Abstract
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and a major preventable cause of stroke and hospitalization. Its prevalence is on the rise worldwide and experts believe it will continue to rise for the foreseeable future, due to the ageing population and increased survival from conditions associated with AF. Despite the fact that oral anticoagulation is effective in preventing strokes due to AF, there is extensive evidence suggesting this therapy remains underused. Barriers to the prescription of anticoagulation include patients’ age per se, comorbidities, inadequate risk stratification, perceived risk of falls and bleeding, and the difficulty in achieving a stable international normalized ratio (INR) on warfarin. Also, asymptomatic patients with AF may not be identified and therefore not be candidates for anticoagulation. Physicians need continued better education on the identification of patients at risk of stroke and management of oral anticoagulation. This article reviews the barriers to anticoagulation in patients with AF in the United Kingdom and considers how those barriers may be overcome.
Keywords: Atrial fibrillation, Stroke, Thromboembolism, Anticoagulation, Barriers, Warfarin, New oral anticoagulants
Abbreviations
- AF
atrial fibrillation
- BAFTA trial
Birmingham Atrial Fibrillation Treatment of the Aged trial
- ECG
electrocardiogram
- GRASP-AF trial
The Guidance on Risk Assessment and Stroke Prevention in Atrial Fibrillation Trial
- INR
international normalized ratio
- LA
left atrial
- RCPE
Royal College of Physicians of Edinburgh
- RE-LY trial
Randomized Evaluation of Long Term Anticoagulant Therapy trial
- ROCKET-AF trial
Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation trial
- TTR
time in therapeutic range
- UK
United Kingdom
- WASPO trial
Warfarin versus Aspirin for Stroke Prevention in Octogenarians with Atrial Fibrillation trial
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia [1] and a major preventable cause of stroke [2] and hospitalization [3–5]. Approximately 1 in 100 of the general population are thought to have AF, although the prevalence exceeds 1 in 10 in elderly cohorts [6,7]. Its prevalence is on the rise worldwide and experts believe it will continue to rise for the foreseeable future, due to the aging population and increased survival from conditions associated with AF. Stroke and peripheral thromboembolism are major complications of AF [8]. This arrhythmia confers, on average, a fivefold risk of stroke, and is responsible for one fifth of all strokes. This risk increases with age. Strokes caused by AF tend to be more frequently fatal, disabling and recurring when compared to other causes of stroke. Furthermore, it is noteworthy that paroxysmal AF carries the same stroke risk as persistent or permanent forms of the arrhythmia [9], irrespective of symptomatic status. Close to 20% of patients with AF are asymptomatic [10] and, in some of these patients, AF is first diagnosed at the time of a stroke.
Despite the fact that oral anticoagulation is effective in preventing strokes due to AF, there is extensive evidence suggesting that this therapy remains underused [7,11-14]. In fact, although current guidelines clearly define indications for anticoagulation treatment and its vast impact in the prevention of stroke, barriers to the implementation of oral anticoagulation continue to exist among physicians and patients.
This article reviews the barriers to anticoagulation in patients with AF in the United Kingdom (UK), as well as potential strategies to overcome these barriers.
Epidemiology of atrial fibrillation in the UK and the rest of the world: an increasing public health challenge?
Based on a 1998 survey of 211 general practices representing a total population of 1.4 million patients in England and Wales, 1.28% of the total population of the UK has AF [7,15]. A more recent study in England revealed that the overall prevalence of AF among practices uploading data from 2009 to 2012 was 1.76% [16]. The prevalence of AF roughly doubles with each advancing decade of age, from 0.5% at age 50–59 years to almost 9% at age 80–90 years [17]. The incidence of AF in a cohort of 15,406 adults aged 45–64 years living in the west of Scotland and screened between 1972–1976 and 1977–1979 [18] was 0.54 cases per 1000 person years (or 0.05%) and, during a 20-year follow-up, 3.5% of this cohort was admitted/discharged from hospital with a diagnosis of AF. The rate of incident hospitalization for AF was 1.9 cases/1000 person-years. Incidence of AF in the UK is estimated at up to 0.3% [7,15–19].
There were approximately 8.8 million adults with AF in the European Union in 2010 and this number will more than double by the year 2060 [19]. In 2010, the number of adults aged ⩾55 years with AF could reflect 1.8% of the total population, and this number will rise to 3.5% by 2060. This increase will be particularly dramatic for adults over age 75 (from 5.6 million in 2010 to 13.8 million in 2060) [20] (Fig. 1). Several studies have also projected a doubling of the number of adults with AF in the United States by the year 2050 [21,22]. A systematic review of the published literature on the epidemiology of AF in regions of the world outside North America and Europe reported prevalence of AF varying among countries, with different ranges in community and hospital-based studies, 0.1–4% and 2.8–14%, respectively [23].
Figure 1.

Individuals with atrial fibrillation in the European Union [Ref. [19]], original figure, with permission].
When analyzing these data, we should take into consideration that the population of the world amounts to seven billion and more than one third live in China and India, where reliable epidemiological data on AF are scarce.
In conclusion, despite a lack of data regarding the epidemiology of AF in non-developed countries, there is enough evidence suggesting AF is an increasingly major public health issue worldwide.
Economic analysis of atrial fibrillation in the UK and the world: what is the cost of this emerging epidemic?
Atrial fibrillation frequently leads to hospitalization and is a cause of costly cardiovascular morbidity including heart failure and stroke. Almost 20% of all strokes are caused by AF. The number of AF related hospitalizations is increasing in both the developed and non-developed world and most patients with AF require long term pharmacological treatment to decrease the risk of complications. Consequently, AF imposes a substantial and growing economic burden on health care systems.
A previous study has shown that the direct cost of AF to the UK’s National Health Service (NHS) was between £244 and £531 million in 1995, 0.6–1.2% of overall health care expenditure in the UK, but the amount doubled until the year 2000 (AF accounted for 0.9–2.4% of NHS expenditure in 2000) [24]. These are very conservative estimates since additional costs such as those related to stroke rehabilitation and warfarin or aspirin-related hemorrhage were not considered. Furthermore, as they included hospitalizations where AF was the primary diagnosis, admissions due to heart failure or stroke caused by AF, where this condition would be coded in a secondary position, were not considered. These admissions would cost an additional £221 million and £228 million, respectively [24]. The direct cost of AF-related stroke is up to £24.000 per patient in the UK [25].
