Graphical abstract
Keywords: Mitochondria, Permeability transition, Reactive oxygen species, F-ATP synthase, Cancer
Highlights
-
•
The mitochondrial permeability transition pore (PTP) is a key effector in the pathways to cell death.
-
•
The PTP forms from F-ATP synthase and is regulated by several signaling pathways.
-
•
Tumor cells desensitize the PTP to Ca2+ and reactive oxygen species increasing their resistance to death.
-
•
The PTP is a target for anticancer chemotherapeutics.
Abstract
This review covers recent progress on the nature of the mitochondrial permeability transition pore (PTP) – a key effector in the mitochondrial pathways to cell death – and on the adaptive responses of tumor cells that desensitize the PTP to Ca2+ and reactive oxygen species (ROS), thereby playing an important role in the resistance of tumors to cell death. The discovery that the PTP forms from dimers of F-ATP synthase; and the definition of the Ca2+- and ROS-dependent signaling pathways affecting the transition of the F-ATP synthase from an energy-conserving to an energy-dissipating device open new perspectives for therapeutic intervention in cancer cells.
1. Introduction
The definition of the permeability transition (PT) dates back to almost 40 years ago, when Haworth and Hunter demonstrated reversible opening of a channel in the inner mitochondrial membrane that could be induced by Ca2+, phosphate, arsenate or oleic acid, resulting in matrix swelling [1–4]. In the following years, the permeability transition pore (PTP) was considered an in vitro artifact, and did not receive very much attention from the scientific community, with some notable exceptions (reviewed in [5]). The interest on the PTP dramatically raised when it was discovered that it could be inhibited by a fungal peptide, cyclosporin A (CsA), following its interaction with a mitochondrial chaperone, the peptidyl-prolyl cis-trans isomerase cyclophilin D (CyP-D; [6–11]). In the same period, mitochondria took center stage in the process of cell death, as it became clear that the effector phases of intrinsic apoptotic pathways were triggered by the release of apoptogenic proteins from mitochondria [12–17]. The PTP was proposed to act as an effector in the process of cell death [18]. Indeed, the PTP is a voltage and Ca2+-dependent, CsA-sensitive, high conductance channel, whose prolonged opening leads to a brisk increase in the permeability of the inner mitochondrial membrane to solutes with molecular mass up to 1500 Da [19]. As a consequence, a bioenergetic catastrophe occurs: equilibration of the proton gradient causes mitochondrial depolarization, followed by respiratory inhibition and generation of reactive oxygen species (ROS), massive release of matrix Ca2+, swelling of mitochondria leading to breaches in the outer mitochondrial membrane that induce the release of intermembrane proteins. Thus, PTP opening prompts the demise of the cell [20–23], and its (dys)regulation turned out to be a crucial step in the pathogenesis of a variety of diverse diseases, encompassing ischemia-reperfusion damage [20,24,25], lysosomal storage diseases [26], liver damage [27,28], many acute and chronic disorders of the central nervous system (reviewed in [29], collagen VI myopathies [30] and cancer (reviewed in [31]). Therefore, attempts to comprehend the molecular structure of the pore gained momentum, but the protein composition of the PTP remained an unsolved conundrum until very recently. This review covers recent advances in the definition of the PTP structure, and its involvement in the tumorigenic process.
2. Toward a definition of PTP structure
Electrophysiological experiments have shown a channel, termed the “mitochondrial megachannel” (MMC) in the inner membrane endowed with high-conductance (≈1–1.3 nS) and all the key regulatory features of the PTP: inhibition by CsA, adenine nucleotides, Mg2+, acidic pH, and reducing agents; induction by Ca2+ and voltage sensitivity [32–35]. Taken together, these observations leave little doubt that the MMC is the PTP [36]. Dissection of the protein composition of the PTP was much more complex. At the turning of the century, a multimolecular PTP model was proposed that included proteins of all mitochondrial compartments, as it was postulated that these could aggregate at “contact sites” between the outer and the inner membranes [37]. Following this model, PTP components included CyP-D in the matrix, the adenine nucleotide translocator (ANT) in the inner membrane, the mitochondrial creatine kinase in the intermembrane space, the porin/voltage-dependent anion channel (VDAC) in the outer membrane, the isoform II of hexokinase (HK II) and the peripheral benzodiazepine receptor (PBR, now termed TSPO), together with Bcl-2 family proteins, externally associated on the mitochondrial surface. Despite its popularity, rigorous genetic analyses ruled out the possibility that any of these proteins could be part of the core of the PTP ([38–40]; for detailed reviews on this topic, see [5,22]). However, the same genetic analyses demonstrate that CyP-D is a crucial regulator of the PTP. Indeed, ablation of CyP-D renders the PTP insensitive to CsA and doubles the threshold Ca2+ load required to open the PTP in the presence of phosphate anion (Pi) [41–44]. Notably, CyP-D can undergo several post-translation modifications, including phosphorylation [45], acetylation [46], and nitrosylation [47,48]; CyP-D can also interact with a variety of proteins, such as Bcl-2 [49], the Ser/Thr kinase GSK-3 [45], the chaperones Hsp90, TRAP1 [50] and Hsp60 [51], and the FOF1 ATP synthase [52]. These dynamic changes of CyP-D affect the PTP, providing a remarkable level of flexibility in its modes of regulation.
An alternative model considered that the mitochondrial Pi carrier would form the PTP following its interaction with CyP-D and ANT [53]; however, this model still awaits genetic testing, while results obtained by patch-clamp analysis of the reconstituted Pi carrier do not match with electrophysiological PTP features. It was also proposed that the PTP originates by clusters of misfolded proteins [54], but this theory does not explain the strict PTP regulation by pH, voltage and CsA (discussed in [5,22]).
Recently, our laboratory has proposed that the PTP is formed by dimers of the FOF1 ATP synthase [55]. The mitochondrial ATP synthase is a large multiprotein complex composed by 15 subunits, and it is formed by the catalytic, soluble F1 domain, which protrudes in the matrix, and by the inner membrane FO domain which allows the translocation of protons; the two domains are connected by a lateral and a central stalk [56]. The ATP synthase is a rotary enzyme: in respiring mitochondria rotation of the γ subunit in the central stalk, caused by proton flow across the FO subunit induces conformational changes in the F1 subunit that elicit ATP synthesis, while the lateral stalk acts as a stator [57]. The functional unit of the enzyme is believed to be constituted by ATP synthase dimers, which associate in long rows of oligomers that shape cristae in the inner mitochondrial membrane [57–61].
