Abstract
Background
Mitral regurgitation (MR) is a frequent finding in patients with aortic stenosis (AS). The objective of this study is to assess the change in MR severity following transcatheter aortic valve implantation (TAVI).
Methods
MR changes were assessed by comparing transthoracic echocardiography before and after the procedure.
Results
The prosthetic aortic valve was successfully implanted in 65 patients. The number of patients with pre-procedure MR was reduced from 58 (85.3%) to 43 (63.2%) (p < 0.001). Vena contracta width was decreased from 0.47 ± 0.28 to 0.25 ± 0.21, (p = 0.043). About 59.4% (19/32) of those who had moderate to severe MR and 85.7% (12/14) of those with severe MR experienced a significant improvement in MR after the procedure (p < 0.001). Improvement in MR was independent of prosthetic valve type with 54.2% in CoreValve and 43.9% in Edwards SAPIEN, p = 0.424; valve sizes were 25.8 ± 1.9 in those who improved vs. 25.0 ± 1.9 mm in those who did not improve, p = 0.105; femoral approach was 51.2% and apical approach was 41.7%, p = 0.457; MR etiology was 48.1% in organic and 48.6% in functional, p = 0.968; and operative risk was 50.0% in EuroScore >20 and 48.6% in EuroScore <20, p = 0.356.
Conclusions
TAVI is associated with a significant improvement in MR, especially in severe types. The lack of influence of MR improvement by the etiology of MR, the type of valve implanted, and the operative risk need to be confirmed in a larger multi-center study.
Keywords: TAVI, Mitral regurgitation, Aortic stenosis
Abbreviations
- TAVI
transcatheter aortic valve implantation
- AS
aortic stenosis
- MR
mitral regurgitation
- AVR
aortic valve replacement
- LVEF
left ventricular ejection fraction
Background
Transcatheter aortic valve implantation (TAVI) has been recognized as an alternative therapeutic option for patients with severe symptomatic aortic stenosis (AS), and high surgical risk [1,2]. Thirty to forty percent of patients with symptomatic AS are inoperable, and almost half of them fulfill the criteria for TAVI [3,4]. Compared to standard surgical aortic valve repair, TAVI procedure is shown to have comparable survival among patients with severe AS who are considered suitable candidates for surgery [5], and to significantly improve survival among patients with severe AS who are not considered suitable candidates for surgery [6]. After 10 years of experience, TAVI is now considered a standard therapeutic option for AS patients with prohibitive risk for standard surgical management [4,7].
Mitral regurgitation (MR) is a frequent finding in patients with severe AS [8]. MR is shown to increase mortality risk after surgical aortic valve replacement (AVR) [9]. However, MR may improve significantly following isolated surgical AVR [10]. The change in MR severity after TAVI procedure was examined in several studies with generally positive findings [11–18]. Patients who experienced MR improvement in these studies ranged between 17% and 28% while those who experienced MR worsening ranged between 11% and 22% [11,12,14]. The exact predictors of such improvement are not well understood [11,13]. Moreover, whether MR improvement is associated with better survival after TAVI procedure is unclear. The objective of the current study is to assess the change in MR severity following TAVI as well as to identify patient and procedural characteristics associated with MR improvement.
Methods
Patient population
The study population consists of 65 consecutive patients who underwent successful TAVI using the CoreValve (Medtronic CoreValve, Minneapolis, Minnesota) or Edwards SAPIEN (Edwards Lifesciences, Inc, Irvine, CA) heart valves between April 2009 and November 2011. Patients were considered as TAVI candidates if they had severe symptomatic native aortic stenosis (AS) and were at high or prohibitive surgical risk. Severe AS was defined as aortic valve area <1 cm2 or <0.6 cm2/m2, a mean aortic-valve gradient of ⩾40 mmHg or a peak aortic-jet velocity of ⩾4.0 m/s, measured by echocardiography. High or prohibitive surgical risk was considered when the patient was >80 years with life expectancy of >12 months, patients with >70 years with Logistic EuroScore >20 [19], or concomitant comorbid conditions assessed and agreed to by both an independent cardiologist and a cardiovascular surgeon.
Procedures were carried out under conscious sedation or general anesthesia via transfemoral approach for CoreValve aortic valve and via transfemoral or transapical approaches for Edwards SAPIEN heart-valve. Transfemoral was the approach of choice where feasible. The selection of the type and size of the valve was based on the measurements of the aortic valve annulus obtained by CT angiography, and transthoracic echocardiography. All patients gave informed consent, and the study was approved by the Institutional Ethics Committee.
