Abstract
Dendritic cells, particularly those residing in the spleen, are thought to orchestrate acquired immunity to malaria, but it is not known how the splenic dendritic cell population responds to malaria infection and how this response compares with the responses of other antigen-presenting cells. We investigated this question for Plasmodium chabaudi AS infection in C57BL/6 mice. We found that dendritic cells, defined here by the CD11c marker, migrated from the marginal zone of the spleen into the CD4+ T-cell area within 5 days after parasites entered the bloodstream. This contrasted with the results observed for the macrophage and B-cell populations, which expanded greatly but did not show any comparable migration. Over the same time period dendritic cells showed upregulation of CD40, CD54, and CD86 costimulatory molecules that are required for successful T-cell activation. In dendritic cells, the peak intracellular gamma interferon expression (as shown by fluorescence-activated cell sorting) was on day 5, 2 days earlier than the peak expression in B-cells or macrophages. These findings show that splenic dendritic cells are actively engaged in the earliest phase of malarial infection in vivo and are likely to be critical in shaping the subsequent immune response.
Efforts to develop a vaccine against malaria, which kills over a million African children each year, would be greatly assisted by a more complete understanding of the cellular events that occur shortly after the parasites enter the bloodstream, which determine the quality and quantity of the immune response to parasite antigens. Dendritic cells (DCs) are thought to play a crucial role, both as highly efficient presenters of antigen to helper T cells and in determining the balance of cell-mediated immunity and antibody-mediated immunity by steering the T-cell population towards a Th1 or Th2 response. The DC population within the spleen is of particular interest, as the spleen is responsible for filtering the blood to remove parasitized erythrocytes and is the central factory of the immune response against the parasite.
Enlargement of the spleen is a hallmark of malaria in humans. In murine models it is one of the phenotypic characteristics associated with host resistance to malaria (25, 29), and resolution of infection in an immunologically naïve animal requires an architecturally intact spleen (33). The complex architecture of the spleen facilitates close contact between blood-borne antigens and immune cells, which are segregated according to their functions into distinct splenic compartments (32).
The first contact between circulating antigens and immune cells occurs in the marginal sinus zone, which is rich in macrophages and DCs that have the capacity to ingest foreign material and present it to helper T cells, thereby initiating an acquired immune response (32). The majority of T cells reside in a different compartment, the periarteriolar lymphoid sheath. It is thought that after exposure to circulating antigens, DCs migrate into the periarteriolar lymphoid sheath, where they present the antigens that they have ingested in the marginal zone to T cells. There is growing experimental evidence which supports this model in the context of inert microbial antigens (5, 27), live bacteria (15, 34), and infection with leishmania and trypanosomiasis parasites (19, 20).
There is some evidence that DCs play an important role in antigen presentation during malaria infection. In vitro experiments have shown that the mouse parasite Plasmodium chabaudi can activate DCs directly to produce Th1 cytokines and upregulate costimulatory molecule expression (26), while in Plasmodium yoelii infections DCs are capable of initiating a protective immune response against the liver stage by activating sporozooite-specific CD8+ and CD4+ T cells (6). A study in which antigen-presenting cell (APC) function during blood-stage P. yoelii infection was examined showed that CD11b+ cells could present malarial antigen, upregulate surface expression of costimulatory molecules, and support Th1 cytokine production by CD4+ T cells (16). The role of these cells is also underscored by the fact that it has been shown that adhesive phenotypes of Plasmodium falciparum have evolved ways to subvert maturation and antigen presentation by human DCs (31).
In this study we examined the response of the splenic DC population to infectious challenge with live blood-stage malaria parasites. We compared the nature and timing of this response with the nature and timing of the response of macrophages and B cells in order to explore the notion that DCs are the key players in early antigen presentation. Immunohistology was used to examine how each population was distributed within the spleen, and fluorescence-activated cell sorting (FACS) analysis was used to quantify population sizes, intracellular cytokine expression, and surface expression of costimulatory molecules for antigen presentation.
MATERIALS AND METHODS
Murine malaria model.
Female C57BL/6 mice were infected when they were 6 to 8 weeks old with P. chabaudi AS by using freshly passaged parasites from a stock provided by David Walliker (Edinburgh University). A total of 105 parasitized red blood cells in 200 μl of phosphate-buffered saline (PBS) was injected intraperitoneally, and the subsequent course of parasitemia was determined by examining thin blood films from tail tip snips and counting a minimum of 200 red cells stained with Diff-Quick (Triangle Bio-medical Sciences Ltd., Skelmersdale, United Kingdom).
