Abstract
Acute myocardial infarction (AMI) is usually seen in the setting of atherosclerosis and its associated risk factors. Myocardial infarction in the young poses a particular challenge, as the disease is less likely, due to atherosclerosis. We report the case of a 37-year-old female patient who presented with ST segment elevation anterolateral AMI. The only abnormality on routine blood investigation was raised hemoglobin and hematocrit. After further testing, she was diagnosed according to the World Health Organization (WHO) criteria with polycythemia vera. This case illustrates the importance of recognizing polycythemia vera as an important cause of thrombosis, which can present initially as AMI, and to emphasize the early recognition of the disease in order to initiate appropriate management strategies.
Keywords: Polycythemia vera (PV), Myocardial infarction
Case report
A 37-year-old Egyptian woman presented to our Emergency Department with severe retrosternal compressive chest pain over a three-hour period. The pain was radiating to both shoulders and was associated with profuse sweating.
She had no previous history of hypertension, diabetes mellitus or hyperlipidemia. She was a lifetime non-smoker. She took no recreational drugs and did not have family history of cardiac disease.
On physical examination, she looked plethoric with conjunctival congestion. Her temperature was 37 °C, pulse 90/min (regular), blood pressure (BP) 130/85 mmHg, respiratory rate 18/min and saturation in room air 99%. Her chest, heart and abdominal examination revealed no abnormality. The peripheral pulses were all intact.
The ECG on admission (Fig. 1) showed ST segment-elevation in anterolateral leads (V1–V6 and I-AVL), so the impression was acute ST segment elevation myocardial infarction. Due to the unavailability of a cardiac catheterization facility at our hospital or at a nearby facility, the patient was thrombolyzed with reteplase 10 units IV bolus, then 10 units given over 30 min, after ruling out contraindications. She was started on enoxaparin 1 mg/kg, aspirin 81 mg, clopidogrel 75 mg, lisinopril 10 mg, bisoprolol 10 mg and simvastatin 20 mg.
Figure 1.
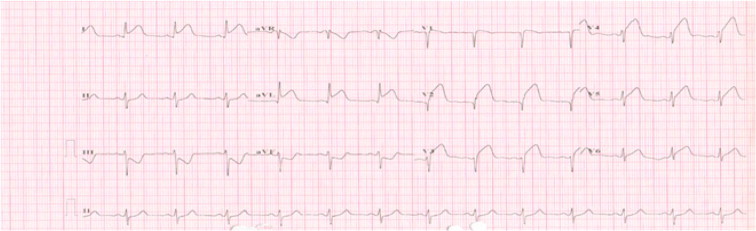
Twelve-lead ECG showing ST segment elevation in chest leads from V1 to V6 and limb leads I; AVL indicating extensive anterolateral AMI.
Laboratory investigations on admission were as follows (normal range is shown in brackets): WBC 11 × 109/L (4–11 × 109/L); neutrophils 58.8%; lymphocytes 33%; Hb 164 g/L (118–148 g/L); hematocrit 0.501 L/L (0.36–0.44 L/L); MCV 83.4 FL (82–98 FL); and platelets 448 × 109/L/L (150–450 × 109/L). Her coagulation profile, renal function test, liver function test, and electrolytes were all within normal ranges.
The maximum troponin was 34.73 μg/L (upper limit of normal 0.3 μg/L); lipid profile showed triglyceride of 3.97 mmol/L (0–1.7 mmol/L); HDL 0.86 mmol/L (1.29–1.69 mmol/L); and LDL 1.53 mmol/L (0–3.4 mmol/L).
In pursuit of the cause for her polycythemia, further tests were done. The arterial blood gas showed no hypoxia, the erythropoietin level was 1.11 mU/ml (4.30–29 mU/ml), and the JAK-2 V617F mutation was positive. Cytogenetic study for BCR/ABL1 rearrangement was negative. The tests for ANA, lupus anticoagulant, anticardiolipin antibody and antib2 glycoprotein were negative. The abdominal ultrasound was normal with no hepatosplenomegaly. The patient’s echocardiography showed ejection fraction of 60% with no intracardiac thrombus.
The bone marrow aspirate and biopsy (Fig. 2) showed increase in trilineage hematopoiesis with an increased number of megakaryocytes present in loose clusters with a degree of pleomorphism. These findings are morphologically in keeping with myeloproliferative disorder. Based on the clinical picture and laboratory results, the patient was diagnosed with polycythemia vera (PV) fulfilling the WHO diagnostic criteria (Table 1), and started on hydroxyurea 15 mg/kg, aspirin 81 mg, and scheduled for regular phlebotomy to keep hematocrit less than 45%.
