) described significantly increased levels of TNF-α, IL-1β, IL-6, IL-8, and leukotriene B4 in surgically obtained bone fragments from patients with acute osteomyelitis.
In this work we found that apoptosis of neutrophils from osteomyelitis patients is decreased in relation to controls and that this seems to be due to the high levels of circulating IL-6.
(These results were presented in part at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002 [V. Asensi, A. Meana, J. Fierer, J. A. Carton, J. A. Maradona, V. Alvarez, E. Coto, J. Paz, A. Dieguez, and J. M. Arribas, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 644, 2002].)
MATERIALS AND METHODS
Patients.
Fifty-two patients (34 men and 18 women, with a mean age of 46.9 ± 15.1 years) admitted between January 1998 and December 2000 to the Hospital Central de Asturias and to other three affiliated hospitals of the same region in northern Spain were studied. Patients with acute (20 cases) and chronic (32 cases) osteomyelitis were included in the study and monitored for 1 year. Osteomyelitis was diagnosed by clinical, roetgenographic, tomographic, and isotopic bone imaging criteria. Osteomyelitis was considered chronic if it was present for more than 3 months and was considered cured if did not relapse during a year of follow-up. Surgical and sinus tract pus samples were cultured from all of the osteomyelitis patients. In addition, a group of 20 blood bank donors, matched for age and sex with the patients, were used as controls. Each participant gave informed consent for the study, which was approved by the ethics committee of the Hospital Central de Asturias.
Neutrophil isolation.
Ten milliliters of peripheral blood was simultaneously collected for each assay from one or more osteomyelitis patients and one or two healthy donors in glass tubes containing potassium-EDTA. Neutrophils were separated by the following consecutive steps: (i) sedimentation in 3% dextran T-500 (Pharmacia, Uppsala, Sweden) in 0.9% NaCl, (ii) standard Ficoll-Hypaque (Lymphoprep; Nicomed Pharma, Oslo, Norway) gradient centrifugation, (iii) hypotonic lysis of the remaining red blood cells by resuspension in 0.2% NaCl, and (iv) resuspension in Ham's medium (Biochrom KG, Berlin, Germany). The cells were counted in an autoanalyzer (Coulter, Izasa, Spain), adjusted to 0.5 × 107 to 1 × 107/ml and kept on ice (4°C) until they were used. Cells collected from the gradient interface were >95% neutrophils by Coulter identification and >95% viable by trypan blue exclusion.
Collection of sera.
Five milliliters of blood of each patient and control was collected in a siliconized glass tube, kept for 2 h at room temperature, and centrifuged at 1,000 × g for 5 min. Fresh sera from patients and controls were used, except for determination of cytokines levels. For these enzyme-linked immunosorbent assays (ELISAs), serum aliquots previously stored at −70°C were used.
Culture conditions for human PMN.
Neutrophils (0.5 × 107 to 1 × 107) were incubated at 37°C in a water bath in an Eppendorf tube with 200 μl of serum or Ham's medium for 12 h, and then apoptosis was measured.
In some experiments, human recombinant IL-6 (Becton Dickinson, Bedford, Mass.) was used at final concentrations of 0.1, 1.0, and 10 ng/ml and added to 200 μl of control serum. Then, 0.5 × 107 to 1 × 107 neutrophils were added to each sample and incubated at 37°C in a water bath for 12 h, and apoptosis was measured.
Neutralization of IL-6 with antibody.
IL-6 present in serum was neutralized with mouse monoclonal anti-IL-6 (Sigma, St. Louis, Mo.). To neutralize each 0.2 μg of IL-6 present in serum as measured by ELISA, 1.15 μg of anti-IL-6 (IL-6/anti-IL-6 ratio, 1:5.75) was added as previously described (1), and the mixtures were incubated for 1 h at 37°C before use.
Flow cytometry determination of apoptosis: propidium iodide assay.