Echocardiography
Transthoracic echocardiography was performed before and after TAVI procedure. All echocardiograms were obtained with the patient in a stable hemodynamic condition. In the apical three- or five-chamber view, the peak and mean pressure gradients across the aortic valve were measured. The annulus diameter was measured at the level of insertion of the leaflets in the longitudinal view. Aortic valve area was calculated using the continuity equation approach [20]. Quantification of the left ventricular ejection fraction (LVEF) was performed using left ventricular end-diastolic and end-systolic dimensions [21]. The color-flow Doppler signal was used to assess the presence and the grade of aortic regurgitation.
MR assessment
There were two independent expert readers and, in case of disparity, a third reader to assess the degree of MR. MR was assessed by visual inspection, calculating vena contracta, and by using color-flow mapping of the regurgitant jet [22]. The vena contracta (contracted zone of the Doppler color flow image of the jet at its passage through an incompetent mitral valve) was measured from the long axis apical view. Mitral regurgitation was graded according to the European Society of Cardiology guidelines and the American Society of Echocardiography recommendations [23,24]. Therefore, MR was assessed by visual inspection and by using color-flow mapping of the regurgitant jet. The maximum jet area was measured by planimetry where it reached its greatest percentage of Left Atrial area, in the four-chamber apical view, with the use of Curad offline analysis package. MR jet area was then expressed as a percentage of the left atrium area (MR percentage), and its severity was graded as follows: none ⩽5%, mild 5–20%, moderate 20–40%, and severe ⩾40%. Organic MR etiology was defined as the presence of moderate to severe calcifications or degeneration of the mitral annulus and/or leaflets; while functional MR etiology was defined as the presence of LV dysfunction with the absence of morphological abnormalities of the mitral apparatus [9,25].
Survival
Survival was defined at 30 days and overall (any time) during the study. Survival was followed up through to November 2011.
Statistical analysis
Data were presented as frequencies and percentages for categorical variables and mean ± standard deviations for continuous variables. The McNemar–Bowker test was used to evaluate significant differences of categorical variables (MR grade) before and after TAVI procedure. Paired t-test or the Wilcoxon signed-rank test, as appropriate, were used to analyze significant differences of continuous variables before and after TAVI procedure. Chi-square or Fisher’s tests, as appropriate, were used to evaluate significant differences of categorical variables between those with and without MR improvement. Unpaired Student’s t-test or the Mann–Whitney test as appropriate, were used to determine significant differences of continuous variables between the two study groups. Mortality-free survival was examined using the Kaplan–Meier estimator. All p-values were two-tailed. P-value <0.05 was considered significant. SPSS software (release 17.0, SPSS Inc., Chicago, U.S.) was used for all statistical analyses.
Results
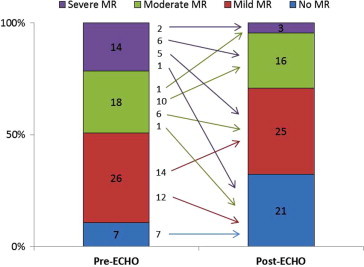
Sixty-five consecutive patients underwent successful TAVI procedure between April 2009 and November 2011. The number of patients with MR was reduced after TAVI from 58 (85.3%) to 43 (63.2%), p < 0.001. Twelve of 14 patients (85.7%), seven of 18 patients (38.9%), and 12 of 26 patients (46.2%) with severe, moderate and mild MR respectively had improved MR (p < 0.001). This represented 47.7% of those who had any degree of MR and 59.4% of those who had moderate to severe MR. Only one patient (1.5%) experienced worsening of the MR grade (from moderate to severe). See Table 1 and Fig. 1. Vena contracta width was decreased from 0.47 ± 0.28 to 0.25 ± 0.21, p = 0.043.
Table 1.
Change in mitral regurgitation (MR) following transcatheter aortic valve implantation (TAVI).
| Pre-procedure | Post-procedure | p-value | |
|---|---|---|---|
| MR degree | |||
| No MR | 7 (10.8%) | 21 (32.3%) | <0.001⁎ |
| Mild MR | 26 (40.0%) | 25 (38.5%) | |
| Moderate MR | 18 (27.7%) | 16 (24.6%) | |
| Severe MR | 14 (21.5%) | 3 (4.6%) | |
| MR change | |||
| Improved | 31 (47.7%) | ||
| Unchanged | 33 (50.8%) | ||
| Worsen | 1 (1.5%) | ||
McNemar–Bowker test.