Figure 2.
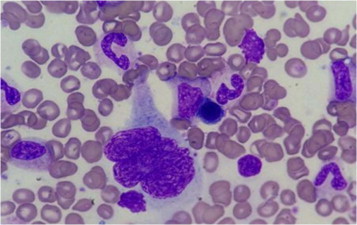
Bone marrow aspirate with Romanowsky stain showing a megakaryocyte surrounded by myeloid elements and erythroid precursors.
Table 1.
WHO criteria for diagnosing Polycythemia Vera [3,20].
| A criteria | |
| I. | Elevated red cell mass >25% above mean normal predicted value, or hemoglobin >18.5 g/dL in men, 16.5 g/dL in women |
| II. | No cause of secondary erythrocytosis, including familial erythrocytosis, hypoxia, high oxygen affinity hemoglobin, truncated erythropoietin or inappropriate erythropoietin production by tumor |
| III. | Splenomegaly |
| IV. | Clonal genetic abnormality other than Philadelphia chromosome or BCR-ABL fusion gene in marrow cells. |
| V. | Endogenous erythroid colony formation in vitro |
| B criteria | |
| I. | Thrombocytosis >400 × 109/L |
| II. | Leukocytosis >12 × 109/L |
| III. | Bone marrow biopsy showing panmyelosis with prominent erythroid and megakaryocytic proliferation |
| IV. | Low serum erythropoietin |
Diagnosis requires the presence of first 2 A criteria plus either any 1 other A criterion or 2 B criteria.
Four weeks later, the patient had myocardial perfusion scintigraphy, which showed no evidence of myocardial ischemia. CT coronary angiography was normal.
Discussion
Polycythemia vera (PV) is a primary disorder of bone marrow stem cells resulting in overproduction of red cells and, to a lesser extent, neutrophils and platelets. The median age of diagnosis is 60 years, though the disorder can occur in all age groups [1]. PV occurs with a slight predominance in men. The incidence is 2.3 per 100,000 persons per year [2].
It can be discovered incidentally after routine blood investigation or may present with signs and symptoms related mainly to thrombosis and/or hemorrhage. Thrombotic events in both the venous and arterial systems are common. Nineteen percent of 1213 patients followed by the Gruppo Italiano Studio Policitemia suffered a thrombotic event, of which 50% were arterial [9]. The pathophysiology of thromboembolic events has not been illustrated in PV, but many factors are involved including the increase in hematocrit and blood hyperviscosity, stimulation of platelet aggregation and thrombogenesis, the presence of leukocytosis and intimal proliferation [4–5].
Benita reported a 30-year-old male patient who died due to myocardial infarction as initial manifestation of PV. On autopsy, the vessels showed neither arteriosclerotic changes nor thrombotic occlusion but a marked intimal proliferation leading to multiple occlusions [6].
Coronary events are not uncommon during the course of PV. Rossi et al. followed 149 patient diagnosed with PV for 10 years and found that 11.4% had myocardial infarction [7]. Another report by Malak et al. concluded that 4% of all patients with myeloproliferative disorder die from myocardial infarction [8].
Despite the association of PV with coronary artery disease, the presentation of PV as AMI is very rare. The authors found some reports [10–18] documenting PV presenting as AMI. Not all papers were available for review but the majority of cases were young males with minimal coronary occlusion. Goethals described the difficulty of maintaining a patent coronary artery due to repeated thrombosis [15]. Despite evidence of the effectiveness of new therapies in reducing the risk of stent thrombosis, early recognition and improved perfusion in PV are critical, as stent thrombosis is common [19].
The major goal of treatment is to prevent thrombotic events. Our patient was scheduled for regular phlebotomies to keep hematocrit less than 45%. In addition, she was commenced on hydroxyurea 15 mg/kg and aspirin 81 mg/kg. The use of angioplasty and stenting is challenged by the development of stent thrombosis. Hydroxyurea is an important component of the treatment of such patients.
In conclusion, there is no doubt that MI accounts for a substantial percentage of morbidity and mortality in patients with PV. Although relatively rare, AMI can be the initial manifestation of myeloproliferative disease. Attending physicians should keep a high index of suspicion for PV, especially when a young person presents with myocardial infarction in the absence of atherosclerotic risk factors, as initiation of early management will alter patient prognosis.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hussain Bahbahani, Email: drhbahbahani@gmail.com.
Khaled Aljenaee, Email: k.jenaee@gmail.com.
Abdelhaleem Bella, Email: abella@ud.edu.sa.