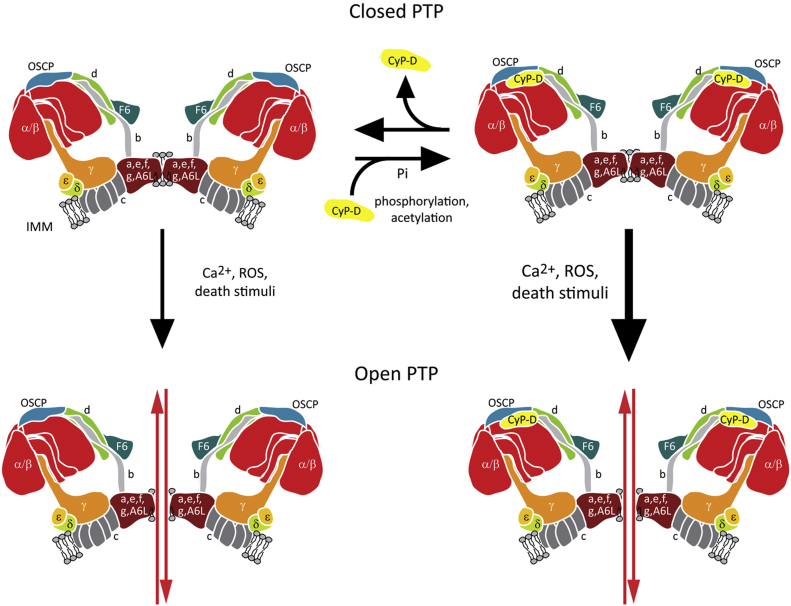
CyP-D interacts with ATP synthase subunits b, d and OSCP, which are part of the lateral stalk of the enzyme; CyP-D interaction requires Pi and partially inhibits ATP synthase activity, which can be reverted by CsA, as it dissociates CyP-D form the enzyme [52]. CyP-D shares the site of OSCP interaction with Bz-423, a compound that sensitizes the PTP to Ca2+, and we could demonstrate that this sensitization is blocked at Pi concentrations that favor the interaction between CyP-D and OSCP, which constitutes a first functional connection between OSCP and the PTP [55]. More direct evidence that the PTP is constituted by ATP synthase, notably in its dimeric form, came from patch-clamp experiments performed on enzyme dimers purified by blue native gel electrophoresis. The measured currents closely matched the features of the MMC channel, and were induced by Bz-423 in a Ca2+-sensitive way [55]. We proposed that the PTP forms at the interface between two adjacent FO subunits (Fig. 1), with OSCP acting as an inhibitor and CyP-D as an inducer, which would lower Ca2+ concentration required to promote PTP opening after substituting Mg2+ binding to the enzyme [5]. In accord with this model, our laboratory has recently demonstrated that purified ATP synthase from yeast forms a channel with properties similar to the mammalian PTP but of somewhat lower conductance; and that ablation of e and g ATP synthase subunits, which are required for dimer formation, leads to a marked resistance to PTP opening [62].
Fig. 1.
Model of PTP structure. The PTP is formed at the interface between two ATP synthase dimers. Phosphorylation and acetylation of CyP-D favor its interaction with ATP synthase and pore opening following increases in Ca2+, ROS or a variety of death stimuli. IMM, inner mitochondrial membrane.
It has been argued that detection of PTP opening in ρ0 cells [63], which lack mitochondrial DNA and therefore ATP synthase subunits a and A6L, speaks against involvement of dimers in pore formation [64,65], but this is an erroneous conclusion based on a misinterpretation of the literature. Wittig et al. [66] have shown that dimers of ATP synthase do form in ρ0 cells, and have concluded that “ATP synthase is almost fully assembled in spite of the absence of subunits a and A6L”. Higher order ATP synthase structures are less stable after digitonin treatment of ρ0 cells; yet, and although their amount was smaller, dimeric and even tetrameric forms of the large assembly intermediate were preserved under the conditions used for clear native electrophoresis, suggesting to the Authors that the ATP synthase associated further into higher order structures in the mitochondrial membrane [66].
Others have found that PTP induction by Ca2+ and oxidants is related to the expression level of the c subunit of ATP synthase. The c subunit forms a ring-shaped oligomer in the FO subunit that is directly involved in proton flux, and it was shown that its transient depletion by RNA interference prevents PTP opening, while its overexpression strongly induces it [67]. This study does not bear on the mechanism of PTP formation, however, because it did not investigate the consequences of knock-down of the c subunit on other components of the ATP synthase.
Reconstitution of the purified c-subunit ring was reported to form a voltage-sensitive channel leading to Ca2+-dependent mitochondrial depolarization and cell death, whereas both effects would be inhibited in cells depleted of the c subunit [68]. It was suggested that the PTP channel forms within the c ring after Ca2+-dependent extrusion of F1, i.e., of the γ subunits. We note that displacement of F1 requires quite drastic conditions, such as treatment with 2 M urea [69]; yet the FOF1 complex can be easily reconstituted indicating that the γ subunits can reinsert into FO. Thus, it is hard to see how Ca2+ could cause release of F1, and then create a channel that cannot be closed by F1 [68].
Alavian et al. [68] have reported that the “FO channel” can instead be blocked by the purified β subunit, and suggested that this is the mechanism for pore closure in situ [68]. Structural studies have established that subunit β does not interact with the c ring, however [57–61]; and a second, major problem with this proposal is the source of matrix free β subunit, given the extreme resistance of the F1 subcomplex to denaturation. It should be noted that channel openings were also observed with preparations of ATP synthase, and that these could be inhibited by CsA after the addition of Ca2+ [68]. If the mechanism of pore opening is “expulsion” of F1 by Ca2+, it is not easy to see how the current would be inhibited by CsA, because the latter inhibits the PTP by removing CyP-D from F1, not FO [52,55]. It should also be noted that earlier patch-clamp studies of highly purified c subunit yielded very different results, as currents were activated by cGMP and inhibited rather than activated by Ca2+ [70–72]. It is legitimate to wonder whether in the preparation of Alavian et al. other F-ATP synthase components were present that could explain this major discrepancy.
Given that in our hands the PTP readily forms from gel-purified ATP synthase dimers but not monomers, and that inactivation of subunits e and g in yeast increases resistance of the PTP to Ca2+, we still favor the idea that the pore forms at the interface between two monomers in the dimeric enzyme [55,62].
3. PTP and tumors
Prolonged PTP opening constitutes the point of no return toward the demise of the cell, irreversibly committing it either to apoptosis, provided that enough ATP is present to activate caspases, or to necrosis, elicited by massive Ca2+ release in the cytosol and by impairment of mitochondrial function. The capability to escape cell death induction following exposure to stress conditions is a mainstay of cell progression to malignancy [73,74], and most chemotherapeutics are based on the possibility to selectively reinstate apoptotic programs in neoplastic cells. Therefore, a detailed comprehension of PTP structure, and of its regulation in cancer constitute attractive approaches to develop anti-neoplastic strategies aimed at PTP induction.