The estimated direct and indirect economic burden of all-cause stroke in the USA was US$34.3 billion in 2008 [26], with AF-related stroke theoretically responsible for one fifth of that amount, probably more given its more disabling nature compared to other causes of stroke. We should also consider the cost of more recent treatments for AF, such as catheter ablation. Although some authors consider catheter ablation a cost-effective intervention for the treatment of paroxysmal AF [27,28], the most determining input variables for a cost-effectiveness assessment of catheter ablation of AF are the impact on stroke and hospitalization rates. Some studies have suggested successful catheter ablation for AF may eventually reduce stroke risk [29–31], but there is no evidence based on randomized studies that this therapeutic approach will indeed decrease thromboembolic risk.
In conclusion, the massive epidemiological and economic impact of AF is a major concern worldwide. An effective treatment of conditions associated with AF, in order to prevent occurrence of this arrhythmia and its complications, especially stroke and heart failure, are essential.
Use of anticoagulants in the management of atrial fibrillation in the UK
The Guidance on Risk Assessment and Stroke Prevention in Atrial Fibrillation (GRASP-AF) tool searches general practice clinical information to identify patients with history of AF, reviews their risk profiles, and provides insights into the prevalence and management of AF in England [16]. According to GRASP-AF, the proportion of patients with AF who were prescribed anticoagulants and antiplatelet agents was 49.3% and 42.5%, respectively, with 6.9% of patients being prescribed both agents. In total, 57.0% and 83.7% of the AF population had a CHADS2 score of ⩾2 and ⩾1, respectively. The prescription of both anticoagulation and antiplatelet agents increased with increasing CHADS2 score for scores 0–3, and plateaued thereafter to reach 58.1% for anticoagulation and 46.5% for antiplatelet drugs at a score of 6. In total, 34.0% of patients with AF who had a CHADS2 score ⩾2 were not reported as having been prescribed an anticoagulant, with no recording of a contraindication or patient refusal. The uptake of anticoagulant therapy increased with age for patients aged less than 80 years, but decreased in patients aged 80 years and over. By contrast, antiplatelet drug prescription continued to increase with age in patients aged 80 years and over. Among high-risk patients with CHADS2 ⩾2, the prescription of anticoagulation was 47.4% for patients aged 80 years and over compared with 64.5% for younger patients. Overall, this study suggested that less than half (49.3%) of patients with a history of AF received an anticoagulant and that anticoagulant uptake increases through CHADS2 score 0–3 and thereafter reaches a plateau [16]. These results are similar to those of another recent study [12] and represent an improvement compared to what had previously been described [32]. Nevertheless, if the treatment threshold for anticoagulation is a CHADS2 score ⩾1, this would include 84% of the AF population, which is significantly higher than the percentage of patients currently given anticoagulation. Furthermore, their study showed an age-dependent inequality in the prescription of anticoagulants and antiplatelet agents, which is also a concern considering elderly patients are at the highest risk of stroke. The NICE guidelines suggest that 17.400 strokes could be avoided annually by following their proposed treatment program when compared with no treatment. With the current level of treatment preventing around 10,300 strokes, they proposed that a higher level of treatment in accordance with their recommendations could result in an additional 7100 strokes being prevented per year.
A few studies have addressed the use of antithrombotic therapy in patients with AF outside Europe and North America. The use of warfarin was very low in China (reported at 0.5% and 2.7% in two separate studies) [33,34], but high in Japan (70.1%) [35]. Aspirin was employed as antithrombotic therapy in at least one third of the patients and a variable percentage was not receiving any kind of antithrombotic treatment. Older patient age was associated with lower prescription of oral anticoagulants [36].
In conclusion, although the rate of prescription of anticoagulants in the UK for patients with AF at moderate to high risk of stroke has been increasing gradually and is higher than in other regions of the globe, there is still a significant margin for improvement.
Barriers to anticoagulation
A recent meta-analysis of quantitative studies revealed that less than 70% of high-risk patients receive adequate oral anticoagulation therapy [37]. Potential barriers to appropriate treatment include inadequate risk stratification, the (advanced) age of the patient, perceived bleeding risk and risk of falling, the pitfalls of warfarin, such as the requirements for regular monitoring, frequent dosage adjustments, dietary restrictions and susceptibility to drug interactions, the high cost of the new oral anticoagulants, the difficulty in identifying asymptomatic AF patients, and the lack of appropriate education. Based on a number needed to treat from the Birmingham Atrial Fibrillation Treatment of the Aged (BAFTA) study of 50 to prevent one thromboembolic event or intracranial haemorrhage [38], and on observations from the study by Cowen et al. [16], it is estimated that 24 in excess of 3000 strokes could potentially be prevented annually if these individuals were commenced on anticoagulation in preference to antiplatelet drugs [16].
Atrial fibrillation and stroke: which patients to treat?
Risk stratification is currently based on clinical risk scores: either the CHADS2 or the CHA2DS2-VASc score are recommended [8]. These algorithms are based on the presence (or absence) of risk factors for stroke and thromboembolism, including congestive heart failure, hypertension, age, diabetes mellitus, history of stroke, evidence of atherosclerotic disease and gender. CHADS2 represents a very simple and easy to remember means of assessing stroke risk. However, it has been shown to be less reliable in the identification of low risk patients (those with a score of zero), who may not be truly low risk (1.9%/year risk of thromboembolic events) [39]. On the other hand, CHA2DS2-VASc is able to identify a truly low risk cohort of patients as a score of zero may associate with an annual stroke risk of 0% [40]. However, this algorithm is usually over inclusive, which is a concern given that some of the subjects who are given oral anticoagulation based on this score would never experience an event if they remained untreated but will have an increased risk of fatal bleeding whilst being on anticoagulation. Despite ease of use, these risk scores have shown a limited capability in the prediction of stroke, with low areas under the curve [40,41]. Moreover, they share some risk factors with scores developed to estimate hemorrhagic risk (age, history of hypertension and stroke) [42], which adds further complexity to decision making.