Spleen cell preparations.
For flow cytometry, spleens were collected in ice-cold RPMI 1640 (Sigma, Poole, United Kingdom) supplemented with 10 μg of brefeldin A (Sigma) per ml and then incubated at 37°C for 30 min with RPMI 1640 containing 10 μg of brefeldin A per ml, 299 μg of Liberase CI purified enzyme blend (Roche, Lewes, United Kingdom) per ml, and 200 μg of DNase I (Roche) per ml before they were gently passed through a 70-μm-pore-size sterile cell strainer (Falcon, BD Biosciences, Franklin Lakes, N.J.) by using ice-cold Dulbecco's PBS without calcium and magnesium but with 0.05 mM EDTA and 10 μg of brefeldin A per ml. Cell suspensions were centrifuged at 200 × g for 7 min at 4°C and resuspended in ice-cold PBS-EDTA-brefeldin A. The viable cell count was determined by staining with trypan blue. Aliquots containing 40 × 106 cells were resuspended in RPMI 1640 containing 10% fetal calf serum, penicillin (10 IU/ml), streptomycin (100 μg/ml), glutamine (2 mM), and 10 μg of brefeldin A per ml and incubated for 4 h at 37°C in the presence of 5% CO2 in Teflon petri dishes (Techmate Ltd., Milton Keynes, United Kingdom) prior to flow cytometry.
For immunohistology, spleens were snap frozen on dry ice and stored at −70°C until they were processed.
Flow cytometry.
In each flow cytometry experiment, three spleens were pooled to obtain data for each time point. All antibodies were supplied by Pharmingen (Oxford, United Kingdom) unless indicated otherwise.
For analysis of the surface phenotype, fresh cells were blocked with anti-mouse CD16/32 FcγIII/II receptor and then stained with fluorescein isothiocyanate-conjugated murine monoclonal antibodies against CD45R/B220, peridinin chlorophyll-α protein (PerCP)-conjugated murine monoclonal antibodies against CD11c, and fluorescein isothiocyanate-conjugated rat anti-mouse F4/80 (Serotec, Oxford, United Kingdom). Phycoerythrin-conjugated monoclonal antibodies against CD54, CD80, and CD86 were used to stain for the costimulatory molecules in the same way that cells were stained for phenotype analysis. Cells were incubated with antibodies in the dark at 4°C for 30 min, washed twice, fixed for 30 min in 1% formalin-PBS, washed twice, resuspended in FACS buffer, and analyzed within 24 h.
The levels of intracellular expression of the cytokines and surface expression of costimulatory molecules are presented below as the ratio of the level of expression in a malaria-infected mouse to the level of expression in a normal mouse for malaria-infected and normal mouse experiments that were performed concurrently on each individual day. Mouse intracellular cytokine-positive control cells were used to ensure that intracellular staining for gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) was working (MiCK-1 catalog number 554652; lot number M060492; Pharmingen, BD Biosciences, Oxford, United Kingdom).
For analysis of cytokine expression, cells were cultured for 4 h in the presence of 10 μg of brefeldin A per ml, stained to determine the surface phenotype, and then fixed and permeabilized by using Cytofix/Cytoperm (Pharmingen) before they were stained with APC-conjugated monoclonal rat anti-mouse interleukin-10 (IL-10), anti-mouse TNF, and anti-mouse IFN-γ. The cells were stained at 4°C in the dark for 30 min, washed twice, resuspended in FACS buffer, and analyzed within 24 h.
Flow cytometry was performed with a FACSCalibur machine (Becton Dickinson, Oxford, United Kingdom). At least 300,000 events were collected for DC phenotyping; at least 100,000 events were collected for intracellular cytokine analysis; and at least 50,000 events were collected for all other observations. Data were analyzed by using CellQuest v3.3 (Becton Dickenson).
Immunohistology.