Figure 1.
Change in mitral regurgitation (MR) following transcat.
Patients’ demographic and clinical characteristics by post-procedure MR improvement are summarized in Table 2. About 42% of both groups had organic MR, and the average EuroScore (used to assess surgical risk in the studied cohort) was 23.1 ± 16.4. MR improvement was not associated with MR etiology (48.1% in organic vs. 48.6% in functional, p = 0.968) nor operative risk (50.0% in EuroScore >20 vs. 48.6% in EuroScore <20, p = 0.356). Review of medical history revealed that patients with improved MR were less likely to have hypertension (61% vs. 84%, p = 0.039) than those without improved MR. There was a trend of lower prevalence of diabetes (58% vs. 78%, p = 0.087); and myocardial infarction (13% vs. 31%, p = 0.080), compared to those without MR improvement. No differences were noted between the two patient groups in their medical histories of chronic obstructive pulmonary disease, coronary artery disease, coronary artery bypass graft, stroke, carotid disease, atrial fibrillation, or peripheral vascular disease.
Table 2.
Characteristics of patients undergoing TAVI by post-procedure MR improvement.
| Improved (N = 31) | Not-improved (N = 34) | p-value | |
|---|---|---|---|
| Age (years) | 78.7 ± 10.2 | 77.6 ± 10.1 | 0.648 |
| Gender | 0.180 | ||
| Male | 17 (54.8%) | 13 (38.2%) | |
| Female | 14 (45.2%) | 21 (61.8%) | |
| Body mass index | 28.5 ± 7.2 | 31.1 ± 5.6 | 0.109 |
| Normal | 8 (25.8%) | 7 (20.6%) | 0.229 |
| Overweight | 13 (41.9%) | 9 (26.5%) | |
| Obese | 10 (32.3%) | 18 (52.9%) | |
| Blood pressure (mmHg) | |||
| Systolic | 136.5 ± 25.2 | 135.8 ± 25.9 | 0.908 |
| Diastolic | 66.3 ± 11.7 | 67.3 ± 11.8 | 0.751 |
| Heart rate | 74.8 ± 12.3 | 73.0 ± 14.7 | 0.609 |
| EuroScore for operative risk | 26.3 ± 19.3 | 19.9 ± 12.5 | 0.126 |
| Low risk (⩽20) | 17 (48.6%) | 18 (51.4%) | 0.356 |
| High risk (>20) | 14 (50.0%) | 14 (50.0%) | |
| MR cause | 0.968 | ||
| Functional | 18 (48.6%) | 19 (51.4%) | |
| Organic | 13 (48.1%) | 14 (51.9%) | |
| Medical history | |||
| Hypertension | 19 (61.3%) | 27 (84.4%) | 0.039 |
| Diabetes | 18 (58.1%) | 25 (78.1%) | 0.087 |
| Chornic obstructive pulmonary disease | 7 (22.6%) | 10 (31.2%) | 0.438 |
| Coronary artery disease | 14 (45.2%) | 20 (60.6%) | 0.216 |
| Myocardial infarction | 4 (12.9%) | 10 (31.2%) | 0.080 |
| CABG | 4 (12.9%) | 7 (20.6%) | 0.409 |
| Stroke | 3 (9.7%) | 5 (15.6%) | 0.708 |
| Carotid disease | 8 (25.8%) | 9 (27.3%) | 0.894 |
| Atrial fibrillation | 8 (26.7%) | 4 (13.3%) | 0.197 |
| Peripheral vascular disease | 17 (58.6%) | 19 (63.3%) | 0.711 |
| Percutaneous coronary intervention | 11 (35.5%) | 10 (30.3%) | 0.659 |
| Hemoglobin (g/dl) | 12.1 ± 1.3 | 12.2 ± 1.7 | 0.815 |
| Anemia | 13 (44.8%) | 11 (37.9%) | 0.594 |
| Platelets count (1000/μl) | 266.0 ± 65.5 | 252.5 ± 76.5 | 0.480 |
| Serum creatinine (mg/dl) | 1.09 ± 0.78 | 1.05 ± 0.52 | 0.882 |
| Hospital stay (days) | 20.9 ± 18.6 | 14.9 ± 9.0 | 0.284 |
Prosthetic valve characteristics and echocardiographic findings by post-procedure MR improvement are summarized in Table 3. Echocardiographic findings were evaluated within an average of 4.4 ± 3.8 months after TAVI with 84% done within the first six months. The improvement in MR was 54.2% in CoreValve compared to 43.9% in Edwards (p = 0.424), and was 51.2% in femoral approach compared to 41.7% in transapical approach (p = 0.457). Both the peak and mean pressure gradients across the aortic valve were similarly improved among patients with and without MR improvement. The reduction in pulmonary artery pressure after the procedure was seen more commonly among patients with MR improvement (8.3 ± 13.8 vs. 0.7 ± 14.0 mmHg, p = 0.049). LV ejection fraction did not change among patients with or without MR improvement (−1.5 ± 7.1 vs. 0.8 ± 7.2 mmHg, p = 0.214).