Multiple studies have tried to find practical ways of improving stroke prediction. A biomarker sub-study of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial showed that elevations of troponin I and NT-proBNP are common in patients with AF and independently related to increased risks of stroke and mortality [43]. These parameters added prognostic power to CHADS2 and CHA2DS2-VASc. In fact, a group of patients with a CHADS2 score of 0–1 and elevated biomarkers had a higher annual rate of a composite of thromboembolic events than those with higher CHADS2 scores and undetectable biomarkers, and some patients with higher CHADS2 scores and undetectable Troponin I levels could also be correctly reclassified as low risk [43]. C-reactive protein and Troponin I have also been shown to associate with left atrium appendage thrombus and dense spontaneous echocardiographic contrast [44,45], and preliminary data from the RE-LY trial suggested a relationship between D-dimers [46] and interleukin-6 [47], and clinical events. In addition, recent evidence suggests renal dysfunction is an important risk factor for stroke in AF patients, irrespective of the CHADS2 or CHA2DS2-VASc scores [48,49].
Likewise, several transthoracic echocardiographic parameters have shown promising value in the prediction of stroke/thromboembolism [50–54] or surrogate markers of stroke [55–59]. These include left atrial (LA) size measured on M-mode [50], indexed LA volume [51], left ventricular ejection fraction [57], E/E′ ratio [52] and peak systolic LA strain rates assessed through speckle-tracking [54]. The presence of LA appendage thrombi, dense spontaneous echocardiographic contrast, LA appendage peak flow velocities <20 cm/s or complex aortic plaque associates with increased thromboembolic risk [60,61]. These abnormalities can be predicted by LA volume, troponin I values, AF episode duration, history of stroke or embolism, and C-reactive protein [59]. The roles of echocardiography in thromboembolic risk assessment in patients with nonvalvular AF and as a predictor of the incidence and progression of AF have been thoroughly addressed before [62,63].
In conclusion, although the traditional CHADS2 or CHA2DS2-VASc scores are a reliable and practical way to estimate thromboembolic risk in patients with AF, future studies or guidelines may offer definite recommendations to refine risk stratification based on the addition of biomarkers or echocardiographic parameters.
Age
Elderly patients with AF are given anticoagulation less often than their younger counterparts [16]. For CHADS2 scores 1–6, the proportion prescribed an anticoagulant was lower in those aged 80 years and over than in those aged less than 80 years. Conversely, for patients with a CHADS2 score from 1 to 6, the proportion with AF prescribed an antiplatelet agent was higher in those aged 80 years and over and remained relatively constant across scores (Fig. 2). This contrasts with the unequivocal evidence demonstrating that elderly patients are at a higher risk of all-cause stroke, including AF-related stroke. Also, the results of the BAFTA study revealed that warfarin is superior to aspirin in stroke prevention in the elderly and does not associate to a significantly higher risk of severe bleeding [38]. The authors studied 973 AF patients aged 75 years or over (mean age 81.5) and concluded that treatment with warfarin (vs. aspirin) associated to a lower risk of a composite endpoint of fatal or disabling stroke (ischemic or hemorrhagic), intracranial hemorrhage, or clinically significant arterial embolism (Table 1). The Warfarin versus Aspirin for Stroke Prevention in Octogenarians with Atrial Fibrillation (WASPO) trial specifically addressed the efficacy of adjusted doses of warfarin (INR 2–3) versus 300 mg of aspirin in octogenarian patients. The primary endpoint, a composite outcome of combined death, thromboembolism, major bleeding and withdrawal from assigned treatment, was more frequent in the aspirin group [64]. Although patient age is independently associated with the risk of stroke and cardiovascular events, van Walraven et al. concluded that anticoagulation remains highly beneficial for preventing these outcomes in elderly patients with AF, despite a slight decrease of the relative risk reduction with oral anticoagulation in older individuals [65]. Importantly, there was a marked decrease of stroke risk reduction with antiplatelet treatment in elderly patients with AF, and no significant interaction between oral anticoagulants or antiplatelets and patient’s age regarding serious hemorrhage or cardiovascular death was seen. Together, these studies support the hypotheses that not only is elderly age not a contraindication to oral anticoagulation in AF patients, but also antiplatelet treatment is clearly insufficient to prevent stroke in this context.
Figure 2.
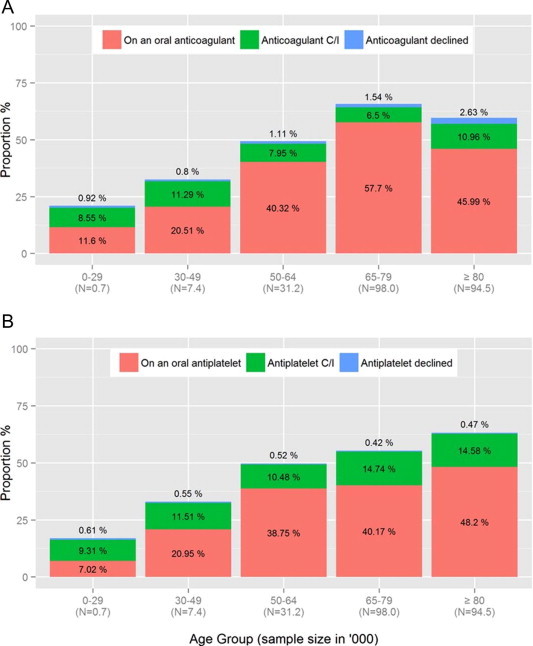
Proportion of atrial fibrillation patients prescribed anticoagulation and antiplatelet agents among general practices in England, according to Cowan C and colleagues, Heart 2013 [Ref. [16]], original figure, with permission].
Table 1.
Results of the BAFTA trial [Ref. [38]].
| Warfarin (n = 448) |
Aspirin (n = 485) |
Warfarin vs. aspirin |
|||||
|---|---|---|---|---|---|---|---|
| n | Risk per year (%) | n | Risk per year (%) | RR (95% CI) | p | ||
| Stroke | 21 | 1.6 | 44 | 3.4 | 0.46 (0.26–0.79) | 0.003 | |
| By severity | Fatal | 13 | 1.0 | 21 | 1.6 | 0.59 (0.27–1.24) | 0.14 |
| Disabling non-fatal | 8 | 0.6 | 23 | 1.8 | 0.33 (0.13–0.77) | 0.005 | |
| Type of stroke | Ischemic | 10 | 0.8 | 32 | 2.4 | 0.30 (0.13–0.63) | 0.0004 |
| Hemorrhagic | 6 | 0.5 | 5 | 0.4 | 1.15 (0.29–4.77) | 0.83 | |
| Unknown | 5 | 0.4 | 7 | 0.5 | 0.69 (0.17–2.51) | 0.53 | |
| Other intracranial bleeding | 2 | 0.2 | 1 | 0.1 | 1.92 (0.10–113.3) | 0.65 | |
| Systemic embolism | 1 | 0.1 | 3 | 0.2 | 0.32 (0.01–3.99) | 0.36 | |
| Total number of events | 24 | 1.8 | 48 | 3.8 | 0.48 (0.28–0.80) | 0.0027 | |
Reasons for this risk-treatment paradox in elderly patients include the high prevalence of co-morbid factors, impaired cognition with limited compliance, use of concomitant medications, the difficulty in getting stable therapeutic INRs, frequent falls and the perceived risk of bleeding.