References
- 1.Tefferi A. Polycythemia vera: a comprehensive review and clinical recommendations. Mayo Clin Proc. 2003;78(2):174–194. doi: 10.4065/78.2.174. [DOI] [PubMed] [Google Scholar]
- 2.Stuart B.J., Viera A.J. Polycythemia vera. Am Fam Physician. 2004;69(9):2139–2144. [PubMed] [Google Scholar]
- 3.Cao M., Olsen R.J., Zu Y. Polycythemia vera: new clinicopathologic perspectives. Arch Pathol Lab Med. 2006;130(8):1126–1132. doi: 10.5858/2006-130-1126-PV. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert H.S. Current management in polycythemia vera. Semin Hematol. 2001;38(1 Suppl. 2):25–28. doi: 10.1016/s0037-1963(01)90137-4. [DOI] [PubMed] [Google Scholar]
- 5.Berk P.D., Wasserman L.R., Fruchtman S.M., Goldberg J.D. Treatment of polycythemia vera: a summary of clinical trials conducted by the Polycythemia Vera Study Group. In: Wasserman L.R., Berk P.D., Berlin N.I., editors. Polycythemia vera and the myeloproliferative disorders. WB Saunders; Philadelphia: 1995. pp. 166–194. [Google Scholar]
- 6.Hermanns B., Handt S., Kindler J., Füzesi L. Coronary vasculopathy in polycythemia vera. Pathol Oncol Res. 1998;4(1):37–39. doi: 10.1007/BF02904693. [DOI] [PubMed] [Google Scholar]
- 7.Rossi C., Randi M.L., Zerbinati P., Rinaldi V., Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med. 1998;244(1):49–53. doi: 10.1046/j.1365-2796.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- 8.Malak S., Labopin M., Saint-Martin C., Bellanne-Chantelot C., Najman A. French group of familial myeloproliferative disorders. Long term follow up of 93 families with myeloproliferative neoplasms: life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis. 2012;49(3–4):170–176. doi: 10.1016/j.bcmd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Gruppo Italiano Studio Policitemia Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995;123(9):656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gouri A., Yakhlef A., Dekaken A., Bentorki A.A. Acute myocardial infarction revealing a polycythemia vera. Ann Biol Clin (Paris) 2012;70(4):489–491. doi: 10.1684/abc.2012.0735. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.F., Armstrong G.P., Henderson R.A., Ruygrok P.N. Polycythaemia vera presenting as ST-elevation myocardial infarction. Heart Lung Circ. 2005;14(1):51–53. doi: 10.1016/j.hlc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Wirth L. Myocardial infarction as the initial manifestation of polycythemia vera. Mil Med. 1960;125:544. [PubMed] [Google Scholar]
- 13.Vacca J.B., Thoma G.E., Jr. Myocardial infarction as the initial manifestation of polycythemia vera. AMA Arch Intern Med. 1959;103(6):974–977. doi: 10.1001/archinte.1959.00270060126017. [DOI] [PubMed] [Google Scholar]
- 14.Tekin M., Gökaslan S., Diker E., Aydoğdu S. Development of acute coronary syndrome in three patients with essential thrombocythemia or polycythemia vera. Turk Kardiyol Dern Ars. 2008;36(1):35–38. [Article in Turkish] [PubMed] [Google Scholar]
- 15.Goethals P., Evrard S., Dubois C. Recurrent coronary stent thrombosis. Acta Cardiol. 2000;55(6):371–373. doi: 10.2143/AC.55.6.2005769. [DOI] [PubMed] [Google Scholar]
- 16.Chan A.W., Drobac M., Sternberg L. The management of acute myocardial infarction in a patient with polycythemia rubra vera during the thrombolytic era–does it make a difference? Can J Cardiol. 1997;13(1):59–63. [PubMed] [Google Scholar]
- 17.Rykov V.A., Letunova O.N. Polycythemia vera, complicated by myocardial infarction. Arkh Patol. 1995;57(3):73–74. [Article in Russian] [PubMed] [Google Scholar]
- 18.Skribnik Ela., Oteva E.A. Rare types of myocardial infarction in young patients. Klin Med (Mosk) 1991;69(11):32–35. [Article in Russian] [PubMed] [Google Scholar]
- 19.Zhang Y., Tang H.Q., Li J., Fu Z.X. Efficacy and safety of triple-antiplatelet therapy after percutaneous coronary intervention: a meta-analysis. Chin Med J (Engl) 2013;126(9):1750–1754. [PubMed] [Google Scholar]
- 20.Tefferi A. Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era. Hematology Am Soc Hematol Educ Program. 2006:240–245. doi: 10.1182/asheducation-2006.1.240. [DOI] [PubMed] [Google Scholar]


