Abstract
Congenital ventricular diverticulum is a rare cardiac malformation. We present the case of a 57-year-old man who underwent cardiac catheterization for suspected unstable angina. No coronary artery disease was diagnosed and a left ventricular diverticulum was incidentally found. Coronary CT and cardiac MRI were performed in order to confirm the diagnosis of a muscular type diverticulum and to exclude a post-ischemic aneurysm.
Keywords: Congenital left ventricle diverticulum, Left ventricular catheterization, Coronary CT, Cardiac MRI
Introduction
Congenital ventricular diverticulum is a rare cardiac malformation, usually diagnosed on routine echocardiography examinations. The increasing use of non invasive techniques such as CT and MRI can help in the evaluation of the myocardial morphology, contraction of the wall and its assessment, and in order to rule out differential diagnoses. We report a case of a patient with incidental finding of left ventricular diverticulum, which was evaluated with various diagnostic imaging methods in order to obtain all data necessary to perform a correct diagnosis.
Informed consent was obtained from the patient before performing each diagnostic examination.
Case report
A 57-year-old male with a localized precordial pain was referred to our cardiology unit.
His clinical history reported repeated episodes of angina-like chest pain symptoms in the recent period, and a significant familiar history of coronary artery disease. Other risk factors for cardiovascular accidents included: metabolic syndrome, smoking habit, and chronic administration of non-steroidal anti-inflammatory drugs due to rheumatoid arthritis. Electrocardiogram (ECG), echocardiographic evaluation, and myocardial creatine kinase value level were normal. However, due to clinical suspicion of unstable angina, a coronary angiography was performed as suggested by the cardiologist. Conventional coronary angiography was performed using an Innova 2100 angiographer with multiple projections (General Electric Medical System, Milwaukee, WI, USA). No stenoses were detected, but a subsequent ventriculography, obtained in 30° right anterior oblique (RAO) projection, revealed a wall appendix characterized by rapid contrast media filling in diastole (Fig. 1A), contracting in synchrony with the ventricle wall (Fig. 1B–D), a narrow neck and no thrombosis in the lumen. Due to its features, the diagnosis of left ventricular congenital diverticulum was hypothesized.
Figure 1.

A 57-year-old male with congenital left ventricular diverticulum. Left ventriculogram obtained in 30° right anterior oblique projection shows an elongated contrast filled outpouching in end-diastolic phase (A), with complete emptying during the systolic phase (B–D), arising from the left ventricular apex (white arrows). This was indicative of cardiac diverticulum.
The patient underwent a coronary CT to exclude the possible post-ischemic nature of the wall appendix. The examination was performed using a 64-slice scanner (LightSpeed VCT, General Electric Medical System, Milwaukee, WI, USA) with a retrospective synchronization technique. A dose of 80 ml of non-ionic iodinated contrast medium and 40 ml of saline solution were administered at a rate of 5 ml/s. Parameters for the contrast-enhanced scan were set as follows: beam collimation 64 × 0.625 mm; slice thickness 0.625 mm; reconstruction increment 0.625 mm; table feed 2.9 mm/rotation; tube rotation 0.35 s; tube voltage 120 kV; intensity 400–650 mA (automatic dose modulation); D-FOV 25 cm; and S-FOV cardiac small, cranium-caudal scan direction. Scan duration was 5.5 s. Image reconstruction was obtained using multiple temporal windows from 40% to 80% of the cardiac cycle, corresponding to the R–R interval or mid- to end-diastole. The coronary CT scan confirmed the presence of the appendix similar to a ventricular diverticulum of about 12 × 7 mm during the cardiac cycle with no signs of atheromatous disease in coronary arteries (Fig. 2A–F).
Figure 2.