3.1. PTP and the redox equilibrium of cancer cells
Cancer cells exhibit changes in their redox status, with elevated ROS levels caused both by changes in their signaling pathways, metabolic networks and mitochondrial function, and by exposure to fluctuating conditions of oxygen and nutrient supply in the surrounding environment [75]. This increased level of ROS must be kept under tight control by enhancing anti-oxidant defenses [76], in order to avoid the detrimental effects of oxidants on a variety of cellular structures, which eventually lead to cell death [77]. This is particularly important in early tumorigenic phases, whereas at later neoplastic stages high ROS levels could be tolerated, as they increase cell motility and invasiveness and promote genetic instability, thus contributing to increase malignancy of the growing neoplasm [78]. In these conditions, the eventual cellular fate may be the result of a delicate balance between ROS generation and scavenging, and cancer cells are likely to be more vulnerable than normal ones to further oxidative insults that outpace their anti-oxidant defense mechanisms. Thus, agents that prompt oxidative damage may represent a strategy for selectively targeting cancer cells, while sparing normal ones [79].
PTP induction is a key effector of death elicited by oxidative stress [29]. Indeed, various pro-oxidant agents are able to induce PTP opening. Oxidants increase intracellular Ca2+ release from the endoplasmic reticulum and inhibit Ca2+ extrusion from the plasma membrane [80], and mitochondrial Ca2+ enhances OXPHOS, and therefore generation of ROS as by-products. Above a certain level, mitochondrial Ca2+ or membrane hyperpolarization might also produce ROS through inhibition of respiratory complexes and eventually by PTP induction, which completely stops electron flow along the respiratory chain and causes release of mitochondrial GSH [81,82]. A transient PTP induction may in turn induce short bursts of ROS in the so-called ROS-induced ROS release (RIRR) [83,84]. Therefore, strategies to elicit PTP opening can be envisioned as promising anti-neoplastic approaches, even if the possibility of noxious side effects, e.g., on the nervous system or cardiac tissues, must be carefully considered [85]. Conversely, CsA inhibition of the PTP promotes skin cancer in transplant patients by allowing keratinocyte survival under conditions of genotoxic stress, highlighting the key role of pore inhibition in tumor development [86].
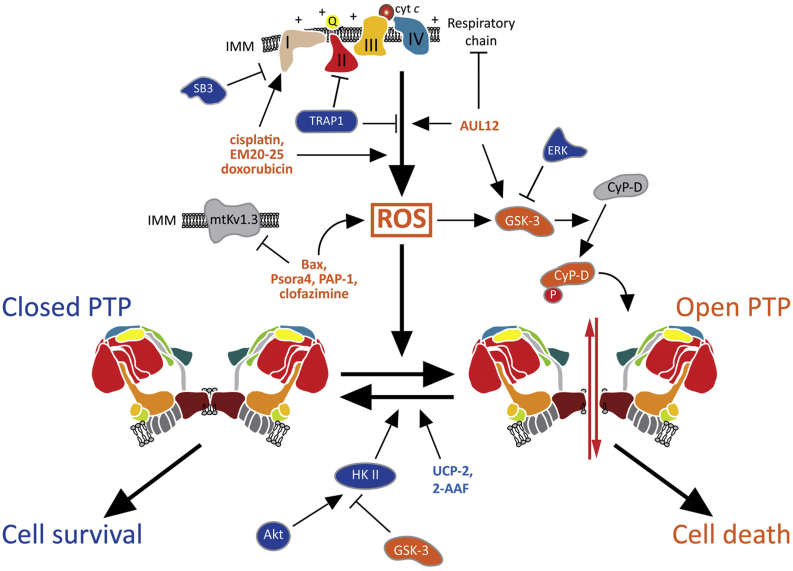
Several molecules can induce ROS-dependent PTP opening (Fig. 2). Inhibition of a mitochondrial voltage-dependent K+ channel, termed mtKV1.3, induces ROS production and a CsA-sensitive mitochondrial depolarization, indicating PTP opening. Inhibition can be elicited by a specific residue on the pro-apoptotic protein Bax [87] or by the membrane-permeant, selective inhibitors Psora-4, PAP-1 and clofazimine, which induce death in several human and mouse cancer cell lines and in primary B-chronic lymphocytic leukemia cells [88,89]; notably, intraperitoneal injection of clofazimine reduced by 90% the size of a melanoma in an orthotopic mouse model, without any side effect on healthy tissues [88]. Such a selective effect of these drugs probably depends on their targeting the altered redox equilibrium of neoplastic cells.
Fig. 2.
Mechanisms of PTP regulation in tumor cells. A variety of factors control tumor cell viability by modulating PTP opening, mainly by modulating ROS levels. A ROS surge elicits PTP opening and cell death, whereas ROS inhibition keeps the pore locked and protects cells from noxious stimuli. PTP inducers are indicated in orange, PTP inhibitors in blue. I-IV: respiratory complexes; 2-AAF: 2-acetylaminofluorene; Q: coenzyme Q; cyt c: cytochrome c; HK II: hexokinase II; IMM: inner mitochondrial membrane; SB3: serpin B3; UCP-2: uncoupling protein 2.
The uncoupling protein-2 (UCP-2) mediates proton leak across the inner mitochondrial membrane [90]. UCP-2 overexpression was reported in a number of tumor types [91], where it protects from oxidative stress, since a depolarizing proton leak is expected to diminish superoxide production [92]. Moreover, a high level of UCP-2 inhibits apoptosis induced by chemotherapeutics [91] and leads to development of tumors in vivo in an orthotopic model of breast cancer [93]. It is reasonable to envision that UCP-2 might shield tumor cells from ROS-induced PTP opening and death.
3.2. Chemotherapeutics and the PTP
A large number of compounds that induce the PTP, mainly by inducing oxidative stress, are under scrutiny as potential chemotherapeutics. Many of them, such as the plant-derived alkaloid berberine [94], the plant hormone methyljasmonate [95], the monocyclic sesquiterpene alcohol α-bisabolol [96], the naphthoquinone shikonin [97], the triterpenoid betulinic acid [98], the constituent of turmeric powder curcumin [99], the polyphenolic compounds resveratrol [100] and honokiol [101], and others, are natural compounds that have been tested on tumor cell lines and in vivo in preclinical animal models (for a complete review, see [82,85]). Some of these molecules, including methyljasmonate, resveratrol and curcumin are particularly promising and are currently undergoing clinical or pre-clinical trials [102].