Falls and bleeding risk
Elderly patients with AF are frequently not given anticoagulation therapy on the basis of a high perceived falling and/or bleeding risk. Falls may lead to major complications in older individuals, including subdural hematomas, intracerebral hemorrhage and hip fracture bleeding, which can be life-threatening. Some authors have suggested that in AF patients with additional risk factors for falling, such as sedative use, cognitive impairment, disability of the lower extremity, gait disturbance or foot abnormalities, especially those with multiple risk factors, extreme caution in prescribing anticoagulation should be warranted [66]. One study analyzed the risk of bleeding from falls in elderly patients (at least 65 years of age) who are anticoagulated for AF and concluded that a person taking warfarin would need to fall about 295 times in one year for warfarin not to be considered the optimal therapy [67]. This study had some limitations, including the fact that it studied elderly patients in community dwelling settings at no particularly increased risk of falls; it did not consider other serious bleeding complications beyond subdural bleeding; and it was not a primary evidence study or meta-analysis. Nevertheless, a study by Donzé et al. revealed that patients at high risk of falls who are put on oral anticoagulants do not have an increased risk of major bleeding. In their study, only three major bleeds directly related to a fall were reported in a cohort of 308 patients at high risk of falling, followed for 12 months [68]. A systematic review of the use of anticoagulation in elderly patients with AF at particular high risk of falls concluded that the benefits of warfarin outweigh its risks even in patients who fall, and therefore warfarin should be used, rather than aspirin or no therapy, in elderly patients at risk of falls [69]. Nevertheless, this general recommendation should not replace clinical judgment.
Physicians’ most frequent reason for not prescribing warfarin is a perception that patients are at high risk of bleeding. However, a previous study suggested that clinicians are no better at predicting bleeding risk than chance [70]. Bleeding risk perceptions may not correspond to actual risk of bleeding, and eligible patients may fail to receive treatment because of faulty judgments. According to information from trials and registry data, bleeding events are five to eight times less likely than ischemic strokes in AF patients [65]. Several studies have suggested that a perceived high bleeding risk should not necessarily preclude the prescription of anticoagulation in patients at moderate to high risk of stroke. A previous systematic review concluded that major bleeding rates for patients receiving antiplatelet therapy were similar to those receiving anticoagulation, while the former associated to a higher rate of ischemic stroke than the latter [71]. These findings are similar to those of the BAFTA study [63]. A large multicenter study that enrolled very old patients concluded that the rate of bleeding is low (1.87 major hemorrhages per 100 patient-years; 26 fatal bleeding events, rate of 0.27 per 100 patient-years) [72]. Tincani et al. followed ninety patients aged 90 years or older with nonvalvular AF and taking anticoagulants, and found low rates of bleeding and thromboembolism. Importantly, all the events occurred when the international normalized ratio (INR) was outside the target range or after anticoagulation had been stopped [73]. Therefore, prescribing aspirin rather than anticoagulation based on a perceived high bleeding risk is likely to result in poorer outcomes, as the former is not necessarily safer in regards to bleeding risk but confers a much lower degree of protection against stroke.
Conversely, one could argue that bleeding rates in prospective studies or registries that require written informed consent for participation and are less likely to enroll the more acutely ill or frail patients are not representative of real-life clinical practice. It is worth remembering that the risk of both hemorrhage and stroke are highest when AF is newly diagnosed and during the initiation of anticoagulation medication, and therefore clinical trials designed to evaluate the safety and efficacy of new anticoagulant drugs should include patients without prior exposure to anticoagulation. As major bleeding events, especially intracranial bleeds, may be devastating when occurring, physicians should be able to accurately estimate the bleeding risk of their patients. This task is not straightforward, as many of the known risk factors for bleeding overlap with stroke risk factors. Bleeding risk factors include advanced age, uncontrolled hypertension, history of myocardial infarction or ischemic heart disease, cerebrovascular disease, anemia or a history of bleeding, renal dysfunction, and the concomitant use of other drugs such as antiplatelet agents. To date, several risk scores have been developed to estimate bleeding risk in AF patients [17,74–76], and the use of the HAS-BLED score [17] is recommended by international guidelines. Despite the limitations of these risk stratification schemes (wide variation in the proportion of patients considered to be low, intermediate and high risk, a lack of common definition of major bleeding, different lengths of follow-up and modest areas under the curve in the prediction of bleeding), they offer a starting point for physicians to consider bleeding when initiating oral anticoagulation and, importantly, to think about correctable risk factors, such as hypertension, renal dysfunction (to a degree), unstable INRs and concomitant aspirin or nonsteroidal anti inflammatory drugs.
It should be noted that randomized studies assessing the efficacy and safety of the new oral anticoagulants, such as dabigatran [77], rivaroxaban [78] and apixaban [79], suggested these could eventually associate to a lower risk of bleeding compared to warfarin. An indirect comparison analysis of dabigatran (2 doses), apixaban and rivaroxaban for their relative efficacy and safety against each other did not find profound differences in efficacy between them, although dabigatran 150 mg BID was superior to rivaroxaban for some efficacy endpoints (such as a composite of stroke and systemic embolism), whereas major bleeding was significantly lower with dabigatran 110 mg or apixaban [80]. Future studies may eventually confirm or refute these preliminary findings.
In conclusion, although bleeding complications can be devastating, most patients with a high CHA2DS2-VASC score benefit from oral anticoagulation even if their bleeding risk is high, given their much higher absolute risk of stroke compared to hemorrhage. Only in rare patients with a relatively low stroke risk and a very high risk of bleeding may the withholding of oral anticoagulation be considered. The HAS-BLED model may help identify those individuals at highest bleeding risk where prevention measures should be applied, including a better blood pressure control, more regular INR measurements (or eventually the use of one of the new oral anticoagulants) and avoidance of antiplatelet drugs.