Cryostat sections (thickness, 8 μm) of frozen spleens were mounted on glass microscope slides, fixed in acetone, and stored at −20°C. Sections were blocked with endogenous peroxidase block reagent (Dako, Ely, United Kingdom) prior to staining with primary antibody, washed, incubated with secondary antibody, washed, flooded with diaminobenzidine (Sigma), and counterstained with hematoxylin (Sigma). Antibodies were supplied by Pharmingen unless indicated otherwise. The primary antibodies were hamster anti-mouse CD11c, rat anti-mouse F4/80, rat anti-mouse CD4, and rat anti-mouse CD45R/B220. The secondary antibodies were horseradish peroxidase-conjugated mouse anti-hamster immunoglobulin G (IgG) (Santa Cruz, Calne, United Kingdom) and horseradish peroxidase-conjugated mouse anti-rat IgG. Purified rat IgG2a, rat IgG2b, and hamster IgG1 were used as isotype control antibodies in all immunohistochemistry experiments.
RESULTS
Size of the APC population in the spleen.
We performed three infectious challenge experiments, and in each experiment three malaria-infected mice per time point sampled and three uninfected age-matched controls were used. Figure 1A shows the time course of infection, which was similar to that previously described for this experimental model (29). Typically, parasites were first observed on thin blood smears on day 4 of infection, the average peak density (28%; standard deviation, 1.7%) was reached on day 8, and then the size of the population declined rapidly. Parasites disappeared from the blood by approximately day 14.
FIG. 1.
Changes in spleen cell numbers and parasitemia during P. chabaudi AS infection in C57BL/6 mice. Each time point represents at least three independent experiments performed with at least three mice per experiment, and the error bars indicate standard deviations. (A) Percentage of parasitemia. The dashed line indicates the time period during which spleens were collected. (B) Total number of nucleated spleen cells. The asterisks indicates that the P value is <0.005, as determined by a two-tailed t test. (C) Total number of F4/80-positive cells (macrophages) and CD45R/B220-positive cells (B cells). An asterisk indicates that the P value is < 0.005, as determined by a two-tailed t test. (D) Total number of CD11c+ cells in the spleen. There was a significant increase in the number of CD11c+ cells by day 7 (P < 0.001), and the number began to decrease by day 9.
Figure 1B shows how the total number of nucleated spleen cells changed in response to infection. Three spleens were pooled before the cells were counted, and the value for each time point represents the average of three experiments. The total spleen cell numbers more than doubled during the course of infection, from an average of 453 × 106 cells (standard deviation, 4× 106 cells) before infection to 1,046 × 106 cells (standard deviation, 17× 106 cells) at day 9 (P < 0.005, as determined by a two-tailed t test).
Flow cytometry was used to determine how the number of APCs changed in response to infection in three experiments in which three mice per time point were examined. As shown in Fig. 1C, the number of macrophages (defined by the F4/80 marker) increased fourfold and the number of B cells (defined by CD45R/B220) increased threefold during the first 9 days of infection. Both of these increases were highly statistically significant (P < 0.005, as determined by a by two-tailed t test).