Moreover, some widely used chemotherapeutics act at least in part by inducing the PTP in a ROS-dependent way. Taxol (paclitaxel), whose main mechanism of action relies upon microtubule stabilization, was shown to prompt ROS generation and PTP opening both in purified liver mitochondria and in liver BRL-3A cells [103]. In pancreatic cancer cells, cisplatin, whose mode of action mainly depends on the formation of adducts with DNA that lead to apoptosis [104], was shown to trigger a CyP-D-dependent necrosis [105], whereas hepatocellular carcinoma cells selected for cisplatin resistance could undergo cell death after PTP induction [106]. These data suggest that both paclitaxel and cisplatin have complex interactions with the target cell, and PTP opening can contribute to their anti-neoplastic effect. In the same line, we have recently found that several chemotherapeutics, including cisplatin, doxorubicin and the BH3 mimetic EM20–25 [107], induce a rapid rise of ROS levels in hepatoma cells, leading to PTP opening and cell death [108]. Notably, overexpression of a serine protease inhibitor of the serpin family, called SERPINB3 (SB3), installs an anti-oxidant defense mechanism in these neoplastic cells: SB3 locates inside mitochondria, mainly during oxidative stress, where it inhibits respiratory complex I, thus blocking ROS generation following chemotherapeutic treatment and protecting cells from PTP opening [108]. Indeed, PTP inhibition is an important adaptation that acts as a tumor-promoting event in the model of hepatocarcinogenesis caused by 2-acetylaminofluorene in rats [109].
3.3. GSK-3 and the PTP in cancer
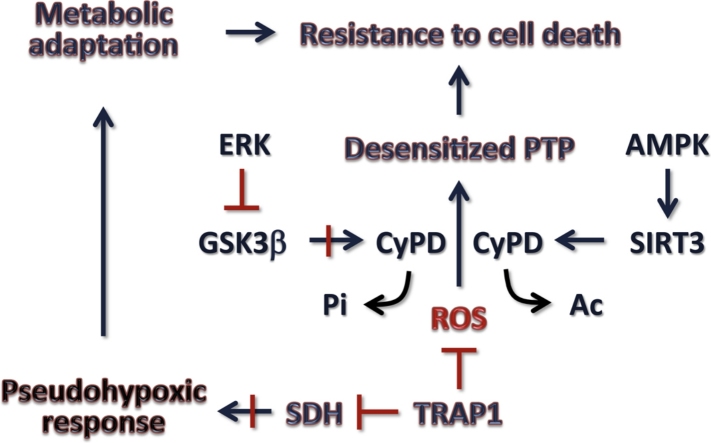
The above data highlight the importance of PTP regulation in tumorigenesis, and indicate respiratory complexes as a primary source of ROS in neoplastic cells. A further connection among PTP induction, ROS generation and respiratory complexes came from studies on the serine/threonine kinase GSK-3. GSK-3 is a kinase crucially involved in a variety of biological processes; it targets more than 50 substrates, and it is placed at the crossroads of multiple transduction pathways, in multimeric complexes with scaffold proteins, other kinases and substrates. Notably, GSK-3 is constitutively active and exerts a tonic inhibitory effect on its targets, and can be both inhibited by phosphorylation on a Ser residue, or further activated by phosphorylation on a Tyr residue [110,111]. A fraction of the enzyme, called mGSK-3, is located inside mitochondria [112], where it down-modulates both the Krebs cycle [113] and oxidative phosphorylation [114] and constitutes an integration point to funnel a multiplicity of survival pathways to target(s) at or in close proximity to the PTP [115,116].
A first connection between respiration, GSK-3 and oxidative stress in tumor cells came from the observation that activation of the mitochondrial pool of GSK-3 enhances ROS production and apoptosis following treatment of human neuroblastoma cells with complex I inhibitors [114,117]. It was then demonstrated by us as well as others that in several tumor cell models mGSK-3 phosphorylates CyP-D, facilitating PTP opening [45,118]. CyP-D is a direct target of GSK-3, as a recombinant GSK-3 could phosphorylate CyP-D in vitro; and the effect of GSK-3 on the PTP was increased after CyP-D overexpression and absent in CyP-D knock-out cells [45]. Deregulation of oncogenic signaling induced constitutive activation of the kinase ERK in a variety of tumor models [119]. We observed that a fraction of active ERK locates in mitochondria, where it inhibits GSK-3 by serine phosphorylation; this results in ablation of CyP-D phosphorylation and in inhibition of PTP opening [45]. Therefore, this mitochondrial kinase pathway contributes to the resilience to undergo death that characterizes neoplastic cells, and can be modulated by drugs acting on any of its constituents. We also found an ERK/GSK-3 dependent modulation of the PTP through CyP-D phosphorylation in ρ0 cells [63]. These cells can be considered as an extreme case of the metabolic rearrangements that occur in tumor cells, as they must completely rely on glycolysis for their metabolic needs. As already mentioned, despite the absence of subunits A6L and a of ATP synthase (which are encoded by mitochondrial DNA) the PTP can be detected in ρ0 cells [63].
Recently we have demonstrated that the Gold(III)-dithiocarbamato complex AUL12, gold-based chemotherapeutic of new generation designed with the specific aim of improving selectivity, bioavailability, and efficacy of platinum-based compounds, diminishing their toxic side effects [120], elicits a rapid burst of mitochondrial superoxide levels following inhibition of the respiratory complex I [121]. ROS generated at complex I enhance activation of GSK-3 by inducing its tyrosine phosphorylation. Active GSK-3 has a double effect of on PTP induction: the mitochondrial pool of the enzyme facilitates PTP opening through phosphorylation of CyP-D, whereas the cytosolic pool interacts with Bax and prompts its mitochondrial translocation, where it contributes to PTP induction and tumor cell death [121]. Notably, AUL12 is much less toxic on non-transformed cells and it displays very low systemic toxicity after in vivo administration [122].
In summary, several functional connections link in a complex network respiration, oncogenic signaling cascades, mGSK3, ROS levels and the PTP in tumor cells, making GSK-3 an appealing target for drug discovery, even if the pleiotropy of its functions exposes to the risk of undesired side effects.
3.4. Mitochondrial hexokinase II and the PTP
Hexokinase (HK) is the first enzyme of glucose metabolism, which converts glucose to glucose-6-phosphate. Four mammalian hexokinase isoforms exist, and isoform II (HK II) is the one predominantly expressed in malignant cells. Several peculiar features of HK II explain this switch: it harbors a very high affinity for glucose; it shows two nearly identical protein domains that can catalyze the phosphorylation of glucose, whereas in other hexokinase isoforms one of these domains has lost its catalytic role; and, together with HK I, it displays a N-terminal mitochondrial anchoring domain, which allows HK II binding to the outer mitochondrial membrane in order to maximally exploit mitochondrial ATP for glucose phosphorylation [123]. All these elements make HK II a central component of the metabolic rewiring of neoplastic cells toward an efficient glucose utilization, which has profound implications for tumor progression. Indeed, many cancer types increase the use of glucose, uncoupling it from oxygen availability (the Warburg effect; [124–126]). This metabolic rearrangement favors neoplastic cell expansion in the hypoxic conditions encountered in the core of the tumor mass [127] and provides anti-oxidant defenses and anabolic building blocks by inducing the pentose phosphate pathway [128].
Several lines of evidence functionally connect HK II to the PTP, even if the different localization of these two elements (external mitochondrial surface vs. inner mitochondrial membrane) suggests the importance of intermediate molecules whose nature is currently unknown. Indeed, release of HK II from mitochondria prompts apoptosis in hepatocellular carcinoma and glioma cells [129,130]; and detachment of HK II from mitochondria by a selective peptide induces PTP opening and the subsequent apoptosis in several tumor cell models [131].