Patients with asymptomatic atrial fibrillation: if we do not detect it, how can we treat it?
Research has shown that, even in patients with documented symptomatic AF, asymptomatic recurrences are common [81]. In fact, asymptomatic arrhythmia events occur more frequently than symptomatic ones in patients with paroxysmal AF, with up to 90% of paroxysmal AF episodes being asymptomatic [81,82]. Among patients with AF-related stroke, the cerebrovascular event may be the first symptom of AF in almost one fourth of the cases. Although one could speculate that transient asymptomatic episodes of AF could be less dangerous than sustained ones, several studies suggested the annual risk of stroke in those with paroxysmal AF is similar to that of patients with permanent AF, and subclinical/asymptomatic atrial tachyarrhythmias are independently associated with higher risk of ischemic stroke and systemic embolism [83,84]. In patients with history of shortness of breath, palpitations, syncope, chest discomfort or cerebrovascular event, manual pulse palpation should be performed to determine the presence of an irregular pulse that could indicate underlying AF. An electrocardiogram should then be performed on all patients, regardless of symptomatic status, in whom a diagnosis of AF is suspected based on the detection of an irregular pulse. Hobbs et al. had ECGs performed in 177 asymptomatic individuals with risk factors for AF, an irregular pulse and no known history of AF and concluded that 17.5% were in AF [85]. Insights from the Royal College of Physicians of Edinburgh (RCPE) UK Consensus Conference on the management of AF included the need for appropriate detection and thromboprophylaxis of AF for the prevention of stroke, with emphasis on screening for AF in people aged 65 years or older in the context of a wider national screening program [86]. Where paroxysmal AF is suspected, including after ischemic stroke or transient ischemic attack, longer ECG monitoring periods (at least 24 h) or event recorders should be used, which is pertinent given the high incidence of asymptomatic AF paroxysms and the fact that AF can be found in one in 20 patients presenting with an acute ischemic stroke [86,87]. Different screening methods include the interrogation of devices implanted for different reasons, such as pacemakers and defibrillators, or the implantation of loop recorders when the identification of AF will potentially have a major clinical impact. A novel algorithm analyzing signals recorded using an iPhone 4S accurately distinguished pulse recordings during AF from sinus rhythm [88].
In conclusion, physicians should be alerted to the fact that asymptomatic AF carries a similar risk of thromboembolic events to symptomatic AF. The identification of subclinical AF through a comprehensive screening program or using ECG monitoring systems is of paramount value in the prevention of AF-related complications.
Pitfalls of warfarin: is it worth the trouble?
Anticoagulation is a life-saving treatment for many patients with AF. Warfarin has been the only option for decades, but more recently several new oral anticoagulants have become available. Whether these allegedly safer options will have a major impact in the management of AF still remains to be determined. However, their promises of added convenience seem to be well-founded. Food does not influence their metabolism, drug–drug interactions are uncommon, predictable anticoagulant effect is achieved with fixed doses without the need for routine laboratory monitoring and, because of their rapid onset of action, parenteral anticoagulant bridging therapy is not necessary when initiating anticoagulation. The so-called “pitfalls” of warfarin may partly account for a lower than optimal rate of prescription of anticoagulation in AF patients who otherwise would be entitled to anticoagulation treatment. Due to the marked inter-individual dose response and day-to-day variation in dose response within individuals, treatment with warfarin requires dose adjustment with measurement of the INR. Several studies indicate a clear association between a low time in therapeutic range (TTR) and an increased risk of vascular events and major hemorrhage in patients on Warfarin [89–91]. A meta-regression analysis of recently published studies reported a mean TTR in all studies of 64% [92], although a wide variation was seen, occasionally with TTRs as low as 29%. A marked benefit against stroke and total vascular events for patients on warfarin treated at centers with mean TTRs above 65% has been documented; [91] no apparent benefit was found for patients treated at centers achieving mean TTRs below 65%. Their findings suggested a threshold TTR (65%) below which the benefit of warfarin over clopidogrel plus aspirin is questionable [91] (Fig. 3). Practices, centers, and regions need to assess the TTR achieved in their own patients and to set a minimum target TTR of 60–65%. The use of warfarin when the TTR is considerably lower than 65% is probably detrimental. The use of anticoagulation clinics and computerized decision support algorithms has been shown to improve TTR [93,94].
Figure 3.

Cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death for patients treated at centers with a time in therapeutic range (TTR) below or above 65%, according to Connolly SJ and associates, Circulation 2008 [Ref. [91]].
The choice between warfarin and one of the new oral anticoagulants is not straightforward. Table 2 includes a list of recommendations that may help in the decision-making process.
Table 2.
General recommendations regarding the choice between warfarin and one of the new oral anticoagulants, according to European guidelines [95].
| Patients who are stable on warfarin and whose INR values are mostly in the therapeutic range need not be switched |
| Patients who are noncompliant with warfarin should not be switched to the new agents because missed doses of these shorter acting anticoagulants can be more detrimental than missed doses of warfarin |
| Patients with valvular AF or mechanical heart valves, with significant hepatic dysfunction or a creatinine clearance below 30 mL/min should receive warfarin |
| If one of the new oral anticoagulants is prescribed in patients with moderate renal dysfunction, rivaroxaban or apixaban seem better choices given their lower degree of renal excretion when compared to dabigatran |
| As gastrointestinal bleeding seems more common with dabigatran and rivaroxaban than with warfarin, patients with a recent history of gastrointestinal bleeding and a moderate to high risk of AF-related stroke should be given apixaban or warfarin. If the latter is prescribed, more frequent INR measurements (every two weeks) should be recommended |
| Dyspepsia occurs in up to 10% of patients on dabigatran, so patients with upper gastrointestinal complaints (other than bleeding) may do better on apixaban, rivaroxaban or warfarin |
| In patients who have suffered an ischemic stroke on warfarin, dabigatran 150 mg twice daily may be considered |
| Patients at lower risk for stroke but nonetheless with indication for oral anticoagulation are better suited for dabigatran or apixaban, as such patients were not included in the ROCKET-AF trial |
| When one expects the potential need to reverse the anticoagulant effect, such as in patients scheduled for AF ablation, the lack of a specific antidote for any of the new oral anticoagulants may be of concern for some operators |
Legends: AF, atrial fibrillation; ROCKET-AF trial, Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation trial.