Table 3.
Prosthetic valve characteristics and echocardiographic findings among patients undergoing TAVI by post-procedure MR improvement.
| Improved (N = 31) | Not-improved (N = 34) | p-value | |
|---|---|---|---|
| Valve type | 0.424 | ||
| Edwards SAPIEN | 18 (43.9%) | 23 (56.1%) | |
| CoreValve | 13 (54.2%) | 11 (45.8%) | |
| Valve approach | 0.457 | ||
| Femoral | 21 (51.2%) | 20 (48.8%) | |
| Transapical | 10 (41.7%) | 14 (58.3%) | |
| Prosthesis valve size (mm) | 25.8 ± 1.9 | 25.0 ± 1.9 | 0.105 |
| Aortic valve area (cm2) | 0.59 ± 0.14 | 0.61 ± 0.14 | 0.493 |
| Aortic annulus diameter (mm) | 22.2 ± 1.8 | 21.5 ± 1.9 | 0.124 |
| Pre-mean AV gradient (mm Hg) | 52.2 ± 13.6 | 47.1 ± 11.9 | 0.112 |
| Post-mean AV gradient (mm Hg) | 11.4 ± 6.6 | 10.5 ± 4.7 | 0.529 |
| Amount of mean AV change | 40.8 ± 15.1 | 36.6 ± 11.8 | 0.222 |
| p-value for change | <0.001 | <0.001 | |
| Pre-peak AV gradient (mm Hg) | 91.6 ± 22.9 | 83.2 ± 20.7 | 0.123 |
| Post-peak AV gradient (mm Hg) | 20.0 ± 9.1 | 21.2 ± 8.9 | 0.611 |
| Amount of peak AV change | 71.6 ± 23.8 | 61.5 ± 20.3 | 0.071 |
| p-value for change | <0.001 | <0.001 | |
| Pre-LV ejection fraction (%) | 51.1 ± 11.8 | 50.0 ± 13.0 | 0.716 |
| Post-LV ejection fraction (%) | 52.5 ± 11.3 | 48.8 ± 13.2 | 0.235 |
| Amount of LV ejection fraction change | −1.5 ± 7.1 | 0.8 ± 7.2 | 0.214 |
| p-value for change | 0.256 | 0.544 | |
| Pre-pulmonary artery pressure (mmHg) | 44.9 ± 14.5 | 41.6 ± 16.9 | 0.419 |
| Post-pulmonary artery pressure (mmHg) | 37.0 ± 13.2 | 41.0 ± 18.8 | 0.372 |
| Amount of PAP change | 8.3 ± 13.8 | 0.7 ± 14.0 | 0.049 |
| p-value for change | 0.005 | 0.793 |
There were no significant differences in post-procedural complications between the two MR improvement groups (Table 4). The incidence of bleeding requiring blood transfusion, complete heart block requiring pacemaker implantation, vascular injury, myocardial infarction, cerebrovascular accident, acute renal failure, pericardial effusion, sepsis, and wound infection were similar in both groups. The 30-day mortality period was not statistically different between the two MR improvement groups, and the cause of death was cardiac (60.0%) rather than non-cardiac (40.0%).
Table 4.