HK II integrates a variety of signals that determine its association/dissociation with mitochondria, and therefore survival or death, respectively, of tumor cells. Inactivation of cytosolic GSK-3 through phosphorylation by the survival kinase Akt, whose signaling is constitutively induced in most tumor types, favors mitochondrial association of HK II, whereas GSK-3 activation elicits the release of HK II from the outer mitochondrial membrane, increasing susceptibility to cell death [132,133]. Moreover, Akt can directly phosphorylate mitochondrial HK II, inhibiting the release of apoptogenic proteins [134]. We have recently observed that the myotonic dystrophy protein kinase (DMPK) forms a multimeric complex with HK II and with the active form of the Tyr kinase Src on the outer mitochondrial membrane, and that this complex protects cells from oxidative stress and from the ensuing PTP opening [135]. In these experiments, detachment of HK II from mitochondria amplifies the oxidative stress caused by placing cells in conditions of serum and glucose depletion. The antioxidant function of HK II relies on its mitochondrial binding as such, and is independent of its enzymatic activity. Indeed, it occurs in the absence of glucose and it is enhanced by 5-thio glucose, which blocks HK II by competing for glucose at the active site of the enzyme [135]. Therefore, the antioxidant function of mitochondrial HK II, which was also observed by others [136], could have a key survival role by maintaining the PTP locked. One possible mechanism to explain how HK II takes part in ROS and PTP regulation might follow HK II interaction with the fructose-2,6-bisphosphatase TIGAR, which occurs in tumor cell mitochondria under hypoxia and limits mitochondrial ROS levels, thus protecting from cell death [137].
The importance of targeting mitochondrial HK II as anti-neoplastic strategy is highlighted by the observation that HK II is required for tumor initiation and maintenance in mouse models of K-Ras-driven lung cancer, as its ablation using HK II conditional knock-out mice inhibits tumor growth without side effects [138]. HK II can be dissociated from mitochondria by the enzyme inhibitor 3-bromopyruvate, which causes release of apoptogenic proteins from mitochondria and eventually cell death [139], and by HK II-based peptides [63,131,135], whose high degree of selectivity toward tumor cells warrants development of clinical trials. However, further studies are needed to understand if all compounds that target mitochondrial HK II (e.g., metformin [140,141]) can lead to PTP opening.
3.5. Mitochondrial chaperones that inhibit PTP in tumor cells
In recent years it has been proposed that other molecular chaperones, in addition to CyP-D, might take part in PTP regulation of tumor cells. These and other observations raise the intriguing possibility that PTP activity is dynamically regulated by a variety of post-translational events, such as phosphorylations, nitrosylations, acetylations, and by multichaperone platforms that maintain the appropriate folding of pore components and regulators.
TRAP1, a member of the Hsp90 protein family, is the most intensively studied chaperone among those involved in pore regulation. TRAP1 expression is restricted to the mitochondrial matrix and inner membrane, and it is increased in the majority of tumor types examined to date [142]. It was shown that TRAP1 protects several normal and transformed cell types from oxidative damage, even if the molecular mechanism of this anti-oxidant activity was not clarified [143–145]. Moreover, TRAP1 prevents the oxidative damage caused by cerebral ischemia in a rat model [146], and inhibits PTP opening and death of hypoxic cardiomyocytes [147]. In several tumor types, TRAP1 expression levels correlate with malignant progression [148,149], and TRAP1 protects cancer cells from toxicity of various antineoplastic agents [144,150,151].
We have recently reported that TRAP1 interacts with succinate dehydrogenase (SDH), aka respiratory complex II, inhibiting succinate oxidation. The consequent succinate accumulation stabilizes the transcription factor HIF1, triggering an oncogenic program required for tumor growth both in vitro and in xenograft mouse models [152]. As also confirmed by others, down-modulation of SDH activity leads to a decrease in respiration [152,153], which could affect the redox equilibrium of tumor cells. Notably, SDH is an important site of ROS generation, and blocking the catalytic site of the SDHA subunit markedly inhibits ROS generation [154–156]. We have recently reported that inhibition of SDH by TRAP1 promotes tumor growth by protecting neoplastic cells from oxidative insults and from the ensuing lethal opening of the PTP, and this anti-oxidant activity of TRAP1 becomes particularly important in conditions of nutrient paucity that mimic those encountered in the tumor mass during the process of unrestrained malignant growth [157]. Thus, TRAP1 is oncogenic not only by prompting accumulation of the oncometabolite succinate and the ensuing pseudohypoxic response (i.e., HIF1 activation in normoxic conditions), but also by shielding tumor cells from ROS-induced PTP opening and death.
It must be highlighted that a decrease in TRAP1 expression levels was observed in some high grade tumors, when increasing intracellular ROS could facilitate cancer cell invasion and genetic instability (reviewed in [142]). In further accord with a role in PTP regulation, it was reported that TRAP1, and also the mitochondrial pools of Hsp90 and Hsp60, interact with CyP-D and prevent its ability to induce the pore in tumor cell models [50,51]. Further work is required to dissect the mode of action of each of these molecular chaperones on the PTP.
4. Summary and conclusions
The key discovery that the PTP forms from F-ATP synthase opens entirely new perspectives to the molecular definition of its role in pathophysiology. The signaling pathways that affect the transition of the F-ATP synthase from an energy-conserving to an energy-dissipating device are of great relevance to cell death and survival, and are exploited by cancer cells to boost their resistance to apoptosis and necrosis. We have little doubt that the rapidly increasing understanding of pore structure and function will bring about the design and validation of PTP-active compounds to treat cancer and degenerative diseases.
Acknowledgements
This study was supported by grants from PRIN/MIUR and FIRB/MIUR (grant numbers RBAP11S8C3 and RBLA03S4SP), from Progetti di Ateneo (grant number CPDA123598) and Progetti Strategici dell’Università di Padova (Models of Mitochondrial Diseases) and from the Associazione Italiana per la Ricerca sul Cancro (grant numbers 8722 and 13392). We thank Elena Trevisan and Marco Ardina for invaluable technical assistance.
Contributor Information
Andrea Rasola, Email: rasola@bio.unipd.it.
Paolo Bernardi, Email: bernardi@bio.unipd.it.