Unlike the numbers of B cells and macrophages, which increased continuously during our experiments, the total number of CD11c+ cells increased to a level that was significantly greater than the normal level on day 7 postinfection (2.1 × 106 cells [standard deviation, 0.23 × 106 cells]; P < 0.001, as determined by a t test) and then decreased somewhat by day 9 (Fig. 1D).
Migration of DCs within the spleen.
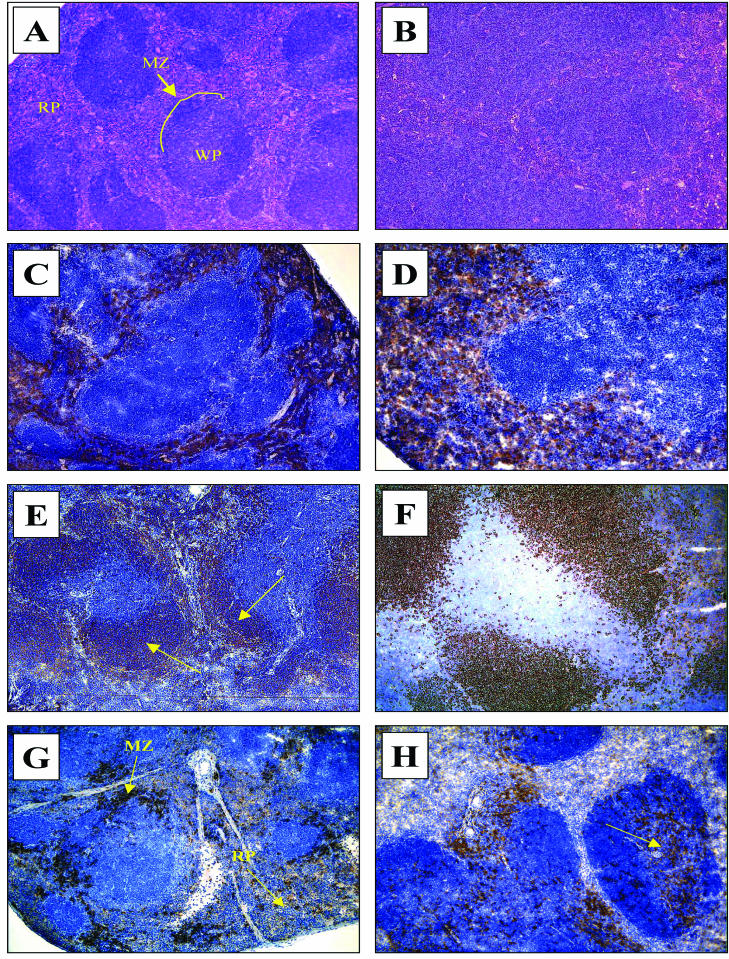
Histological features are shown in Fig. 2, which shows the massive splenic hyperplasia following malaria infection. In broad terms, the histological structure of the spleen is divided into red pulp and white pulp. This zonal distinction is completely lost by day 9 of an infection when tissue is examined by routine methods (hematoxylin and eosin) (compare Fig. 2A and B). Immunohistochemical staining for specific cell types, however, resulted in a different picture. There was a clear retention of structure along cell phenotype lines. In normal spleen tissue F4/80+ cells were very clearly restricted to the red pulp (Fig. 2C). During infection the red pulp underwent rapid and remarkable hyperplasia, and this may in part have been due to expansion of the F4/80+ population. Interestingly, despite the loss of the red pulp-white pulp distinction when preparations were examined by routine histological methods by day 9 of the infection, the F4/80+ cells very clearly continued to delineate this zone, and this phenotype was not observed in the T-cell area at any time (Fig. 2D). CD45R/B220+ cells had a wider distribution (Fig. 2E), but despite the fact that there was up to a sixfold increase in the number of these cells, the number of cells did not increase in the T-cell area of the white pulp (Fig. 2F). Similar changes were observed for CD4+ T cells (data not shown).
FIG. 2.
Hematoxylin and eosin staining (A and B) and immunohistochemical histology (C to H) on days 0 and 9 of the spleens of C57BL/6 mice with a P. chabaudi AS infection. Magnification for all panels, ×100. (A) Normal spleen stained with hematoxylin and eosin. Note the normal architecture and clear distinction between the red pulp (RP) and the white pulp (WP). MZ, marginal zone (line). (B) Spleen tissue stained withhematoxylin and eosin on day 9 of an infection. Note the almost complete disruption of the normal architecture, especially the architecture of the normal red pulp-white pulp distinction. (C) Normal distribution of F4/80+ cells in an uninfected mouse spleen confined to the red pulp. (D) Distribution of F4/80+ cells on day 9 of a malaria infection. Note that despite hyperplasia, the phenotype maintains a strict red pulp distribution even late in the infection. (E) Normal distribution of CD45R/B220+ cells in an uninfected mouse spleen (the arrows indicate brown-stained cells). (F) Distribution of CD45R/B220+ cells on day 9 of a malaria infection. Note that despite the hyperplasia, the white pulp distribution is not appreciably different. (G) Normal distribution of CD11c+ cells (the arrows indicate brown-stained cells) in the red pulp and marginal zone in the spleen of an uninfected mouse. (H) Distribution of CD11c+ cells on day 5 of a malaria infection. Note the redistribution of positively stained cells in the white pulp area (the arrow indicates brown-stained cells).
In contrast, CD11c+ cells showed a striking pattern of migration within the spleen. At the start of infection they were largely confined to the red pulp and marginal zone (Fig. 2G), but by day 5 a substantial number had moved to the CD4+ area of the white pulp, an area in which they were previously absent (Fig. 2H).
IFN-γ expression by APCs.