Post-procedure complications among patients undergoing TAVI by post-procedure MR improvement.
| Improved | Not-improved | p-value | |
|---|---|---|---|
| (N = 31) | (N = 34) | ||
| Blood transfusion | 9 (30.0%) | 11 (33.3%) | 0.777 |
| CHB requiring pacemaker | 8 (26.7%) | 6 (18.2%) | 0.418 |
| Vascular | 3 (10.0%) | 5 (15.6%) | 0.709 |
| Cerebrovascular accident | 2 (6.5%) | 2 (5.9%) | 1.000 |
| Pericardial effusion | 4 (13.8%) | 3 (9.1%) | 0.696 |
| Acute renal failure | 1 (3.3%) | 4 (12.5%) | 0.355 |
| Sepsis | 3 (9.7%) | 0 (0.0%) | 0.108 |
| Wound infection | 1 (3.3%) | 4 (12.1%) | 0.357 |
| 30-days mortality | 0 (0.0%) | 2 (5.9%) | 0.493 |
Thirty-day survival was 94.1% and overall survival was 88.2% during an average 12.5 ± 9.9 months of follow up. The Kaplan–Meier mortality-free survival for the entire cohort and by MR improvement groups is shown in Fig. 2. There was no significant difference in survival by MR improvement groups (p-value for Log Rank test was 0.507). Likewise, survival was not significantly different in relation to the EuroScore operative risk group, type of prosthesis, or procedural approach (p-values for Log Rank test were 0.742, 0.191, and 0.215, respectively; data are not shown).
Figure 2.
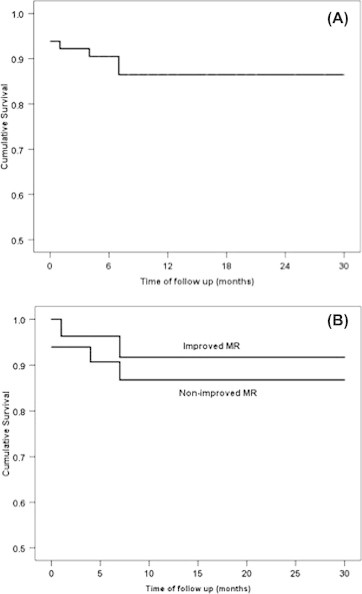
Kaplan–Meier for mortality-free survival for the whole cohort (A), by MR improvement group (B). Significant survival differences were tested by Log Rank test.
Discussion
We report a significant improvement in MR after TAVI procedure. The improvement was more evident in those with significant MR: 85.7% of those who had severe MR, and 59.4% of those who had moderate to severe MR. MR improvement was observed in a number of studies following the implantation of balloon expandable Edwards valves [12,13,15–18]. In a multicenter registry of 1,007 patients undergoing TAVI using CoreValve, MR improvement was observed in 47% and 35% of patients with severe and moderate MR, respectively [16]. In two Canadian centers, MR was improved in 55% of patients with moderate or severe MR one year after TAVI using different types of valves [18]. Durst et al. [13] showed improvement in MR (evaluated by expert readers and by the vena contracta in 35 patients) over time after TAVI. Those who had moderate to severe MR at baseline (53%) decreased to 38% after three months and 19% after six months. Additionally, Hekimian et al. [12] showed significant improvement in MR grade (assessed using integration of color Doppler jet area, vena contracta, and proximal isovelocity surface area) in 28% of the patients at seven days (N = 99 patients), with no further improvement observed at one month (N = 60 patients). Similarly, Webb et al. [15] reported a significant improvement in MR at discharge with mild insignificant improvement thereafter. The proportion of patients who had moderate to severe MR at baseline (N = 24, 53%) declined to 33%, 31%, 25% and 24% at discharge, and at one, six, and 12 months, respectively.
A couple of studies failed to show any significant MR improvement following the implantation of CoreValve prosthesis [11,14]. Tzikas et al. [11] reported no overall change in the degree of MR evaluated in 74 patients using color-flow Doppler at discharge and at the first outpatient visit after TAVI. There were more patients with worsened MR (22%) than with improved MR (17%). This may be partially explained by the fact that our population had more cases of moderate to severe MR than the Tzikas study (49% vs. 19%). Those who had moderate to severe MR in both studies had the highest improvement frequency (59% in our study compared to 60% in the Tzikas study). Those who had mild MR had higher improvement in our study compared to the Tzikas study (46% vs. 8%). It is to be noted that our study had more functional MR than organic MR patients (58% vs. 42%) compared to the Tzikas study (50% vs. 50%). Similarly, Gotzmann et al. [14] reported no overall change in the degree of MR evaluated in 39 patients at 30 days and at six months after TAVI. At six months, 10 patients (26%) had improved MR while eight patients (21%) had worsened MR. The lack of MR improvement in the Gotzmann study is difficult to explain due to both studies having the same proportion of severe to moderate MR (49% each), and the lack of differentiation between functional and organic causes of MR in the Gotzmann study.