Propidium iodide staining was assessed as described by Nicoletti et al. (31). Neutrophils (0.5 × 107 to 1 × 107) were suspended in 1 ml of ice-cold 70% ethanol for 4 h, either used immediately or kept at −20°C for a week, washed three times in phosphate-buffered saline (PBS) at 4°C, and resuspended in 250 μl of PBS and 250 μl of RNase (1 mg/ml) (Sigma). After gentle mixing, 500 μl of propidium iodide (100 μg/ml in PBS) (Sigma) was added, and the cells were incubated in the dark at room temperature for 15 min and stored at 4°C before analysis with a fluorescence-activated cell sorter flow cytometer (Cytoron Absolute; Ortho Diagnostic System, Raritan, N.J.). Mean fluorescence values were determined from a minimum of 104 cells within an analysis region corresponding to nonfragmented neutrophils by using Winlist 2.01 (Verity Software House, Topsham, Maine) for data acquisition analysis.
DNA isolation and gel electrophoresis.
DNA isolation and gel electrophoresis were done as described by Baran et al. (4). For preparation of genomic DNA, neutrophils (0.5 × 107 to 1 × 107) were lysed with 0.5 ml of a lysis buffer (0.6% sodium dodecyl sulfate, 10 mM EDTA, 1 M NaCl), mixed vigorously to obtain a viscous and clear cell lysate, and kept at 4°C for 12 to 24 h. The cell lysate was centrifuged at 14,000 × g for 15 min at 37°C, and the supernatant was treated with 50 μg/ml of RNase, incubated for 30 min at 37°C, and extracted twice with an equal volume (0.5 ml) of phenol-chloroform-isoamyl alcohol (25:24:1). After incubation, the supernatant was centrifuged at 14,000 × g for 5 min at 4°C. DNA in the aqueous phase was precipitated at −20°C in 0.3 M sodium acetate-100% ethanol. Precipitates were pelleted by centrifugation at 14,000 × g for 15 min at 4°C, washed with ice-cold 70% ethanol, and resuspended in 10 μl of Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA). To each sample, 10 μl of 2% gel loading buffer (2% Ficoll 400, 10 mM EDTA, 0.1% sodium dodecyl sulfate, 0.025% bromophenol blue, and 0.025% xylene cyanol) was added. Samples were electrophoresed for 90 min at 5 V/cm in a 2% agarose gel containing 0.5 μg of ethidium bromide per ml in Tris-borate-EDTA buffer (10.8 g of Tris base, 5.5 g of boric acid, 2 mM EDTA [pH 8.2]). DNA was visualized with UV light and photographed. Sizes of DNA fragments in the samples were determined by comparison with standard DNA molecular size markers. All of the reagents in this assay were purchased from Sigma.
Cytokines and CRP.
IL-1-α, IL-6, and TNF-α were measured with ELISA kits (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Cytokine concentrations were calculated by comparing the sample absorbance with the absorbance of pooled serum enriched with increasing amounts of recombinant human cytokine (22). C-reactive protein (CRP) was measured by nephelometry with a BNII reader (Behring, Marburg, Germany).
Statistical analysis.
All values are presented as the mean ± standard deviation. Statistical significance for the means was determined with the Mann-Whitney test, and that for paired variables was determined by the Wilcoxon rank and Pearson's correlation tests. A P value of <0.05 was considered statistically significant.