References
- 1.Haworth R.A., Hunter D.R. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch. Biochem. Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 2.Hunter D.R., Haworth R.A. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch. Biochem. Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 3.Hunter D.R., Haworth R.A. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 4.Hunter D.R., Haworth R.A., Southard J.H. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J. Biol. Chem. 1976;251:5069–5077. [PubMed] [Google Scholar]
- 5.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front. Physiol. 2013;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broekemeier K.M., Dempsey M.E., Pfeiffer D.R. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J. Biol. Chem. 1989;264:7826–7830. [PubMed] [Google Scholar]
- 7.Connern C.P., Halestrap A.P. Purification and N-terminal sequencing of peptidyl-prolyl cis-trans-isomerase from rat liver mitochondrial matrix reveals the existence of a distinct mitochondrial cyclophilin. Biochem. J. 1992;284(Pt 2):381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crompton M., Ellinger H., Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson A.M., Halestrap A.P. Partial inhibition by cyclosporin A of the swelling of liver mitochondria in vivo and in vitro induced by sub-micromolar [Ca2+], but not by butyrate. Evidence for two distinct swelling mechanisms. Biochem. J. 1990;268:147–152. doi: 10.1042/bj2680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolli A., Basso E., Petronilli V., Wenger R.M., Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 11.Woodfield K.Y., Price N.T., Halestrap A.P. cDNA cloning of rat mitochondrial cyclophilin. Biochim. Biophys. Acta. 1997;1351:27–30. doi: 10.1016/s0167-4781(97)00017-1. [DOI] [PubMed] [Google Scholar]
- 12.Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 13.Hegde R., Srinivasula S.M., Zhang Z., Wassell R., Mukattash R., Cilenti L., DuBois G., Lazebnik Y., Zervos A.S., Fernandes-Alnemri T., Alnemri E.S. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 14.Li L.Y., Luo X., Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Kim C.N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Susin S.A., Zamzami N., Castedo M., Hirsch T., Marchetti P., Macho A., Daugas E., Geuskens M., Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verhagen A.M., Ekert P.G., Pakusch M., Silke J., Connolly L.M., Reid G.E., Moritz R.L., Simpson R.J., Vaux D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 18.Crompton M., Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur. J. Biochem. 1988;178:489–501. doi: 10.1111/j.1432-1033.1988.tb14475.x. [DOI] [PubMed] [Google Scholar]
- 19.Bernardi P., Krauskopf A., Basso E., Petronilli V., Blachly-Dyson E., Di Lisa F., Forte M.A. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 20.Baines C.P. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res. Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm S., Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- 22.Rasola A., Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 23.Zorov D.B., Juhaszova M., Yaniv Y., Nuss H.B., Wang S., Sollott S.J. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc. Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths E.J., Balaska D., Cheng W.H. The ups and downs of mitochondrial calcium signalling in the heart. Biochim. Biophys. Acta. 2010;1797:856–864. doi: 10.1016/j.bbabio.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Javadov S., Karmazyn M., Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J. Pharmacol. Exp. Ther. 2009;330:670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 26.Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., Forte M., d‘Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinson J.A., Roberts D.W., James L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soriano M.E., Nicolosi L., Bernardi P. Desensitization of the permeability transition pore by cyclosporin a prevents activation of the mitochondrial apoptotic pathway and liver damage by tumor necrosis factor-alpha. J. Biol. Chem. 2004;279:36803–36808. doi: 10.1074/jbc.M405297200. [DOI] [PubMed] [Google Scholar]
- 29.Rasola A., Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi P., Bonaldo P. Mitochondrial dysfunction and defective autophagy in the pathogenesis of collagen VI muscular dystrophies. Cold Spring Harb. Perspect. Biol. 2013;5:a011387. doi: 10.1101/cshperspect.a011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasola A., Sciacovelli M., Pantic B., Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernardi P., Vassanelli S., Veronese P., Colonna R., Szabo I., Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem. 1992;267:2934–2939. [PubMed] [Google Scholar]
- 33.Kinnally K.W., Campo M.L., Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J. Bioenerg. Biomembr. 1989;21:497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- 34.Petronilli V., Szabo I., Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- 35.Szabo I., Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J. Biol. Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 36.Szabo I., Zoratti M. The mitochondrial megachannel is the permeability transition pore. J. Bioenerg. Biomembr. 1992;24:111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- 37.Zamzami N., Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 38.Baines C.P., Kaiser R.A., Sheiko T., Craigen W.J., Molkentin J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokoszka J.E., Waymire K.G., Levy S.E., Sligh J.E., Cai J., Jones D.P., MacGregor G.R., Wallace D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sileikyte J., Blachly-Dyson E., Sewell R., Carpi A., Menabo R., Di Lisa F., Ricchelli F., Bernardi P., Forte M. Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (TSPO) J. Biol. Chem. 2014;289:13769–13781. doi: 10.1074/jbc.M114.549634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., Molkentin J.D. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 42.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 44.Schinzel A.C., Takeuchi O., Huang Z., Fisher J.K., Zhou Z., Rubens J., Hetz C., Danial N.N., Moskowitz M.A., Korsmeyer S.J. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasola A., Sciacovelli M., Chiara F., Pantic B., Brusilow W.S., Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc. Natl. Acad. Sci. U. S. A. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shulga N., Pastorino J.G. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J. Cell Sci. 2010;123:4117–4127. doi: 10.1242/jcs.073502. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Kohr M.J., Aponte A.M., Sun J., Wang G., Murphy E., Gucek M., Steenbergen C. Characterization of potential S-nitrosylation sites in the myocardium. Am. J. Physiol. Heart Circ. Physiol. 2013;300:H1327–H1335. doi: 10.1152/ajpheart.00997.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen T.T., Stevens M.V., Kohr M., Steenbergen C., Sack M.N., Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J. Biol. Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eliseev R.A., Malecki J., Lester T., Zhang Y., Humphrey J., Gunter T.E. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J. Biol. Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang B.H., Plescia J., Dohi T., Rosa J., Doxsey S.J., Altieri D.C. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh J.C., Siegelin M.D., Dohi T., Altieri D.C. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010;70:8988–8993. doi: 10.1158/0008-5472.CAN-10-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giorgio V., Bisetto E., Soriano M.E., Dabbeni-Sala F., Basso E., Petronilli V., Forte M.A., Bernardi P., Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J. Biol. Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung A.W., Varanyuwatana P., Halestrap A.P. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He L., Lemasters J.J. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 55.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabo I., Lippe G., Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida M., Muneyuki E., Hisabori T. ATP synthase—a marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001;2:669–677. doi: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- 57.Rees D.M., Leslie A.G., Walker J.E. The structure of the membrane extrinsic region of bovine ATP synthase. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21597–21601. doi: 10.1073/pnas.0910365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker L.A., Watt I.N., Runswick M.J., Walker J.E., Rubinstein J.L. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11675–11680. doi: 10.1073/pnas.1204935109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies K.M., Anselmi C., Wittig I., Faraldo-Gomez J.D., Kuhlbrandt W. Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13602–13607. doi: 10.1073/pnas.1204593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strauss M., Hofhaus G., Schroder R.R., Kuhlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008;27:1154–1160. doi: 10.1038/emboj.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas D., Bron P., Weimann T., Dautant A., Giraud M.F., Paumard P., Salin B., Cavalier A., Velours J., Brethes D. Supramolecular organization of the yeast F1Fo-ATP synthase. Biol. Cell. 2008;100:591–601. doi: 10.1042/BC20080022. [DOI] [PubMed] [Google Scholar]
- 62.Carraro M., Giorgio V., Sileikyte J., Sartori G., Forte M., Lippe G., Zoratti M., Szabo I., Bernardi P. Channel Formation by Yeast F-ATP Synthase and the Role of Dimerization in the Mitochondrial Permeability Transition. J. Biol. Chem. 2014;289:15980–15985. doi: 10.1074/jbc.C114.559633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masgras I., Rasola A., Bernardi P. Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim. Biophys. Acta. 2012;1817:1860–1866. doi: 10.1016/j.bbabio.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Bonora M., Wieckowski M.R., Chinopoulos C., Kepp O., Kroemer G., Galluzzi L., Pinton P. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2014 doi: 10.1038/onc.2014.462. [DOI] [PubMed] [Google Scholar]
- 65.Halestrap A.P. The C ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: a critical appraisal. Front. Oncol. 2014;4:234. doi: 10.3389/fonc.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wittig I., Meyer B., Heide H., Steger M., Bleier L., Wumaier Z., Karas M., Schagger H. Assembly and oligomerization of human ATP synthase lacking mitochondrial subunits a and A6L. Biochim. Biophys. Acta. 2010;1797:1004–1011. doi: 10.1016/j.bbabio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 67.Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S., Patergnani S., Rimessi A., Suski J.M., Wojtala A., Wieckowski M.R., Kroemer G., Galluzzi L., Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alavian K.N., Beutner G., Lazrove E., Sacchetti S., Park H.A., Licznerski P., Li H., Nabili P., Hockensmith K., Graham M., Porter G.A., Jr., Jonas E.A. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U. S. A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J. Biol. Chem. 1966;241:2461–2466. [PubMed] [Google Scholar]
- 70.McGeoch J.E., Guidotti G. A 0.1–700 Hz current through a voltage-clamped pore: candidate protein for initiator of neural oscillations. Brain Res. 1997;766:188–194. doi: 10.1016/s0006-8993(97)00618-5. [DOI] [PubMed] [Google Scholar]
- 71.McGeoch J.E., Guidotti G. Batten disease and the control of the Fo subunit c pore by cGMP and calcium. Eur. J. Paediatr. Neurol. 2001;5(Suppl. A):147–150. doi: 10.1053/ejpn.2000.0452. [DOI] [PubMed] [Google Scholar]
- 72.McGeoch J.E., McGeoch M.W., Mao R., Guidotti G. Opposing actions of cGMP and calcium on the conductance of the F(0) subunit c pore. Biochem. Biophys. Res. Commun. 2000;274:835–840. doi: 10.1006/bbrc.2000.3231. [DOI] [PubMed] [Google Scholar]
- 73.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 74.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Grek C.L., Tew K.D. Redox metabolism and malignancy. Curr. Opin. Pharmacol. 2010;10:362–368. doi: 10.1016/j.coph.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., Scrimieri F., Winter J.M., Hruban R.H., Iacobuzio-Donahue C., Kern S.E., Blair I.A., Tuveson D.A. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 78.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 79.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 80.Camello-Almaraz C., Gomez-Pinilla P.J., Pozo M.J., Camello P.J. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 81.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Leanza L., Biasutto L., Manago A., Gulbins E., Zoratti M., Szabo I. Intracellular ion channels and cancer. Front. Physiol. 2013;4:227. doi: 10.3389/fphys.2013.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: an update and review. Biochim. Biophys. Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 84.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leanza L., Zoratti M., Gulbins E., Szabo I. Mitochondrial ion channels as oncological targets. Oncogene. 2014 doi: 10.1038/onc.2013.578. [DOI] [PubMed] [Google Scholar]
- 86.Norman K.G., Canter J.A., Shi M., Milne G.L., Morrow J.D., Sligh J.E. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion. 2010;10:94–101. doi: 10.1016/j.mito.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szabo I., Bock J., Grassme H., Soddemann M., Wilker B., Lang F., Zoratti M., Gulbins E. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14861–14866. doi: 10.1073/pnas.0804236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leanza L., Henry B., Sassi N., Zoratti M., Chandy K.G., Gulbins E., Szabo I. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 2012;4:577–593. doi: 10.1002/emmm.201200235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leanza L., Trentin L., Becker K.A., Frezzato F., Zoratti M., Semenzato G., Gulbins E., Szabo I. Clofazimine, Psora-4 and PAP-1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia. Leukemia. 2013;27:1782–1785. doi: 10.1038/leu.2013.56. [DOI] [PubMed] [Google Scholar]
- 90.Donadelli M., Dando I., Fiorini C., Palmieri M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014;71:1171–1190. doi: 10.1007/s00018-013-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robbins D., Zhao Y. New aspects of mitochondrial Uncoupling Proteins (UCPs) and their roles in tumorigenesis. Int. J. Mol. Sci. 2011;12:5285–5293. doi: 10.3390/ijms12085285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mailloux R.J., Harper M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 93.Ayyasamy V., Owens K.M., Desouki M.M., Liang P., Bakin A., Thangaraj K., Buchsbaum D.J., LoBuglio A.F., Singh K.K. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS ONE. 2011;6:e24792. doi: 10.1371/journal.pone.0024792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L.Y., Wu Y.L., Gao X.H., Guo F. Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells. Biochem. Biophys. Res. Commun. 2014;450:697–703. doi: 10.1016/j.bbrc.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 95.Raviv Z., Cohen S., Reischer-Pelech D. The anti-cancer activities of jasmonates. Cancer Chemother. Pharmacol. 2013;71:275–285. doi: 10.1007/s00280-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 96.Cavalieri E., Bergamini C., Mariotto S., Leoni S., Perbellini L., Darra E., Suzuki H., Fato R., Lenaz G. Involvement of mitochondrial permeability transition pore opening in alpha-bisabolol induced apoptosis. FEBS J. 2009;276:3990–4000. doi: 10.1111/j.1742-4658.2009.07108.x. [DOI] [PubMed] [Google Scholar]