Theoretically, correcting AS abruptly reduces LV systolic pressure, which results in a lower transmitral systolic pressure gradient, which in turn reduces the driving force of MR. Although both the peak and mean pressure gradients across the aortic valve in the current study were similarly reduced among patients with and without improved MR, there was a trend towards improvement of the peak pressure gradient among those with improved MR (p = 0.071). Early improvement in MR after TAVI may be due to an initial and rapid transvalvular pressure reduction, because the afterload is reduced immediately. Without stenosis, there is less pressure gradient between the left ventricle and left atrium, leading to reduced MR in the short term; whereas persistent MR reduction may be secondary to changes in the LV and mitral valve geometry and in positive remodeling that occurs over time [13,18].
MR improvement in the current study was not influenced by MR etiology. A number of reports show MR improvement after TAVI is more often observed in functional MR [13,16,18], while others could detect the influence of etiology [11,12,17]. Survival following TAVI in the current study was high and comparable to previous studies [4,26]. Thirty-day survival in the current study was 94.1% while overall survival during an average of 12.5 months of follow-up was 88.2%. In a meta-analysis of 12 TAVI studies, average 30-day survival was 88.6% (77.0–94.7%), while one year survival was 75.9% (64.1–87%) [26]. The observed lack of association between MR improvement and better survival has been reported and was attributed to the negative effects of other comorbidities [16].
This study, the first reported experience in Saudi Arabia and the region, had a relatively high proportion (49%) of patients with moderate to severe MR (potential candidates of MR improvement) for its evaluation of the association between valve type (CoreValve or Edwards SAPIEN) and MR improvement. Nevertheless, we acknowledge a number of limitations: the retrospective nature of the study design, the relatively small number of patients included, and the single center experience with no external independent adjudication. The semi quantitative assessment of MR degree and inconsistent timing of echocardiography may subject our data to potential bias. Finally, lack of longer-term follow up with more data on LV changes in volumes and pressures is a further limitation.
Conclusion
We report a significant improvement in MR after TAVI procedure, especially among those with severe MR. The lack of influence on MR improvement by MR etiology, the type of valve implanted and operative risks need to be confirmed in a larger multi-center study. Such a study should report prospective detailed echocardiographic evaluation of the mitral valve before and after TAVI to better predict patient and procedural characteristics associated with immediate and long term MR improvement.
The authors have no relationship with industry or financial associations that might pose a conflict of interest in connection with the submitted article.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Cribier A., Eltchaninoff H., Bash A., Borenstein N., Tron C., Bauer F. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 2.Cribier A., Eltchaninoff H., Tron C., Bauer F., Agatiello C., Sebagh L. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43(4):698–703. doi: 10.1016/j.jacc.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Saia F., Marrozzini C., Dall’Ara G., Russo V., Martìn-Suàrez S., Savini C. How many patients with severe symptomatic aortic stenosis excluded for cardiac surgery are eligible for transcatheter heart valve implantation? J Cardiovasc Med (Hagerstown) 2010;11(10):727–732. doi: 10.2459/JCM.0b013e328338940f. [DOI] [PubMed] [Google Scholar]
- 4.Olasińska-Wiśniewska A., Grygier M., Lesiak M., Trojnarska O., Grajek S. Transcatheter aortic valve implantation: the new option for high-risk patients with aortic stenosis. Cardiol J. 2011;18(4):461–468. [PubMed] [Google Scholar]
- 5.Smith C.R., Leon M.B., Mack M.J., Miller D.C., Moses J.W., Svensson L.G. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W., Svensson L.G. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 7.Webb J., Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J. 2011;32(2):140–147. doi: 10.1093/eurheartj/ehq453. [DOI] [PubMed] [Google Scholar]