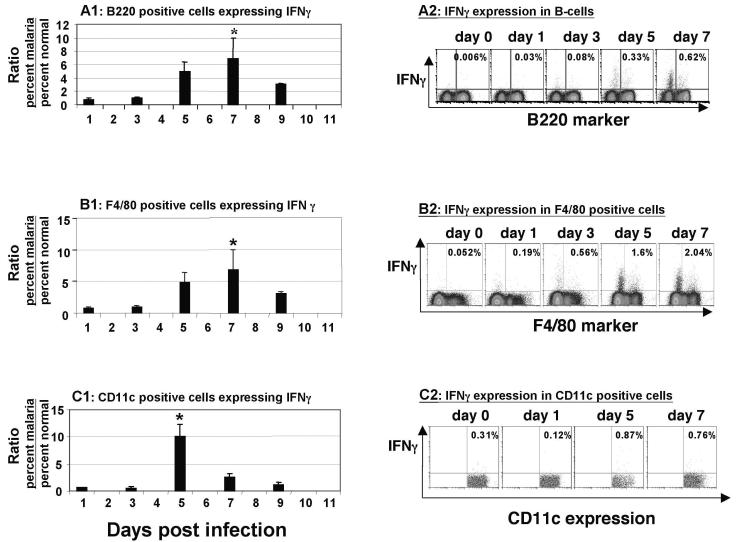
The APC populations delineated by FACS analysis shown in Fig. 1C and D were analyzed to determine cytokine expression. The pattern observed for CD11c+ cells was different from the pattern observed for B cells (CD45R/B220+) and macrophages (F4/80+).
Figure 3A shows that the percentage of DCs expressing IFN-γ rose within 3 days and reached a peak at day 5 before declining. The difference between the peak value at day 5 and the starting value was highly significant (P < 0.005, as determined by a two-tailed t test). While there was a significant IFN-γ peak at this time, the levels of TNF and IL-10 remained static in all cell phenotypes tested. The latter result provided a useful isotype control for the IFN-γ findings, as the IFN-γ and TNF antibodies were the same isotype and both performed as expected in positive control experiments, as described in Materials and Methods.
FIG. 3.
Intracellular expression of IFN-γ. (A1) Ratio (malaria/normal) of the percentages of B cells (CD45/B220+) expressing intracellular IFN-γ during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. The peak expression occurred on day 7 postinfection, and this expression was significantly greater than the baseline expression (P < 0.005). (A2) FACS dot plot representative of a typical series of experiments, showing intracellular expression of IFN-γ in B cells (CD45/B220+). Cells positive for both the phenotype marker and intracellular cytokine are in the top right quadrant. The percentages of cells that were positive for both the phenotype marker and intracellular cytokine are indicated in the quadrants. (B1) Ratio (malaria/normal) of the percentages of macrophages (F4/80+) expressing intracellular IFN-γ during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. The peak expression occurred on day 7 postinfection, and this expression was significantly greater than the baseline expression (P = 0.05). (B2) FACS dot plot representative of a typical series of experiments, showing intracellular expression of IFN-γ in macrophages (F4/80+). Cells positive for both the phenotype marker and intracellular cytokine are in the top right quadrant. The percentages of cells that were positive for both the phenotype marker and intracellular cytokine are indicated in the quadrants. (C1) Ratio (malaria/normal) of the percentages of DCs (CD11c+) expressing intracellular IFN-γ during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. The peak expression occurred on day 5 postinfection, and this expression was significantly greater than the baseline expression (P = 0.05). (C2) FACS dot plot representative of a typical series of experiments, showing intracellular expression of IFN-γ in DCs (CD11c+). Cells positive for both the phenotype marker and intracellular cytokine are in the top right quadrant. The percentages of cells that were positive for both the phenotype marker and intracellular cytokine are indicated in the quadrants.
As shown in Fig. 3B and C, the percentage of macrophages and B cells expressing IFN-γ also increased markedly, but the increase occurred more slowly than the increase for DCs; the value reached a peak at day 7, at which point expression was also significantly greater than the baseline expression (P = 0.05, as determined by a two-tailed t test).
At no time during the period evaluated was there any significant expression of IL-10 in any of the cell phenotypes examined (data not shown). In the CD11c+ cell population there was an insignificant increase in the level of intracellular TNF expression, which peaked on the last day examined (day 7) (data not shown).
Expression of costimulatory molecules by CD11c+ cells.