RESULTS
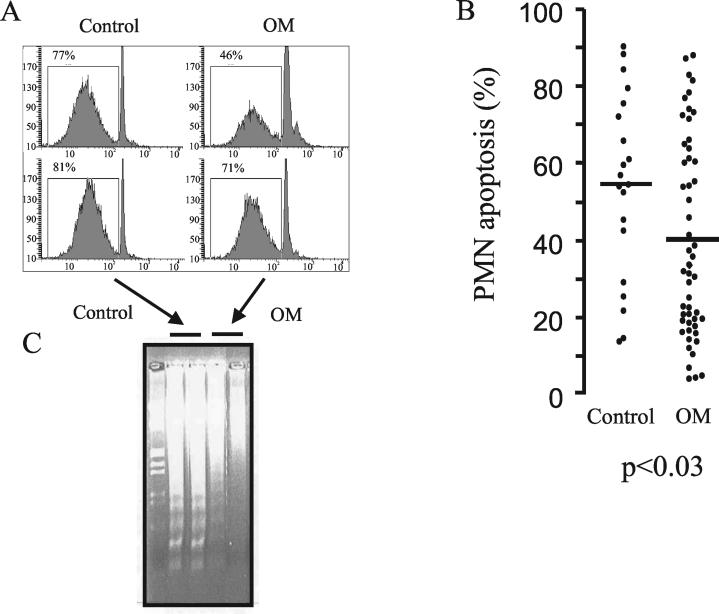
Because neutrophils are so important in the defense against bacterial infections but normally have a short half-life, we determined the rate of spontaneous apoptosis by neutrophils from patients and normal controls. After 12 h of incubation in Ham's medium, apoptosis of neutrophils was determined by propidium iodide staining and flow cytometry. Normally, cells at the beginning of G1 phase show an important peak of apoptosis. However, reduced apoptosis was observed in neutrophils of osteomyelitis patients compared to controls (Fig. 1A). This difference was statistically significant (40.2% ± 25.2% versus 54.5% ± 23.5% for osteomyelitis patients and controls, respectively; P < 0.03) (Fig. 1B). These results were confirmed with DNA neutrophil fragmentation by gel electrophoresis. Control neutrophils exhibited an internucleosomal pattern of DNA fragmentation (DNA laddering) that was much more intense than that of neutrophils from osteomyelitis patients (Fig. 1C).
FIG. 1.
Apoptosis of neutrophils (0.5 × 107 to 1 × 107) of osteomyelitis (OM) patients after 12 h of incubation (37°C) in Ham's medium compared to neutrophils of healthy controls. Results from a representative experiment with neutrophils from two patients and two controls are shown in panels A and C. DNA was stained with propidium iodide, and apoptosis was measured by cytometric analysis (A and B) or by agarose gel electrophoresis (C). Left lane in panel C, molecular size markers. In panel B, the 12-h spontaneous apoptosis, in Ham's medium, of 0.5 × 107 to 1 × 107 neutrophils from 52 osteomyelitis patients and 20 controls, measured by cytometric analysis, is represented. Points and bars represent the individual apoptosis values and their means.
Soluble mediators that are detectable in the inflammatory environment surrounding recruited neutrophils are capable of modulating cell survival, retarding apoptosis (11). Therefore, to determine if the decreased apoptosis of osteomyelitis neutrophils is an intrinsic defect or may be due to released cytokines or growth factors, we tested the effect of control or osteomyelitis sera on the apoptosis of control neutrophils incubated for 12 h. Incubation of control neutrophils with control sera induced a strong increase (25%) in the spontaneous apoptosis (79.1% ± 14.8% in autologous serum versus 54.5% ± 23.5% in Ham's medium). By contrast, incubation with osteomyelitis sera did not modify significantly the apoptotic rate (62.2% ± 18.7%).
So far, our experiments suggested that a factor present in the sera of osteomyelitis patients might be responsible for the antiapoptotic effect. For this reason, we tested the concentrations of some proinflammatory cytokines in serum. Although the mean levels of TNF-α were higher in osteomyelitis patients (n = 52), the increase was not significant (8.7 ± 11.7 pg/ml, versus 2.4 ± 0.62 pg/ml in controls [n = 22]). By contrast, the levels of IL-6 and IL-1α in sera from osteomyelitis patients (IL-6, 8.8 ± 11.9 pg/ml; IL-1α, 3.8 ± 6.4 pg/ml) were significantly higher than those in control sera (IL-6, 1.8 ± 1.2 pg/ml [P < 0.004]; IL-1α, 1.0 ± 2.2 pg/ml [P < 0.02]) We also determined the levels of CRP, an acute-phase protein that is a hallmark of inflammation. As expected, the levels in the sera of osteomyelitis patients (33.7 ± 39.8 μg/ml) were increased (P < 0.0001) in relation to those in the control sera (3.3 ± 0.4 μg/ml) (Table 1).
TABLE 1.