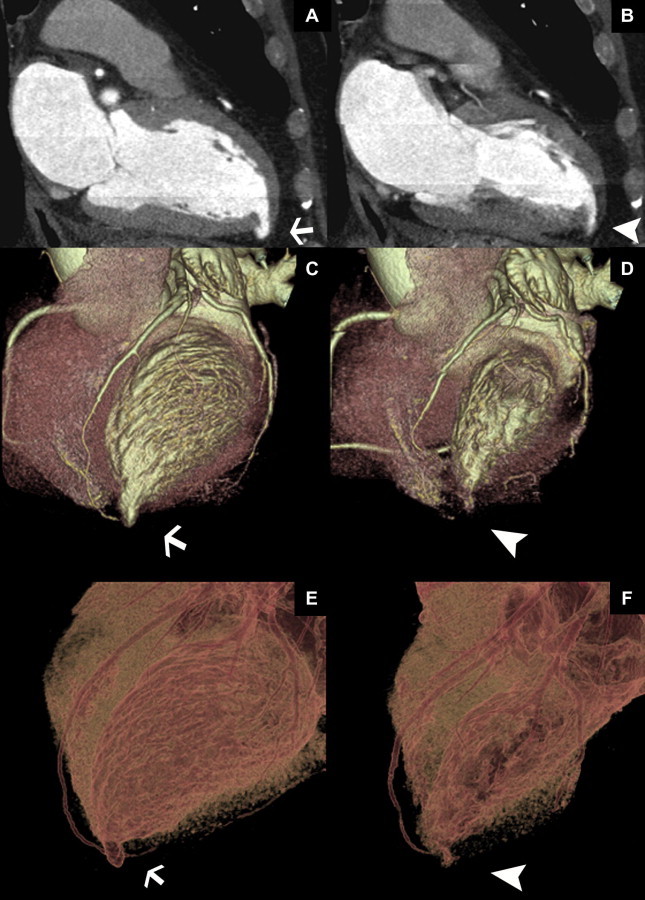
A 57-year-old male with a congenital left ventricular diverticulum. Coronary CT images from 64 row multidetector scanner. Multiplanar reformatted (A, B); volume rendering reconstruction (C, D); and 3D transparency of heart images (E, F) showed the diverticulum located on the apical wall of the left ventricle chamber, which is contracting in synchrony with the ventricle wall (B, D, F). The diverticulum was studied in the diastole, which presented dimensions of about 12 × 7 mm (white arrows) (A, C, E) and during its contraction in systolic phase (arrowheads) (B, D, F). Protocol: 64 slice, LightSpeed VCT, General Electric Medical System, intensity 400–650 mA; slice thickness 0.625 mm, 100 kV, D-FOV 25 cm; 80 ml Iomeron 400, Bracco, Milan, Italy.
In order to study the morphologic characteristics of the congenital malformation and its kinetic features, a contrast-enhanced cardiac magnetic resonance imaging (MRI) was performed (Fig. 3A–F) using a 1.5 T whole body scanner (Gyroscan Intera, Philips Medical Systems, Best, The Netherlands). A 5-element cardiac phased-array receiver surface coil was used for signal reception. A retrospective ECG triggered balanced-TFE (Steady State Free Precession, SSFP) sequence was performed to evaluate left ventriculum (LV) myocardium thickness, kinetic and parietal segmental and global contractility. The sequences were oriented on the short and long axis (atrium-ventricular and four-chamber axis), with the following parameters: TR 3.8 ms; TE 1.8 ms; FA 70°; matrix scan 256 × 256; FOV 400; thickness 10 mm; gap 0 mm; 20 cardiac phases per cycle; and retrospective synchronization. As shown in Fig. 3, the morphology of the lesion was similar to a LV diverticulum characterized by a thinned but contractile wall. We subsequently performed black blood T2 weighted sequences with and without fat saturation (STIR) on the short axis and on the four-chamber axis to assess myocardium signal alterations (Fig. 3F). Technical parameters were: TR 1333 ms; TE 100 ms; TI 290 ms; EPI factor 1; turbo factor 33; matrix scan 512 × 512; slice thickness 8 mm; gap 0.8 mm; prospective diastolic synchronization; and 1 slice per breath-hold. Finally, 15 ml of Gadolinium-DTPA were administrated and a 3D inversion recovery TFE T1 weighted sequence with a delay of 15 min from the injection was performed to evaluate fibrotic tissue in the myocardium (Fig. 3E) (TR 4.6 ms; TE 1.4 ms; FA 15°; matrix scan 256 × 256; FOV 400; slice thickness 14 mm; gap 7 mm; and TI 290 ms). Delayed enhancement images showed no signs of necrosis or fibrous tissue in the circumferential wall of the diverticulum. The cardiac functional parameters were: ejection fraction 60.2%; stroke volume 41.4 ml; cardiac output 3.6 l/min; end-diastolic volume 102.12 ml; end-systolic volume 40.69 ml; and end-diastolic wall mass 97.35 g.
Figure 3.