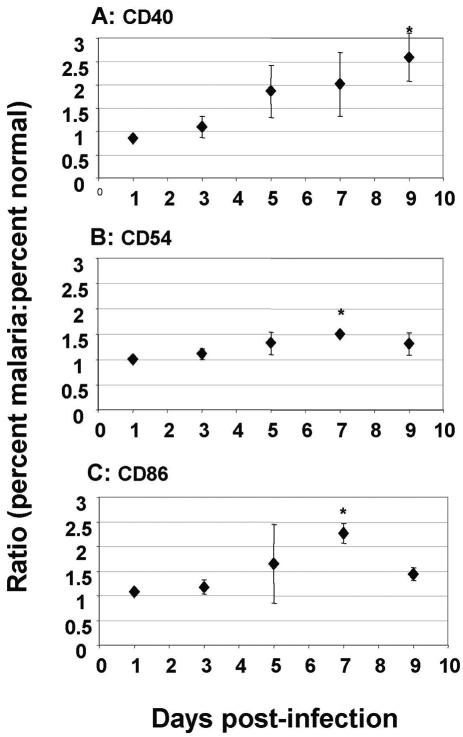
We used FACS to analyze the CD11c+ population for expression of costimulatory molecules that are important in antigen presentation by DCs (Fig. 4). The percentage of cells expressing CD40 increased significantly by day 3 (Fig. 4A) (P = 0.03 for a t test comparison with preinfection). CD86 expression increased significantly by day 7 (Fig. 4C) (P = 0.01), and CD54 expression also increased significantly by day 7 (Fig. 4B) (P = 0.008).
FIG. 4.
Expression of costimulatory molecules. (A) Ratio (malaria/normal) of the percentages of CD11c+ cells expressing CD40 during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. By day 9 the expression was significantly greater than the baseline expression (P = 0.03). (B) Ratio (malaria/normal) of the percentages of CD11c+ cells expressing CD54 during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. By day 9 the expression was significantly greater than the baseline expression (P = 0.008). (C) Ratio (malaria/normal) of the percentages of CD11c+ cells expressing CD86 during the first 9 days of a P. chabaudi AS infection in C57BL/6 mice. By day 7 the expression was significantly greater than the baseline expression (P = 0.03).
DISCUSSION
In this study we were interested in early events that occur in the spleen following an infectious challenge, as these events may be critical in shaping the subsequent immune response. Previous studies in which DC biology in malaria infection was evaluated were in vitro studies and focused on the effect of blood-stage parasites on DC function in the liver (21) or on the role of DCs in T-cell activation against liver stage infection in rodent models (6, 7, 14, 26). In other in vitro studies the workers analyzed the effects of laboratory P. falciparum isolates on human monocyte-derived DCs (31). This study is the first in vivo investigation of DC function in the spleen in a mouse model in response to P. chabaudi blood-stage infection. We found that within 5 days of the onset of an experimental blood-stage malaria infection, a substantial number of DCs appeared in the area of the white pulp that was rich in CD4+ T cells. As the total size of the splenic DC population remained constant over this period, it is highly probable that these cells migrated from the marginal sinus zone or red pulp, where the majority of DCs normally reside and can readily survey blood-borne antigens.
The migration of CD11c+ cells into the T-cell areas of the white pulp contrasts with the migration of the other two APC types, B cells and macrophages, which, although they undergo obvious hyperplasia, do not redistribute over the early time course of infection. F4/80+ cells remain strikingly confined to the red pulp area, and CD45R/B220+ cells show no appreciable change in distribution. The relatively stable localization of the B-cell and macrophage populations seems to rule out the possibility that the change in DC distribution is an artifact of the loss of splenic architecture that occurs as malaria infection progresses.
Using intracellular staining and flow cytometry, we showed that there was a significant peak of intracellular expression of the prototypical Th1 cytokine IFN-γ in DCs on day 5 following blood-stage malaria infection. The absolute percentage of CD11c+ cells positive for IFN-γ in normal mice is around 0.2%, and by day 5 the value was around 0.8%. Although these percentages represent small numbers of cells, it is important to remember that the microanatomical location in the CD4+ T-cell area of these professional APCs may be more important than the absolute number of cells positive for this cytokine. B cells and macrophages showed a peak in expression of this cytokine only 2 days later. This finding is interesting because the cytokine milieu generated by the APCs at the time of antigen presentation significantly influences the T-helper bias of the subsequent T-cell response, as presentation of an antigen within the context of a Th1 cytokine favors a Th1 CD4+ response (10, 12). The expression of IFN-γ by DCs (which has previously been observed in other contexts [11, 22]) may be particularly important in malaria for this reason. Experimental murine malaria models have shown that early production of IFN-γ is a marker for resistance to infection (8), and in vitro studies have shown that DCs produce TNF, another Th1 cytokine, very soon after exposure to malaria antigen in vitro. Workers have obtained similar findings related to Th1 cytokine production by DCs in response to toxoplasma (1) and leishmania (4). It will be important in future work to demonstrate this DC expression of IFN-γ by other methods (such as reverse transcription-PCR and in situ hybridization) to corroborate this finding.