- 8.Brener S.J., Duffy C.I., Thomas J.D., Stewart W.J. Progression of aortic stenosis in 394 patients: relation to changes in myocardial and mitral valve dysfunction. J Am Coll Cardiol. 1995;25(2):305–310. doi: 10.1016/0735-1097(94)00406-g. [DOI] [PubMed] [Google Scholar]
- 9.Barreiro C.J., Patel N.D., Fitton T.P., Williams J.A., Bonde P.N., Chan V. Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: impact on survival and functional outcome. Circulation. 2005;112(Suppl. 9):I443–1447. doi: 10.1161/CIRCULATIONAHA.104.526046. [DOI] [PubMed] [Google Scholar]
- 10.Unger P., Plein D., Van Camp G., Cosyns B., Pasquet A., Henrard V. Effects of valve replacement for aortic stenosis on mitral regurgitation. Am J Cardiol. 2008;102(10):1378–1382. doi: 10.1016/j.amjcard.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Tzikas A., Piazza N., van Dalen B.M., Schultz C., Geleijnse M.L., van Geuns R.J. Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;75(1):43–49. doi: 10.1002/ccd.22197. [DOI] [PubMed] [Google Scholar]
- 12.Hekimian G., Detaint D., Messika-Zeitoun D., Attias D., Iung B., Himbert D. Mitral regurgitation in patients referred for transcatheter aortic valve implantation using the Edwards Sapien prosthesis: mechanisms and early postprocedural changes. J Am Soc Echocardiogr. 2012;25(2):160–165. doi: 10.1016/j.echo.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Durst R., Avelar E., McCarty D., Poh K.K., Friera L.F., Llano M.F. Outcome and improvement predictors of mitral regurgitation after transcatheter aortic valve implantation. J Heart Valve Dis. 2011;20(3):272–281. [PubMed] [Google Scholar]
- 14.Gotzmann M., Lindstaedt M., Bojara W., Mügge A., Germing A. Hemodynamic results and changes in myocardial function after transcatheter aortic valve implantation. Am Heart J. 2010;159(5):926–932. doi: 10.1016/j.ahj.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Webb J.G., Pasupati S., Humphries K., Thompson C., Altwegg L., Moss R. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116(7):755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 16.Bedogni F., Latib A., De Marco F., Agnifili M., Oreglia J., Pizzocri S. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve revalving system: a multicenter registry. Circulation. 2013;128(19):2145–2153. doi: 10.1161/CIRCULATIONAHA.113.001822. [DOI] [PubMed] [Google Scholar]
- 17.Giordana F., Capriolo M., Frea S., Marra W.G., Giorgi M., Bergamasco L. Impact of TAVI on mitral regurgitation: a prospective echocardiographic study. Echocardiography. 2013;30(3):250–257. doi: 10.1111/echo.12050. [DOI] [PubMed] [Google Scholar]
- 18.Toggweiler S., Boone R.H., Rodés-Cabau J., Humphries K.H., Lee M., Nombela-Franco L. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59(23):2068–2074. doi: 10.1016/j.jacc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Roques F., Nashef S.A., Michel P., Gauducheau E., de Vincentiis C., Baudet E. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15(6):816–882. doi: 10.1016/s1010-7940(99)00106-2. [discussion 822–3] [DOI] [PubMed] [Google Scholar]
- 20.Otto C.M. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47(11):2141–2151. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Oh J.K., Seward J.B., Tajik A.J. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2006. The echo manual. [Google Scholar]
- 22.Helmcke F., Nanda N.C., Hsiung M.C., Soto B., Adey C.K., Goyal R.G. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation. 1987;75(1):175–183. doi: 10.1161/01.cir.75.1.175. [DOI] [PubMed] [Google Scholar]
- 23.Vahanian A., Baumgartner H., Bax J., Butchart E., Dion R., Filippatos G. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European society of cardiology. Eur Heart J. 2007;28(2):230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 24.Zoghbi W.A., Enriquez-Sarano M., Foster E., Grayburn P.A., Kraft C.D., Levine R.A. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 25.Moazami N., Diodato M.D., Moon M.R., Lawton J.S., Pasque M.K., Herren R.L. Does functional mitral regurgitation improve with isolated aortic valve replacement? J Card Surg. 2004;19(5):444–448. doi: 10.1111/j.0886-0440.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 26.Figulla L., Neumann A., Figulla H.R., Kahlert P., Erbel R., Neumann T. Transcatheter aortic valve implantation: evidence on safety and efficacy compared with medical therapy. A systematic review of current literature. Clin Res Cardiol. 2011;100(4):265–276. doi: 10.1007/s00392-010-0268-x. [DOI] [PubMed] [Google Scholar]