In conclusion, treatment with Warfarin should not be precluded on the basis of its long-known pitfalls. Instead, one should not forget the unequivocal impact of anticoagulation in the prevention of AF-related stroke. Setting a minimum target TTR of at least 65%, eventually with the use of anticoagulation clinics and computerized decision support algorithms, or prescribing one of the new oral anticoagulants in patients with highly variable/unstable INRs should be strongly considered.
Conclusion
Atrial fibrillation is a major public health issue. Oral anticoagulants are effective in preventing its most severe complication, stroke. However, extensive evidence suggests this therapy remains underused. Patients’ age per se, their comorbidity burden, the perceived risk of falls and bleeding, the difficulty in achieving stable INR control and high time in therapeutic range, a sub-optimal identification of asymptomatic patients with atrial fibrillation, and occasionally insufficient education regarding the benefit of anticoagulation in these patients are known barriers to the prescription of anticoagulation, and eligible patients may fail to receive treatment because of faulty judgments. Physicians need continuously better education on the management of oral anticoagulation and to be aware of the importance of atrial fibrillation and the devastating consequences of not treating it adequately.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Ezekowitz M.D. Atrial fibrillation: the epidemic of the new millennium. Ann Intern Med. 1999;131(7):537–538. doi: 10.7326/0003-4819-131-7-199910050-00011. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Zarifis J., Beevers G., Lip G.Y. Acute admissions with atrial fibrillation in a British multiracial hospital population. Br J Clin Pract. 1997;51(2):91–96. [PubMed] [Google Scholar]
- 4.Stewart S., MacIntyre K., MacLeod M.M., Bailey A.E., Capewell S., McMurray J.J. Trends in hospital activity, morbidity and case fatality related to atrial fibrillation in Scotland, 1986–1996. Eur Heart J. 2001;22(8):693–701. doi: 10.1053/euhj.2000.2511. [DOI] [PubMed] [Google Scholar]
- 5.Carrol K., Majeed A. Trends in mortality and hospital admissions associated with atrial fibrillation in England and Wales. Health Stat Quart. 2001;9:37–44. [PubMed] [Google Scholar]
- 6.Ryder K.M., Benjamin E.J. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1998;84:131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 7.Majeed A., Moser K., Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2001;86(3):284–288. doi: 10.1136/heart.86.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wann L.S., Curtis A.B., Ellenbogen K.A., Estes N.A., Ezekowitz M.D., Jackman W.M. ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on Dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:1144–1150. doi: 10.1161/CIR.0b013e31820f14c0. [DOI] [PubMed] [Google Scholar]
- 9.Friberg L., Hammar N., Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31(8):967–975. doi: 10.1093/eurheartj/ehn599. [DOI] [PubMed] [Google Scholar]
- 10.Page R.L., Tilsch T.W., Connolly S.J., Schnell D.J., Marcello S.R., Wilkinson W.E. Asymptomatic or “silent” atrial fibrillation: frequency in untreated patients and patients receiving azimilide. Circulation. 2003;107(8):1141–1145. doi: 10.1161/01.cir.0000051455.44919.73. [DOI] [PubMed] [Google Scholar]
- 11.Glazer N.L., Dublin S., Smith N.L., French B., Jackson L.A., Hrachovec J.B. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167(3):246–252. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
- 12.Holt T.A., Hunter T.D., Gunnarsson C., Khan N., Cload P., Lip G.Y. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract. 2012;62(603):e710–e717. doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch D.L., O’Neill K., Jackson J., McMahon A., Rumley A., Wallace I. Are atrial fibrillation guidelines altering management? A community based study. Scott Med J. 2005;50(4):166–169. doi: 10.1177/003693300505000409. [DOI] [PubMed] [Google Scholar]
- 14.Waldo A.L., Becker R.C., Tapson V.F., Colgan K.J. NABOR Steering Committee. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence (NICE). Costing report: implementing NICE guidance in England. Atrial fibrillation: the management of atrial fibrillation. NICE Clinical Guideline 36. London, UK: National Institute for Health and Clinical Excellence, 2006.
- 16.Cowan C., Healicon R., Robson I., Long W.R., Barrett J., Fay M. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart. 2013;99(16):1166–1172. doi: 10.1136/heartjnl-2012-303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannel W.B., Wolf P.A., Benjamin E.J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S., Hart C.L., Hole D.J., McMurray J.J. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86(5):516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruigómez A., Johansson S., Wallander M.A., Rodríguez L.A. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol. 2002;55(4):358–363. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 20.Krijthe B.P., Kunst A., Benjamin E.J., Lip G.Y., Franco O.H., Hofman A. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyasaka Y., Barnes M.E., Gersh B.J., Cha S.S., Bailey K.R., Abhayaratna W.P. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. E498–E498. [DOI] [PubMed] [Google Scholar]
- 22.Naccarelli G.V., Varker H., Lin J., Schulman K.L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Lip G.Y., Brechin C.M., Lane D.A. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142(6):1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S., Murphy N.F., Walker A., McGuire A., McMurray J.J. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90(3):286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luengo-Fernandez R., Yiin G.S., Gray A.M., Rothwell P.M. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke. 2013;8(5):308–314. doi: 10.1111/j.1747-4949.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1) doi: 10.1161/CIR.0b013e31823ac046. e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckard N., Davidson T., Walfridsson H., Levin L.A. Cost-effectiveness of catheter ablation treatment for patients with symptomatic atrial fibrillation. J Atr Fibrillation. 2009;1(8):461–470. doi: 10.4022/jafib.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds M.R., Zimetbaum P., Josephson M.E., Ellis E., Danilov T., Cohen D.J. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2(4):362–369. doi: 10.1161/CIRCEP.108.837294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunch T.J., May H.T., Bair T.L., Weiss J.P., Crandall B.G., Osborn J.S. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. 2013;10(9):1272–1277. doi: 10.1016/j.hrthm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Saad E.B., d’Avila A., Costa I.P., Aryana A., Slater C., Costa R.E. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ⩽3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011;4(5):615–621. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- 31.Bunch T.J., Crandall B.G., Weiss J.P., May H.T., Bair T.L., Osborn J.S. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(8):839–845. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher A.M., Rietbrock S., Plumb J., van Staa T.P. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X., Zhang S., Li Y., Detrano R.C., Chen K., Li X. Association of obesity and atrial fibrillation among middle-aged and elderly Chinese. Int J Obes (Lond) 2009;33(11):1318–1325. doi: 10.1038/ijo.2009.157. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z., Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18(5):209–216. doi: 10.2188/jea.JE2008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchiyama S., Shibata Y., Hirabayashi T., Mihara B., Hamashige N., Kitagawa K. Risk factor profiles of stroke, myocardial infarction, and atrial fibrillation: a Japanese Multicenter Cooperative Registry. J Stroke Cerebrovasc Dis. 2010;19(3):190–197. doi: 10.1016/j.jstrokecerebrovasdis.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Research Group for Antiarrhythmic Drug Therapy Survey of atrial fibrillation and thromboembolism in the elderly: a multicenter cooperative study. J Cardiol. 1999;33(1):27–35. [Article in Japanese] [PubMed] [Google Scholar]
- 37.Ogilvie I.M., Newton N., Welner S.A., Cowell W., Lip G.Y. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645. doi: 10.1016/j.amjmed.2009.11.025. e4. [DOI] [PubMed] [Google Scholar]
- 38.Mant J., Hobbs F.D., Fletcher K., Roalfe A., Fitzmaurice D., Lip G.Y. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 39.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 40.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 41.Fang M.C., Go A.S., Chang Y., Borowsky L., Pomernacki N.K., Singer D.E. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51(8):810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 43.Hijazi Z., Oldgren J., Andersson U., Connolly S.J., Ezekowitz M.D., Hohnloser S.H. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125(13):1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 44.Ederhy S., Di Angelantonio E., Dufaitre G., Meuleman C., Masliah J., Boyer-Chatenet L. C-reactive protein and transesophageal echocardiographic markers of thromboembolism in patients with atrial fibrillation. Int J Cardiol. 2012;159(1):40–46. doi: 10.1016/j.ijcard.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Providência R., Paiva L., Faustino A., Botelho A., Trigo J., Casalta-Lopes J. Cardiac troponin I: prothrombotic risk marker in non-valvular atrial fibrillation. Int J Cardiol. 2013;167(3):877–882. doi: 10.1016/j.ijcard.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 46.Eikelboom J., Hijazi Z., Oldgren J., Andersson U., Connolly S.J., Ezekowitz M.D. D-dimer is prognostic for stroke, major bleeding and death during anticoagulation of Atrial Fibrillation – a RELY substudy. Circulation. 2010;122:A18321. [Google Scholar]
- 47.Aulin J.K., Ezekowitz M.D., Andersson U., Connolly S.J., Huber K., Reilly P.A. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(14s1):E91. doi: 10.1016/j.ahj.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa K., Hirai T., Takashima S., Fukuda N., Ohara K., Sasahara E. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107(6):912–916. doi: 10.1016/j.amjcard.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 49.Apostolakis S., Guo Y., Lane D.A., Buller H., Lip G.Y. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34(46):3572–3579. doi: 10.1093/eurheartj/eht328. [DOI] [PubMed] [Google Scholar]
- 50.The Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med. 1992;116(1):6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- 51.Osranek M., Bursi F., Bailey K.R., Grossardt B.R., Brown R.D., Jr, Kopecky S.L. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26(23):2556–2561. doi: 10.1093/eurheartj/ehi483. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.H., Choi S., Chung W.J., Byun Y.S., Ryu S.K., Pyun W.B. Tissue Doppler index, E/E′, and ischemic stroke in patients with atrial fibrillation and preserved left ventricular ejection fraction. J Neurol Sci. 2008;271(1–2):148–152. doi: 10.1016/j.jns.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Shin H.W., Kim H., Son J., Yoon H.J., Park H.S., Cho Y.K. Tissue Doppler imaging as a prognostic marker for cardiovascular events in heart failure with preserved ejection fraction and atrial fibrillation. J Am Soc Echocardiogr. 2010;23(7):755–761. doi: 10.1016/j.echo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Azemi T., Rabdiya V.M., Ayirala S.R., McCullough L.D., Silverman D.I. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J Am Soc Echocardiogr. 2012;25(12):1327–1332. doi: 10.1016/j.echo.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Ayirala S., Kumar S., O’Sullivan D.M., Silverman D.I. Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr. 2011;24(5):499–505. doi: 10.1016/j.echo.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Kleemann T., Becker T., Strauss M., Schneider S., Seidl K. Prevalence and clinical impact of left atrial thrombus and dense spontaneous echo contrast in patients with atrial fibrillation and low CHADS2 score. Eur J Echocardiogr. 2009;10(3):383–388. doi: 10.1093/ejechocard/jen256. [DOI] [PubMed] [Google Scholar]
- 57.Puwanant S., Varr B.C., Shrestha K., Hussain S.K., Tang W.H., Gabriel R.S. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol. 2009;54(22):2032–2039. doi: 10.1016/j.jacc.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 58.Doukky R., Khandelwal A., Garcia-Sayan E., Gage H. External validation of a novel transthoracic echocardiographic tool in predicting left atrial appendage thrombus formation in patients with nonvalvular atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2013;14(9):876–881. doi: 10.1093/ehjci/jes313. http://dx.doi.org/10.1093/ehjci/jes313. [DOI] [PubMed] [Google Scholar]
- 59.Providência R., Faustino A., Paiva L., Trigo J., Botelho A., Nascimento J. Cardioversion safety in patients with nonvalvular atrial fibrillation: which patients can be spared transesophageal echocardiography? Blood Coagul Fibrinolysis. 2012;23(7):597–602. doi: 10.1097/MBC.0b013e3283562d4f. [DOI] [PubMed] [Google Scholar]
- 60.Zabalgoitia M., Halperin J.L., Pearce L.A., Blackshear J.L., Asinger R.W., Hart R.G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1998;31(7):1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 61.The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128(8):639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 62.Providência R., Trigo J., Paiva L., Barra S. The role of echocardiography in thromboembolic risk assessment of patients with nonvalvular atrial fibrillation. J Am Soc Echocardiogr. 2013;26(8):801–812. doi: 10.1016/j.echo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 63.Providência R., Barra S., Paiva L. The role of echocardiography as a predictor of the incidence and progression of atrial fibrillation. J Atr Fibrillation. 2012;5(3):27–36. doi: 10.4022/jafib.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rash A., Downes T., Portner R., Yeo W.W., Morgan N., Channer K.S. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO) Age Ageing. 2007;36(2):151–156. doi: 10.1093/ageing/afl129. [DOI] [PubMed] [Google Scholar]