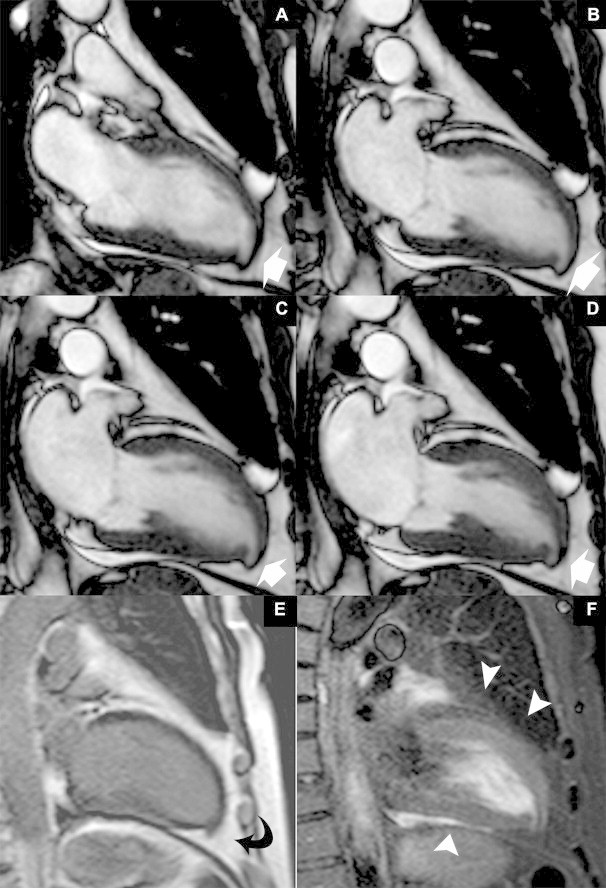
A 57-year-old male with a congenital left ventricular diverticulum. Vertical long axis balanced-TFE images in different cardiac cycle phases (A–D) showed the apical diverticulum with thinned and contractile wall, clearly evident with MRI, associated with the absence of focal lesions in the remaining myocardial tissue (white arrows) (E). Inversion recovery 3D TFE T1, ECG-retrospective synchronization, performed 15 min after contrast media injection showed no intra-myocardial hyper-enhancement area and the presence of myocardial tissue in the outpouching (curved arrow); this confirmed the congenital nature of the diverticulum (F). The absence of hyperintensity in the black blood T2 weighted sequences with fat saturation (STIR) in the myocardium tissue suggests that there is no myocardial edema (arrowheads); 1.5 T magnetic resonance imaging. Protocol: TR 1333 ms, TE 100 ms, TI 290 ms, EPI factor 1, turbo factor 33, matrix scan 512 × 512, slice thickness 8 mm, gap 0.8 mm, diastolic perspective synchronization; 15 ml Dotarem® 0.5 mmol/ml, Guerbet, Villepinte, France.
A gastroscopy was performed showing a peptic ulcer localized at the pyloric antrum. An anti-acid therapy was administered, and the chest pain was resolved.
Discussion
Congenital ventricular diverticulum is a rare cardiac malformation and its first description in the literature dates back to 1816 [1]. In adulthood, it is reported in about 0.4% of cardiac death autopsies, and about 0.26% in non selected patients who have undergone cardiac catheterization [1–5]. Patients are usually asymptomatic. Congenital ventricular diverticulum may cause systemic embolization, fatal ventricular arrhythmias, and sudden death due to ventricle rupture [6–8].
This malformation is characterized by a finger or hook-like appendix of the entire left ventricular wall, beyond the myocardial margin, frequently localized in the left ventricle. It can be differentiated into two types: muscular or fibrous [2,8–11]. The muscular type is more frequent and not prone to rupture [10,12]. It is usually associated with other congenital malformations, particularly with Cantrell’s syndrome [9,13–15]. The fibrous diverticulum is usually located either in the base of the heart or in the subvalvular area, leading to possible aortic or mitral regurgitation. It is characterized by a fibrous layer, a narrow neck, no contractile function, a tendency to rupture and is not associated with other malformations [2,10,12]. Differential diagnosis may include true LV aneurysm and LV pseudoaneurysm (Table 1). LV aneurysm has been strictly defined as a distinct area of abnormal left ventricular diastolic contour with systolic dyskinesia or paradoxical bulging. This pathologic condition involves bulging of the full thickness of the left ventricular wall with thinned fibrous tissue (scar) as remnant of the left ventricular muscle [10,12,14,16–18].
Table 1.