Concentrations of proinflammatory cytokines and CRP in sera from osteomyelitis patients and controlsa
| Group | IL-6 (pg/ml) | IL-1α (pg/ml) | TNF-α (pg/ml) | CRP (μg/ml) | No. of patients |
|---|---|---|---|---|---|
| Controls | 1.8 ± 1.2 | 1.0 ± 2.2 | 2.4 ± 0.62 | 3.3 ± 0.4 | 20 |
| Osteomyelitis patients | 8.8 ± 11.9 | 3.8 ± 6.4 | 8.7 ± 11.7 | 33.7 ± 39.8 | 52 |
| P value | 0.004 | 0.02 | 0.6 | 0.0001 |
IL-6, IL-1α, and TNF-α were measured by ELISA, and CRP was measured by nephelometry. Results are expressed as means and standard deviations.
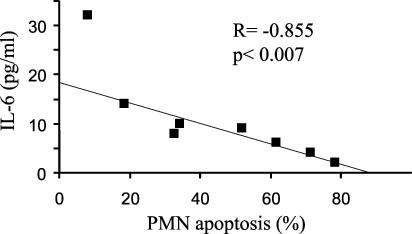
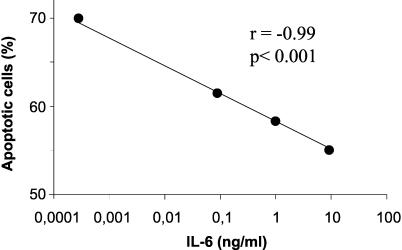
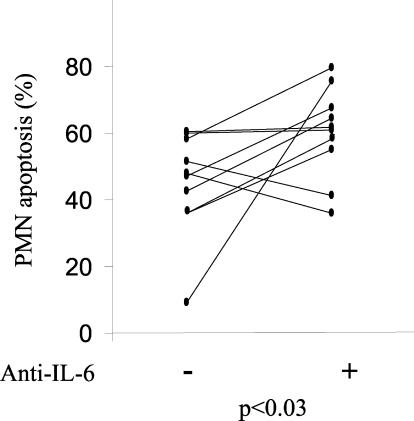
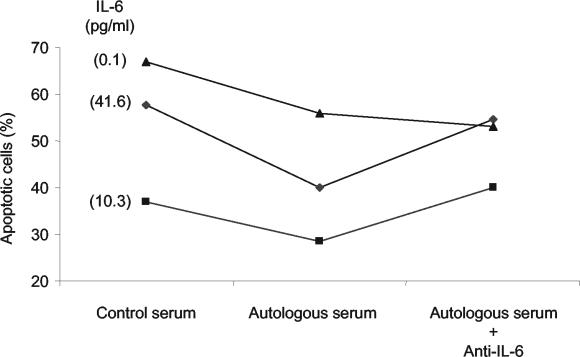
We then correlated the levels of IL-6 in the sera of osteomyelitis patients with the percentage of spontaneous neutrophil apoptosis after a 12-h incubation in Ham's culture medium. We observed a significant inverse correlation (r = −0.855 and P < 0.007 by the Pearson correlation test) between the serum IL-6 levels and apoptosis of neutrophils (Fig. 2). No significant correlation was found for IL-1α or TNF-α. To establish that IL-6 was responsible for the decreased apoptosis of PMN in patients suffering osteomyelitis we performed two additional experiments. First, we incubated control neutrophils with control serum and recombinant IL-6. There was a negative correlation between the IL-6 level and the induction of apoptosis (Fig. 3). Second, we incubated control neutrophils with sera of osteomyelitis patients in the presence or absence of antibodies against IL-6. Apoptosis was determined by using neutrophils of 10 controls in the presence of sera of 10 osteomyelitis patients (Fig. 4). When antibodies against IL-6 were added to the osteomyelitis sera, the antiapoptotic effect was significantly inhibited (P < 0.03 by the Wilcoxon signed rank test) (Fig. 4). Finally, we tested the role of IL-6 present in the patient's serum on apoptosis by inhibition of the cytokine with an anti-IL-6 antibody. The apoptosis of neutrophils from three osteomyelitis patients, two with acute infection and one with cured infection, increased in the presence of a control serum in relation to their autologous sera. Also, the addition of anti-IL-6 antibodies enhanced the rate of apoptosis when they were added to the autologous serum in patients with elevated levels of IL-6 but not in the patient with cured infection, in whom the levels of IL-6 were lower (Fig. 5).