- 65.van Walraven C., Hart R.G., Connolly S., Austin P.C., Mant J., Hobbs F.D. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40(4):1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 66.King D.E., Dickerson L.M., Sack J.L. Acute management of atrial fibrillation: Part II. Prevention of thromboembolic complications. Am Fam Phys. 2002;66(2):261–264. [PubMed] [Google Scholar]
- 67.Man-Son-Hing M., Nichol G., Lau A., Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159(7):677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 68.Donzé J., Clair C., Hug B., Rodondi N., Waeber G., Cornuz J. Risk of falls and major bleeds in patients on oral anticoagulation therapy. Am J Med. 2012;125(8):773–778. doi: 10.1016/j.amjmed.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 69.Garwood C.L., Corbett T.L. Use of anticoagulation in elderly patients with atrial fibrillation who are at risk for falls. Ann Pharmacother. 2008;42(4):523–532. doi: 10.1345/aph.1K498. [DOI] [PubMed] [Google Scholar]
- 70.Alonso-Coello P., Montori V.M., Solà I., Schünemann H.J., Devereaux P., Charles C. Values and preferences in oral anticoagulation in patients with atrial fibrillation, physicians’ and patients’ perspectives: protocol for a two-phase study. BMC Health Serv Res. 2008;8:221. doi: 10.1186/1472-6963-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogilvie I.M., Welner S.A., Cowell W., Lip G.Y. Ischaemic stroke and bleeding rates in ‘real-world’ atrial fibrillation patients. Thromb Haemost. 2011;106(1):34–44. doi: 10.1160/TH10-10-0674. [DOI] [PubMed] [Google Scholar]
- 72.Poli D., Antonucci E., Testa S., Tosetto A., Ageno W., Palareti G. Italian Federation of Anticoagulation Clinics. Bleeding risk in very old patients on vitamin K antagonist treatment: results of a prospective collaborative study on elderly patients followed by Italian Centres for Anticoagulation. Circulation. 2011;124(7):824–829. doi: 10.1161/CIRCULATIONAHA.110.007864. [DOI] [PubMed] [Google Scholar]
- 73.Tincani E., Baldini P., Crowther M.A., Zanasi A., Ferrari P., Cenci A.M. Bleeding rates in patients older than 90 years of age on vitamin K antagonist therapy for nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis. 2009;20(1):47–51. doi: 10.1097/MBC.0b013e32831be9da. [DOI] [PubMed] [Google Scholar]
- 74.Gage B.F., Yan Y., Milligan P.E., Waterman A.D., Culverhouse R., Rich M.W. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Beyth R.J., Quinn L.M., Landefeld C.S. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105(2):91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 76.Shireman T.I., Mahnken J.D., Howard P.A., Kresowik T.F., Hou Q., Ellerbeck E.F. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130(5):1390–1396. doi: 10.1378/chest.130.5.1390. [DOI] [PubMed] [Google Scholar]
- 77.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 78.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 79.Granger C.B., Alexander J.H., McMurray J.J., Lopes R.D., Hylek E.M., Hanna M. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 80.Lip G.Y., Larsen T.B., Skjøth F., Rasmussen L.H. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2012;60(8):738–746. doi: 10.1016/j.jacc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 81.Israel C.W., Grönefeld G., Ehrlich J.R., Li Y.G., Hohnloser S.H. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43(1):47–52. doi: 10.1016/j.jacc.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 82.Page R.L., Wilkinson W.E., Clair W.K., McCarthy E.A., Pritchett E.L. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89(1):224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 83.Hart R.G., Pearce L.A., Rothbart R.M., McAnulty J.H., Asinger R.W., Halperin J.L. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke prevention in atrial fibrillation investigators. J Am Coll Cardiol. 2000;35(1):183–187. doi: 10.1016/s0735-1097(99)00489-1. [DOI] [PubMed] [Google Scholar]
- 84.Healey J.S., Connolly S.J., Gold M.R., Israel C.W., Van Gelder I.C., Capucci A. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 85.Hobbs F.D., Fitzmaurice D.A., Mant J., Murray E., Jowett S., Bryan S. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9(40):iii–iv. doi: 10.3310/hta9400. ix–x, 1–74. [DOI] [PubMed] [Google Scholar]
- 86.Lip G.Y., Ramsay S.G. Insights from the RCPE UK consensus conference on approaching the comprehensive management of atrial fibrillation. Expert Rev Cardiovasc Ther. 2012;10(6):697–700. doi: 10.1586/erc.12.48. [DOI] [PubMed] [Google Scholar]
- 87.Liao J., Khalid Z., Scallan C., Morillo C., O’Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38(11):2935–2940. doi: 10.1161/STROKEAHA.106.478685. [DOI] [PubMed] [Google Scholar]
- 88.McManus D.D., Lee J., Maitas O., Esa N., Pidikiti R., Carlucci A. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm. 2013;10(3):315–319. doi: 10.1016/j.hrthm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White H.D., Gruber M., Feyzi J., Kaatz S., Tse H.F., Husted S. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–245. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 90.Jones M., McEwan P., Morgan C.L., Peters J.R., Goodfellow J., Currie C.J. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvular atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91(4):472–477. doi: 10.1136/hrt.2004.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Connolly S.J., Pogue J., Eikelboom J., Flaker G., Commerford P., Franzosi M.G. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 92.van Walraven C., Jennings A., Oake N., Fergusson D., Forster A.J. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129(5):1155–1166. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 93.Fitzmaurice D.A., Hobbs F.D., Murray E.T., Holder R.L., Allan T.F., Rose P.E. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med. 2000;160(15):2343–2348. doi: 10.1001/archinte.160.15.2343. [DOI] [PubMed] [Google Scholar]
- 94.Manotti C., Moia M., Palareti G., Pengo V., Ria L., Dettori A.G. Effect of computer-aided management on the quality of treatment in anticoagulated patients: a prospective, randomized, multicenter trial of APROAT (Automated PRogram for Oral Anticoagulant Treatment) Haematologica. 2001;86(10):1060–1070. [PubMed] [Google Scholar]
- 95.Heidbuchel H., Verhamme P., Alings M., Antz M., Hacke W., Oldgren J. European heart rhythm association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15(5):625–651. doi: 10.1093/europace/eut083. http://dx.doi.org/10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]