We found significant upregulation of the costimulation molecules (CD40, CD54, and CD86) on the surface of CD11c+ cells. The increased expression is crucial to activation of T cells and is characteristic of the maturation of DCs (3, 18, 28). These molecules have been shown to play an important role in murine malaria models. Blocking the CD80/CD86 signaling pathway disrupts the Th1/Th2 balance in a P. chabaudi AS malaria model (30). Key roles for CD40 and its ligand and for CD54 have also been demonstrated in experiments with the Plasmodium berghei ANKA murine cerebral malaria model, which is rescued from death by antibody inhibition or gene deletion of these molecules (9, 23). Our results provide in vivo evidence that there is CD11c+ cell maturation, as demonstrated by increased expression of these costimulatory molecules. These indications of cell maturation strengthen the assertion that the CD11c+ cells may play a primary role as APCs early in malaria infection. Other workers have previously demonstrated that there is malaria parasite-induced suppression of DC function. Ocana-Morgner et al. have recently shown that blood-stage infection compromises DC-mediated antisporozoite immunity in the liver in a rodent model (21). However, the results of these workers cannot be compared directly to the results of the present study, as the host effector responses against Plasmodium spp. appear to be distinct for liver and blood-stage parasites. Urban et al. demonstrated that the adhesive phenotypes of blood-stage P. falciparum were able to inhibit T-cell activation by DCs in vitro, which was associated with downregulation of costimulatory molecules on DCs (31). Again, these findings are not directly comparable to the results of our study, in which we looked at early events that occur during P. chabaudi infection in vivo.
An issue arising from this study is the phenotypic overlap between DCs and macrophages. CD11c is conventionally used as a marker of murine DCs (17, 24), and F4/80 is conventionally used as a marker of murine macrophages (2, 13). Although our data confirmed that these markers did effectively separate cell populations with different functional properties, it is interesting to consider the cells in which the two markers were coexpressed. This was most evident in the uninfected spleen, where both markers were localized to the red pulp and marginal zone. At this stage we estimated that of approximately 250 × 106 F4/80+ cells and 0.5 × 106 CD11c+ cells, approximately 0.25 × 106 cells expressed both markers. At 5 days after infection, the two populations were more sharply delineated in two respects: (i) a large portion of the CD11c+ cells had migrated into the white pulp, whereas the F4/80+ population remained in the red pulp; and (ii) and a much smaller proportion of CD11c+ cells (approximately 0.12 × 106 cells; data not shown) expressed F4/80. One possible interpretation of these data is that the process of DC migration is associated with a loss of the F4/80+ phenotype.
We concluded that CD11c+ cells move into the T-cell area of the splenic white pulp by day 5 following malaria infection and that they move there earlier than other major APCs in the spleen. CD11c+ cells also express IFN-γ by day 5, 2 days earlier than either of the other types of APCs, and show upregulation of costimulation molecules. In light of these findings, it seems likely that splenic DCs are actively engaged in the earliest phase of malarial infection in vivo and may be critical in shaping the subsequent immune response.
Acknowledgments
The Wellcome Trust, Sainsbury's Scholarship program, and the MRC provided support for this work.