FIG. 2.
Correlation between apoptosis of neutrophils and serum IL-6 levels in osteomyelitis patients. Neutrophils (0.5 × 107 to 1 × 107) from eight osteomyelitis patients were incubated for 12 h in 200 μl of Ham's medium. IL-6 levels in simultaneously obtained serum were measured by ELISA. Neutrophil apoptosis was measured by propidium iodide staining and flow cytometry.
FIG. 3.
IL-6-dependent apoptosis in neutrophils. Neutrophils (0.5 × 107 to 1 × 107) from a healthy control were incubated for 12 h in 200 μl of control serum with increasing concentrations of recombinant human IL-6. The baseline IL-6 concentration in serum was 0.00041 ng/ml. Apoptosis was measured by propidium iodide staining and flow cytometry.
FIG. 4.
IL-6 is involved in the antiapoptotic effect of sera from osteomyelitis patients. Neutrophils from 10 healthy donors were incubated for 12 h in the presence of untreated sera from 10 osteomyelitis patients or sera absorbed with anti-IL-6 antibodies. For the preincubation, the IL-6/anti-IL-6 ratio was 1:5.75. Apoptosis was measured by propidium iodide staining and flow cytometry. Points represent the individual apoptosis values.
FIG. 5.
The protective effect of sera from osteomyelitis patients is lost in the present of antibodies against IL-6. Two patients with acute osteomyelitis (serum IL-6 levels of 41.6 and 10.3 pg/ml) and one with cured osteomyelitis (0.1 pg/ml) were studied. Serum IL-6 levels were measured by ELISA and are indicated in parenthesis. Apoptosis of the neutrophils from each osteomyelitis patient (0.5 × 107 to 1 × 107) after 12 h of incubation with 200 μl of serum from one donor or with the patient's own serum with or without anti-IL-6 antibodies was studied. Apoptosis was measured by propidium iodide staining and flow cytometry. A 1-h preincubation of serum with anti-IL-6 antibodies with a serum IL-6/anti-IL-6 ratio of 1:5.75 was performed for the indicated samples. As an antibody control, neutrophils were incubated with irrelevant antibodies of the same isotype, which did not modify apoptosis.
DISCUSSION
This report documents that neutrophils from osteomyelitis patients have a lower rate of spontaneous apoptosis than do cells from normal controls. Although neutrophils of osteomyelitis patients were obtained from peripheral blood and not from the bony lesions, the tissue cells are derived from circulating cells, so it is likely that they represent the behavior of neutrophils at the infectious loci. The persistence of neutrophils at the inflammatory loci could contribute to the clearance of bacterial infection. However, the retardation of apoptosis and elimination of neutrophils in the site of infection could help to perpetuate the in situ bone inflammatory response by release of their proinflammatory and histotoxic contents (21, 22). In fact, an increased survival of neutrophils has been found to be associated with inflammation (15, 28).
The increased survival of neutrophils from these patients does not seem to be an intrinsic property of the PMN but seems to be due to the action of IL-6 on those cells. Although we cannot exclude a role for other cytokines or growth factors, IL-6 seems to be the main cytokine incriminated in the antiapoptotic effect. There was a clear correlation between higher serum IL-6 levels and longer neutrophil survival; this effect was blocked with anti-IL-6, and it was reproduced with recombinant human IL-6. This cytokine is released during the acute-phase response and in chronic intracellular infections (5), where it plays a critical biologic function in the inflammation, decreasing TNF-α, GM-CSF, and IFN-γ levels and increasing the B-cell differentiation, T-cell proliferation and activation, and antibody secretion (5, 24, 40). It has been reported that, when the spontaneous or endotoxin-induced inflammatory responses of mice in which the IL-6 gene has been inactivated by homologous recombination are compared with those of normal animals, in tissues there are only half of the number neutrophils present in the controls under the same conditions (40). Thus, there is a strong relationship between IL-6 and neutrophils.