Differential diagnoses of congenital left ventricle diverticulum.
| Left ventricular catheterization | Coronary-CT | Cardiac-MRI | |
|---|---|---|---|
| Congenital left ventricular muscular type diverticulum | Wall appendix with rapid contrast media filling, narrow neck, volume reduction during systolic phase and volume increase during diastolic phase | Appendix contracting in synchrony with the ventricle wall, presence of myocardial tissue on the diverticulum, homogeneous myocardial tissue density in the ventricle chamber | Diverticulum with thinned but contractile wall, no signal alterations of the left ventricular wall and no signs of necrosis or fibrous tissue on delayed enhancement images |
| Congenital left ventricular fibrous type diverticulum | Wall appendix with rapid contrast media filling, narrow neck, and no volume change during the different cardiac cycle phases | Appendix with no volume change during the cardiac cycle, presence of fibrous tissue on the diverticulum wall, homogeneous myocardial tissue density in the ventricle chamber | Diverticulum characterized by thinned and fibrous wall, no volume change during the cardiac cycle, no signal alterations of the remaining left ventricular wall with no signs of necrosis or fibrous tissue on delayed enhancement images |
| Left ventricular aneurysm | Wall appendix with rapid contrast media filling, usually with a wide neck and no volume change during the different cardiac cycle phases | Appendix with no volume change during the cardiac cycle, presence of fibrous tissue on the wall, hypodensity on the myocardial tissue related to past ischemic attack, associated coronary artery disease | Area of high signal intensity on delayed enhancement MR images on the aneurysm wall related to the presence of scar or fibrosis, lack of contraction of the myocardial tissue |
| Left ventricular pseudo-aneurysm | Differential diagnosis with aneurysm is not possible | Appendix with no volume change during the cardiac cycle; pseudoaneurysm wall is < 5 mm composed by hypodense fibrous tissue (pericardium); hypodensity can be also related to thrombosis usually present in the wall balloon | Absent myocardial wall in the site of the recent myocardial infarction, replaced by pericardium. Significant enhancement in the surrounding myocardial tissue due to acute myocyte necrosis |
False aneurysms of the left ventricle or left ventricle pseudoaneurysms may be caused by acute contained rupture of the ventricle wall, often after myocardial infarction, or after circumflex coronary arterial occlusion [10,12,14,16–18]. True LV aneurysm and LV pseudoaneurysm are characterized by a common contractile function, exhibiting either akinesis or dyskinesis [10,12,14,16–18].
ECG alterations remain the first clinical sign. However, several studies report an abnormal ECG in only 56% of all patients, leading to an undiagnosed diverticulum [2]. Coronary angiography is the gold standard in the evaluation of coronary artery disease (CAD). Left ventricular catheterization, as part of the examination, is very useful in assessing the morphology and dynamics of the left ventricular chamber.
This heart malformation is characterized by a rapid contrast media filling and a narrow neck [12] (Fig. 1A-D). Multidetector row CT is a non-invasive tool with a high spatial resolution able to exclude CAD, to identify the composition of an eventual plaque and to evaluate cardiac structures besides the coronary arteries [9] (Fig. 2A–F). A further diagnostic evaluation with cardiac MRI (Fig. 3A–F) was performed with the purpose of providing accurate tissue characterization without ionizing radiation exposition, making possible the detection of the presence of myocardial edema, fibrous and necrotic tissue inside the ventricular diverticulum [6,19,20]. In the case of a cardiac diverticulum, a thinned but contractile wall is present, associated to no signal alterations of the left ventricular wall, and no signs of necrosis or fibrous tissue on delayed enhancement images. The cardiac MRI is a fundamental tool for the performance of a differential diagnosis with true aneurysms and left ventricle pseudoaneurysm. In the case of a true aneurysm, the wall of the aneurysm shows delayed enhancement, indicating scar tissue as a result of infarcted myocardial muscle. A pseudoaneurysm is composed only of pericardium and does not show delayed enhancement within the sac, while the border of the aneurysm shows enhancement that indicates a peri-aneurysmal infracted area [16].
The treatment of a congenital left diverticulum is still undefined. If the diverticulum is small and asymptomatic, especially in a muscular type diverticulum as in our case, a conservative treatment with follow-up is often recommended. When symptoms appear, medical or surgical treatment should be started immediately [10,12,14,15].
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Kreysig F. Über die Zufälle und Unterscheidungsmerkmale der Verdickung, Verdünnung und Mürbheit des Herzens. In: Kreysig F (Hrsg). Die Krankheiten des Herzens. Zweiter Theil, zweite Abtheilung. Berlin: Maurer’sche Buchhandlung; 1816. p. 464.