We thank Gareth Turner and Caetona Reis Sousa for their valuable assistance and advice during this study.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 2.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann, T., S. Zimmermann, H. Himmelrich, A. Gumy, O. Egeter, A. K. Sakrauski, I. Seegmuller, H. Voigt, P. Launois, A. D. Levine, H. Wagner, K. Heeg, J. A. Louis, and M. Rocken. 2001. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2:1054-1060. [DOI] [PubMed] [Google Scholar]
- 5.Bourguin, I., M. Moser, D. Buzoni-Gatel, F. Tielemans, D. Bout, J. Urbain, and O. Leo. 1998. Murine dendritic cells pulsed in vitro with Toxoplasma gondii antigens induce protective immunity in vivo. Infect. Immun. 66:4867-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruna-Romero, O., and A. Rodriguez. 2001. Dendritic cells can initiate protective immune responses against malaria. Infect. Immun. 69:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruna-Romero, O., J. Schmieg, M. Del Val, M. Buschle, and M. Tsuji. 2003. The dendritic cell-specific chemokine, dendritic cell-derived CC chemokine 1, enhances protective cell-mediated immunity to murine malaria. J. Immunol. 170:3195-3203. [DOI] [PubMed] [Google Scholar]
- 8.De Souza, J. B., K. H. Williamson, T. Otani, and J. H. Playfair. 1997. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect. Immun. 65:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favre, N., C. Da Laperousaz, B. Ryffel, N. A. Weiss, B. A. Imhof, W. Rudin, R. Lucas, and P. F. Piguet. 1999. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes Infect. 1:961-968. [DOI] [PubMed] [Google Scholar]
- 10.Fearon, D. T., and R. M. Locksley. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50-53. [DOI] [PubMed] [Google Scholar]
- 11.Fukao, T., S. Matsuda, and S. Koyasu. 2000. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J. Immunol. 164:64-71. [DOI] [PubMed] [Google Scholar]
- 12.Goff, W. L., W. C. Johnson, S. M. Parish, G. M. Barrington, W. Tuo, and R. A. Valdez. 2001. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-gamma and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 23:463-471. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch, S., J. M. Austyn, and S. Gordon. 1981. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J. Exp. Med. 154:713-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby, A. C., U. Yrlid, M. Svensson, and M. J. Wick. 2001. Differential involvement of dendritic cell subsets during acute Salmonella infection. J. Immunol. 166:6802-6811. [DOI] [PubMed] [Google Scholar]
- 16.Luyendyk, J., O. R. Olivas, L. A. Ginger, and A. C. Avery. 2002. Antigen-presenting cell function during Plasmodium yoelii infection. Infect. Immun. 70:2941-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maraskovsky, E., K. Brasel, M. Teepe, E. R. Roux, S. D. Lyman, K. Shortman, and H. J. McKenna. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellman, I., and R. M. Steinmman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 19.Moll, H. 1997. The role of chemokines and accessory cells in the immunoregulation of cutaneous leishmaniasis. Behring Inst. Mitt. 99:73-78. [PubMed] [Google Scholar]
- 20.Nargis, M., M. M. Chisty, Y. Ihama, H. Sato, T. Inaba, and H. Kamiya. 2001. Kinetics of Trypanosoma cruzi infection in guinea-pigs, with special reference to the involvement of epidermal Langerhans' cells in the induction of immunity. Parasitology 123:373-380. [DOI] [PubMed] [Google Scholar]
- 21.Ocana-Morgner, C., M. M. Mota, and A. Rodriguez. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piguet, P. F., C. D. Kan, C. Vesin, A. Rochat, Y. Donati, and C. Barazzone. 2001. Role of CD40-CVD40L in mouse severe malaria. Am. J. Pathol. 159:733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran, B., J. Lingappa, M. K. Kennedy, J. Smith, M. Teepe, A. Rudensky, C. R. Maliszewski, and E. Maraskovsky. 1997. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 159:2222-2231. [PubMed] [Google Scholar]
- 25.Rae, D., and M. M. Stevenson. 1988. Changes in size and cell populations in lymphoid organs during Plasmodium chabaudi infection in resistant and susceptible mice. Adv. Exp. Med. Biol. 239:113-120. [DOI] [PubMed] [Google Scholar]
- 26.Seixas, E., C. Cross, S. Quin, and J. Langhorne. 2001. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur. J. Immunol. 31:2970-2978. [DOI] [PubMed] [Google Scholar]
- 27.Sousa, C. R., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R. N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cell development and maturation. Adv. Exp. Med. Biol. 417:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson, M. M., J. J. Lyanga, and E. Skamene. 1982. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect. Immun. 38:80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor-Robinson, A. W., and E. C. Smith. 1999. Modulation of experimental blood stage malaria through blockade of the B7/CD28 T-cell costimulatory pathway. Immunology 96:498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 32.Yadava, A., S. Kumar, J. A. Dvorak, G. Milon, and L. H. Miller. 1996. Trafficking of Plasmodium chabaudi adami-infected erythrocytes within the mouse spleen. Proc. Natl. Acad. Sci. USA 93:4595-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap, G. S., and M. M. Stevenson. 1994. Differential requirements for an intact spleen in induction and expression of B-cell-dependent immunity to Plasmodium chabaudi AS. Infect. Immun. 62:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yrlid, U., M. Svensson, A. Hakansson, B. J. Chambers, H. G. Ljunggren, and M. J. Wick. 2001. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 69:5726-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]