The origin of IL-6 in inflammation, as well as of other factors that induce neutrophil survival, is the macrophages as well as the endothelial and epithelial cells (12). Different authors, as we did, have studied the role of IL-6 in neutrophil survival by adding recombinant IL-6 in vitro. Although most of the reports showed a 15 to 20% longer neutrophil survival, which agrees with the 15.4% in our observations (6, 8, 11, 23, 28, 39), some authors (1, 21) reported an increased apoptosis. This may be related to the different experimental conditions (6). Our data for osteomyelitis patients show an antiapoptotic effect of endogenous IL-6 in vivo in a pathological state.
This delaying apoptotic effect on neutrophils has been related to an increase of neutrophil superoxide generation (7, 15). However, we have not found changes in the neutrophil life span after pretreatment of blood donor neutrophils with antioxidants before their incubation with osteomyelitis serum or when using neutrophils from a chronic granulomatous disease patient, which are congenitally incapable of producing oxidants in response to soluble or particulate stimuli (unpublished observations). Therefore, the antiapoptotic effect of IL-6 on the neutrophil does not seem to be linked to an increase in its capacity for O2− production. These data confirm previous observations by Ottonello et al. (33), in which, using different cytokines and growth factors that included IL-6, a correlation between protection from apoptosis and the PMN oxidative response was not found. The antiapoptotic effect of IL-6 is blocked by platelet-activating factor receptor antagonists and by IL-10 (2, 7). This fact could suggest a membrane-bound mechanism similar to that reported for GM-CSF and IL-8, in which an Fc receptor-dependent activation may activate some mitogen-activated protein kinases that enhance the expression of the antiapoptotic proteins Mcl-1, A1 and Bcl-X, which delay the apoptosis of neutrophils (2). However, it has been shown that IL-6 inhibits constitutive Bax expression (6, 33), which may play a major role in neutrophil survival. Finally, from our results we cannot exclude the effect of other cytokines or growth factors released during inflammation, such as IL-1 (which was also significantly increased in the sera of our osteomyelitis patients) or GM-CSF or IFN-γ (which we did not measure), all of which have a neutrophil antiapoptotic effect (6, 33, 39). In fact, we have found recently that the IL-1α −889 polymorphism was significantly more frequent in osteomyelitis patients than in healthy controls (3).
In summary, there is an extended life span of neutrophils of osteomyelitis patients; these neutrophils could migrate into the infected bone, where, by release of proteolytic enzymes, they could help to perpetuate this infection.
Acknowledgments
This study was supported by research grants IR-00-519-59 and MB-02-519-1 from Oviedo University, Oviedo, Spain; FICYT grant PB02-019; and Fondo de Investigaciones Sanitarias grant PI030282 (all given to Victor Asensi).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Afford, S. C., J. Pongracz, R. A. Stockley, R. A. Crocker, and D. Burnett. 1992. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J. Biol. Chem. 267:21612-21616. [PubMed] [Google Scholar]
- 2.Akgul, C., D. A. Moulding., and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-332. [DOI] [PubMed] [Google Scholar]
- 3.Asensi, V., V. Alvarez, E. Valle, A. Meana, J. Fierer, E. Coto, J. A. Carton., J. A. Maradona, J. Paz, M. A. Dieguez, B. de la Fuente, A. Moreno, S. Rubio, M. J. Tuya, J. Sarasua, and J. M. Arribas. 2003. An IL-1α (−889) promoter polymorphism is a risk factor for osteomyelitis. Am. J. Med. Genet. 119A:132-136. [DOI] [PubMed] [Google Scholar]