- 2.Ohlow M.A., Lauer B., Geller J.C. Prevalence and spectrum of abnormal electrocardiograms in patients with an isolated congenital left ventricular aneurysm or diverticulum. Europace. 2009;11(12):1689–1695. doi: 10.1093/europace/eup323. [DOI] [PubMed] [Google Scholar]
- 3.Yazici M., Demircan S., Durna K., Yasar E. Left ventricular diverticulum in two adult patients. Int Heart J. 2005;46(1):161–165. doi: 10.1536/ihj.46.161. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa K., Makino K., Futagami Y., Fujioka H., Ito M., Hamada M. Isolated congenital left ventricular diverticulum in an adult. A case report. Angiology. 1994;45(8):743–747. doi: 10.1177/000331979404500811. [DOI] [PubMed] [Google Scholar]
- 5.Ohlow M.A. Congenital left ventricular aneurysms and diverticula: definition, pathophysiology, clinical relevance and treatment. Cardiology. 2006;106(2):63–72. doi: 10.1159/000092634. [DOI] [PubMed] [Google Scholar]
- 6.Aguaro G.D., Di Bella G., Strata E., Deiana M., De Marchi D., Pingitore A. Cardiac magnetic resonance findings in isolated congenital left ventricular diverticuli. Int J Cardiovasc Imaging. 2007;23(1):43–47. doi: 10.1007/s10554-006-9120-9. [DOI] [PubMed] [Google Scholar]
- 7.Walton-Shirley M., Smith S.M., Talley J.D. Left ventricular diverticulum: case report and review of the literature. Cathet Cardiovasc Diagn. 1992;26(1):31–33. doi: 10.1002/ccd.1810260108. [DOI] [PubMed] [Google Scholar]
- 8.Archbold R.A., Robinson N.M., Mills P.G. Long-term follow-up of a true contractile left ventricular diverticulum. Am J Cardiol. 1999;83(5):810–813. doi: 10.1016/s0002-9149(98)01003-0. A11. [DOI] [PubMed] [Google Scholar]
- 9.Erol C., Koplay M., Olcay A., Kivrak A.S., Ozbek S., Seker M. Congenital left ventricular wall abnormalities in adults detected by gated cardiac multidetector computed tomography: clefts, aneurysms, diverticula and terminology problems. Eur J Radiol. 2012;81(11):3276–3281. doi: 10.1016/j.ejrad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Cianciulli T.F., Del Carmen Gonzalez Colaso P., Saccheri M.C., Lax J.A., Redruello H.J., Guerra J.E. Left ventricular diverticulum, a rare echocardiographic finding: two adult patients and review of the literature. Cardiol J. 2009;16(1):76–81. [PubMed] [Google Scholar]
- 11.Wang Z.W., Wu H.B., Mao Z.F., Hu X.P. Congenital left ventricular diverticulum, a rare chest fluoroscopy finding: a case report. Chin Med J (Engl) 2011;124(5):783–786. [PubMed] [Google Scholar]
- 12.Cantrell J.R., Haller J.A., Ravitch M.M. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet. 1958;107(5):602–614. [PubMed] [Google Scholar]
- 13.Yang H., Zhu Q., Chen J., Guo N. Congenital left ventricular diverticulum diagnosed by echocardiography. Pediatr Cardiol. 2012;33(4):646–648. doi: 10.1007/s00246-012-0153-7. [DOI] [PubMed] [Google Scholar]
- 14.Makkuni P., Kotler M.N., Figueredo V.M. Diverticular and aneurysmal structures of the left ventricle in adults: report of a case within the context of a literature review. Tex Heart Inst J. 2010;37(6):699–705. [PMC free article] [PubMed] [Google Scholar]
- 15.Sechtem U., Pflugfelder P.W., Gould R.G., Cassidy M.M., Higgins C.B. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163(3):697–702. doi: 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- 16.Kumbasar B., Wu K.C., Kamel I.R., Lima J.A., Bluemke D.A. Left ventricular true aneurysm: diagnosis of myocardial viability shown on MR imaging. AJR Am J Roentgenol. 2002;179(2):472–474. doi: 10.2214/ajr.179.2.1790472. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford J.D., Braunwald E., Cohn P.E. Chronic ischemic heart disease. In: Braunwald E., editor. Heart disease: a textbook of cardiovascular medicine. WB Saunders; Philadelphia: 1988. p. 1364. [Google Scholar]
- 18.Buckberg G.D. Defining the relationship between akinesia and dyskinesia and the cause of left ventricular failure after anterior infarction and reversal of remodeling to restoration. J Thorac Cardiovasc Surg. 1998;116(1):47–49. doi: 10.1016/s0022-5223(98)70241-7. [DOI] [PubMed] [Google Scholar]
- 19.Kim R.J., Wu E., Rafael A., Chen E.L., Parker M.A., Simonetti O. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 20.Srichai M.B., Hecht E.M., Kim D.C., Jacobs J.E. Ventricular diverticula on cardiac CT: more common than previously thought. AJR Am J Roentgenol. 2007;189(1):204–208. doi: 10.2214/AJR.06.1223. [DOI] [PubMed] [Google Scholar]