- 4.Baran, J., K. Guzik, W. Hryniewicz, M. Ernst, H. D. Flad, and J. Pryjma. 1996. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect. Immun. 64:4242-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann, H., and J. Gauldie. 1994. The acute phase response. Immunol. Today 15:74-80. [DOI] [PubMed] [Google Scholar]
- 6.Biffl, W. L., E. E. Moore, F. A. Moore, and C. C. Barnett. 1995. Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent. J. Leukoc. Biol. 58:582-584. [DOI] [PubMed] [Google Scholar]
- 7.Biffl, W., E. E. Moore, F. A. Moore, and C. C. Barnett. 1996. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J. Trauma 40:575-579. [DOI] [PubMed] [Google Scholar]
- 8.Biffl, W. L., E. Moore, F. A. Moore, C. C. Barnett, V. S. Carl, and V. M. Peterson. 1996. Interleukin-6 delays neutrophil apoptosis. Arch. Surg. 131:24-30. [DOI] [PubMed] [Google Scholar]
- 9.Bost, K. L., W. K. Raup, N. C. Nicholson, J. L. Bento, I. Marriott I, and M. C. Hudson. 1999. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of interleukin-6 and interleukin-12 production. J. Infect. Dis. 150:1912-1920. [DOI] [PubMed] [Google Scholar]
- 10.Cierny, G. I., and J. T. Mader. 1984. Adult chronic osteomyelitis. Orthopedics 7:1557-1564. [DOI] [PubMed] [Google Scholar]
- 11.Colotta, F., F. Re, N. Polentaritti, S. Sozzani, and A. Mantovani. 1992. Modulation of granulocyte and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 12.Daffern, D. J., M. A. Jageles, and T. E. Hugli. 1999. Multiple epithelial cell-derived factors enhance neutrophil survival. Regulation by glucocorticoids and tumor-necrosis factor-alpha. Am. J. Respir. Cell. Mol. Biol. 21:259-267. [DOI] [PubMed] [Google Scholar]
- 13.Dibbert, B., M. Weber, W. H. Nikolaizik, P. Vogt, M. H. Schoni, K. Blaser, and H. U. Simon. 1999. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis. A general mechanism to accumulate effector cells in inflammation. Proc. Natl. Acad. Sci. USA 96:13330-13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, C. A. W., J. Jellis, S. P. F. Hughes, D. G. Remick, and J. S. Friedland. 1998. Tumor necrosis factor-α, interleukin 6, and interleukin-8 secretion and the acute-phase response in patients with bacterial and tuberculous osteomyelitis. J. Infect. Dis. 177:1582-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanning, N. F., M. R. Kell, G. D. Shorten, W. O. Kirwan., D. Bouchier-Hayes, T. G. Cotter, and H. P. Redmond. 1999. Circulating granulocyte colony-stimulating factor in plasma of patients with the systemic inflammatory response syndrome delays neutrophil apoptosis through inhibition of spontaneous reactive oxygen species generation. Shock 11:167-174. [DOI] [PubMed] [Google Scholar]
- 16.Fanning, N. F., J. Porter, G. D. Shorten, W. O. Kirwan, D. Bouchier-Hayes, T. G. Cotter, and H. P. Redmond. 1999. Inhibition of neutrophil apoptosis after elective surgery. Surgery 126:527-534. [PubMed] [Google Scholar]
- 17.Henson, P. M., and R. B. Johnston. 1988. Tissue injury in inflammation: oxidants, proteinases and cationic proteins. J. Clin. Investig. 79:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez, L. A., M. B. Grisham, B. Twohig, K. E. Arfors, J. M. Harlan, and D. N. Granger. 1987. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am. J. Physiol. 253:H699-H703. [DOI] [PubMed] [Google Scholar]
- 19.Iwai, K., T. Miyawaki, T. Takizawa, A. Konno, K. Ohta, A. Yachie, H. Seki, and N. Taniguchi. 1994. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes and neutrophils. Blood 84:1201-1208. [PubMed] [Google Scholar]
- 20.Jimenez, M. F., R. W. Watson, J. Parodo, D. Evans, D. Foster, M. Steinberg, O. D. Rotstein, and J. C. Marshall. 1997. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch. Surg. 132:1263-1271. [DOI] [PubMed] [Google Scholar]
- 21.Kaplanski, G., V. Marin, F. Montero-Julina, A. Mantovani, and C. Farnarier. 2003. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24:25-30. [DOI] [PubMed] [Google Scholar]
- 22.Klosterhalfen, B., K. M. Peters, C. Töns, S. Hauptmann, C. L. Klein., and C. J. Kirkpatrick. 1996. Local and systemic inflammatory mediator release in patients with acute and chronic posttraumatic osteomyelitis. J. Trauma 40:372-378. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi, E., and H. Yamauchi. 1997. Interleukin-6 and a delay of neutrophil apoptosis after major surgery. Arch. Surg. 132:209-210. [DOI] [PubMed] [Google Scholar]
- 24.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Blethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339-342. [DOI] [PubMed] [Google Scholar]
- 25.Lee, A., M. K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 26.Lew, D. P., and F. A. Waldvogel. 1997. Osteomyelitis. N. Engl. J. Med. 336:999-1007. [DOI] [PubMed] [Google Scholar]
- 27.Liles, W. C., and S. J. Klebanoff. 1995. Regulation of apoptosis in neutrophils. Fast track to death? J. Immunol. 155:3289-3291. [PubMed] [Google Scholar]
- 28.Matsuda, T., H. Saito, K. Fukatsu, I. Han, T. Inoue, S. Furukawa, S. Ikeda, and A. Hidemura. 2001. Cytokine-modulated inhibition of neutrophil apoptosis at local site augments exudative neutrophil functions and reflects inflammatory response after surgery. Surgery 129:76-85. [DOI] [PubMed] [Google Scholar]
- 29.Matute-Bello, G., W. C. Liles, F. Radella II, K. P. Steinberg, J. T. Ruzinski, M. Jonas, E. Y. Chi, L. D. Hudson, and T. R. Martin. 1997. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 156:1969-1977. [DOI] [PubMed] [Google Scholar]
- 30.Murray, J., J. A. Barbara, S. A. Dunkley,. A. F. Lopez, X. Van Ostade, A. M. Condliffe, I. Dransfield, C. Haslett, and E. R. Chilvers. 1997. Regulation of neutrophil apoptosis by tumor necrosis factor-α: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90:2772-2783. [PubMed] [Google Scholar]
- 31.Nicoletti, I., G. Migliorati, M. C. Oagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 32.Ohta, H., Y. Yatomi, E. A. Sweeny, S. Hakomori, and Y. Igarashi. 1994. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-α in human neutrophils. FEBS Lett. 355:267-270. [DOI] [PubMed] [Google Scholar]
- 33.Ottonello, L., G. Frumento, N. Arduino, M. Bertolotto, P. Dapino, M. Mancini, and F. Dalegri. 2002. Differential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. Role of oxidants, Bax and caspase-3. J. Leukoc. Biol. 72:125-132. [PubMed] [Google Scholar]
- 34.Roesgen, M., G. Hierholzer, and P. M. Hax. 1989. Post-traumatic osteomyelitis. Pathophysiology and management. Arch. Orthop. Trauma Surg. 108:1-9. [DOI] [PubMed] [Google Scholar]
- 35.Savill, J., and C. Haslett. 1995. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin. Cell Biol. 6:385-393. [DOI] [PubMed] [Google Scholar]
- 36.Savill, J. 1997. Apoptosis in resolution of inflammation. J. Leukoc. Bio. 61:375-380. [DOI] [PubMed] [Google Scholar]
- 37.Takeda, Y., H. Watanabe, S. Yonehara, T. Yamashita, S. Saito, and F. Sendo. 1993. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-α. Int. Immunol. 5:691-694. [DOI] [PubMed] [Google Scholar]
- 38.Weinmann, P., P. Gaehtgens, and B. Walzog. 1999. Bcl-X and Bax-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood 93:3106-3115. [PubMed] [Google Scholar]
- 39.Weinmann, P., K. Scharffetter-Kochanek, S. Bardley-Forlow, T. Peters, and B. Walzog. 2003. A role for apoptosis in the control of neutrophil homeostasis in the circulation: insights from CD18-deficient mice. Blood 101:739-746. [DOI] [PubMed] [Google Scholar]
- 40.Xing, Z., J. Gauldie, G. Cox, H. Baumann, M. Jordana, X.-F. Lei, and M. K. Achong. 1998. 1998. IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon, K. S., R. H. Fitzgerald, Jr., S. Sud, Z. Song, and P. H. Wooley. 1999. Experimental acute hematogenous osteomyelitis in mice. II. Influence of Staphylococcus aureus infection on T-cell immunity. J. Orthop. Res. 17:382-391. [DOI] [PubMed] [Google Scholar]