- 97.Han W., Li L., Qiu S., Lu Q., Pan Q., Gu Y., Luo J., Hu X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol. Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 98.Lena A., Rechichi M., Salvetti A., Bartoli B., Vecchio D., Scarcelli V., Amoroso R., Benvenuti L., Gagliardi R., Gremigni V., Rossi L. Drugs targeting the mitochondrial pore act as cytotoxic and cytostatic agents in temozolomide-resistant glioma cells. J. Transl. Med. 2009;7:13. doi: 10.1186/1479-5876-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiu Y., Yu T., Wang W., Pan K., Shi D., Sun H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem. Biophys. Res. Commun. 2014;448:15–21. doi: 10.1016/j.bbrc.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 100.Ma X., Tian X., Huang X., Yan F., Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol. Cell. Biochem. 2007;302:99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]
- 101.Li L., Han W., Gu Y., Qiu S., Lu Q., Jin J., Luo J., Hu X. Honokiol induces a necrotic cell death through the mitochondrial permeability transition pore. Cancer Res. 2007;67:4894–4903. doi: 10.1158/0008-5472.CAN-06-3818. [DOI] [PubMed] [Google Scholar]
- 102.Suh D.H., Kim M.K., Kim H.S., Chung H.H., Song Y.S. Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front. Oncol. 2013;3:41. doi: 10.3389/fonc.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varbiro G., Veres B., Gallyas F., Jr., Sumegi B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radic. Biol. Med. 2001;31:548–558. doi: 10.1016/s0891-5849(01)00616-5. [DOI] [PubMed] [Google Scholar]
- 104.Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., Castedo M., Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 105.Chen B., Xu M., Zhang H., Wang J.X., Zheng P., Gong L., Wu G.J., Dai T. Cisplatin-induced non-apoptotic death of pancreatic cancer cells requires mitochondrial cyclophilin-D-p53 signaling. Biochem. Biophys. Res. Commun. 2013;437:526–531. doi: 10.1016/j.bbrc.2013.06.103. [DOI] [PubMed] [Google Scholar]
- 106.Ling X., Zhou Y., Li S.W., Yan B., Wen L. Modulation of mitochondrial permeability transition pore affects multidrug resistance in human hepatocellular carcinoma cells. Int. J. Biol. Sci. 2010;6:773–783. doi: 10.7150/ijbs.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Milanesi E., Costantini P., Gambalunga A., Colonna R., Petronilli V., Cabrelle A., Semenzato G., Cesura A.M., Pinard E., Bernardi P. The mitochondrial effects of small organic ligands of BCL-2: sensitization of BCL-2-overexpressing cells to apoptosis by a pyrimidine-2,4,6-trione derivative. J. Biol. Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 108.Ciscato F., Sciacovelli M., Villano G., Turato C., Bernardi P., Rasola A., Pontisso P. SERPINB3 protects from oxidative damage by chemotherapeutics through inhibition of mitochondrial respiratory complex I. Oncotarget. 2014;5:2418–2427. doi: 10.18632/oncotarget.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klohn P.C., Soriano M.E., Irwin W., Penzo D., Scorrano L., Bitsch A., Neumann H.G., Bernardi P. Early resistance to cell death and to onset of the mitochondrial permeability transition during hepatocarcinogenesis with 2-acetylaminofluorene. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10014–10019. doi: 10.1073/pnas.1633614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jope R.S., Johnson G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 111.Jope R.S., Yuskaitis C.J., Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chiara F., Rasola A. GSK-3 and mitochondria in cancer cells. Front. Oncol. 2013;3:16. doi: 10.3389/fonc.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hoshi M., Takashima A., Noguchi K., Murayama M., Sato M., Kondo S., Saitoh Y., Ishiguro K., Hoshino T., Imahori K. Regulation of mitochondrial pyruvate dehydrogenase activity by tau protein kinase I/glycogen synthase kinase 3beta in brain. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.King T.D., Clodfelder-Miller B., Barksdale K.A., Bijur G.N. Unregulated mitochondrial GSK3beta activity results in NADH: ubiquinone oxidoreductase deficiency. Neurotox. Res. 2008;14:367–382. doi: 10.1007/BF03033861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Juhaszova M., Zorov D.B., Yaniv Y., Nuss H.B., Wang S., Sollott S.J. Role of glycogen synthase kinase-3beta in cardioprotection. Circ. Res. 2009;104:1240–1252. doi: 10.1161/CIRCRESAHA.109.197996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miura T., Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ. J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 117.Petit-Paitel A., Brau F., Cazareth J., Chabry J. Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS ONE. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Traba J., Del Arco A., Duchen M.R., Szabadkai G., Satrustegui J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca(2+) buffering. Cell Death Differ. 2011;19:650–660. doi: 10.1038/cdd.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 120.Ronconi L., Fregona D. The Midas touch in cancer chemotherapy: from platinum- to gold-dithiocarbamato complexes. Dalton Trans. 2009;48:10670–10680. doi: 10.1039/b913597a. [DOI] [PubMed] [Google Scholar]
- 121.Chiara F., Gambalunga A., Sciacovelli M., Nicolli A., Ronconi L., Fregona D., Bernardi P., Rasola A., Trevisan A. Chemotherapeutic induction of mitochondrial oxidative stress activates GSK-3α/β and Bax, leading to permeability transition pore opening and tumor cell death. Cell Death Dis. 2012;3:e444. doi: 10.1038/cddis.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marzano C., Ronconi L., Chiara F., Giron M.C., Faustinelli I., Cristofori P., Trevisan A., Fregona D. Gold(III)-dithiocarbamato anticancer agents: activity, toxicology and histopathological studies in rodents. Int. J. Cancer. 2011;129:487–496. doi: 10.1002/ijc.25684. [DOI] [PubMed] [Google Scholar]
- 123.Mathupala S.P., Ko Y.H., Pedersen P.L. The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochim. Biophys. Acta. 2010;1797:1225–1230. doi: 10.1016/j.bbabio.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 125.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ward P.S., Thompson C.B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim W., Yoon J.H., Jeong J.M., Cheon G.J., Lee T.S., Yang J.I., Park S.C., Lee H.S. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol. Cancer Ther. 2007;6:2554–2562. doi: 10.1158/1535-7163.MCT-07-0115. [DOI] [PubMed] [Google Scholar]
- 130.Machida K., Ohta Y., Osada H. Suppression of apoptosis by cyclophilin D via stabilization of hexokinase II mitochondrial binding in cancer cells. J. Biol. Chem. 2006;281:14314–14320. doi: 10.1074/jbc.M513297200. [DOI] [PubMed] [Google Scholar]
- 131.Chiara F., Castellaro D., Marin O., Petronilli V., Brusilow W.S., Juhaszova M., Sollott S.J., Forte M., Bernardi P., Rasola A. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS ONE. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 134.Miyamoto S., Murphy A.N., Brown J.H. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 135.Pantic B., Trevisan E., Citta A., Rigobello M.P., Marin O., Bernardi P., Salvatori S., Rasola A. Myotonic dystrophy protein kinase (DMPK) prevents ROS-induced cell death by assembling a hexokinase II-Src complex on the mitochondrial surface. Cell Death Dis. 2013;4:e858. doi: 10.1038/cddis.2013.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun L., Shukair S., Naik T.J., Moazed F., Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol. Cell. Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheung E.C., Ludwig R.L., Vousden K.H. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patra K.C., Wang Q., Bhaskar P.T., Miller L., Wang Z., Wheaton W., Chandel N., Laakso M., Muller W.J., Allen E.L., Jha A.K., Smolen G.A., Clasquin M.F., Robey R.B., Hay N. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Z., Zhang H., Lu W., Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta. 2009;1787:553–560. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marini C., Salani B., Massollo M., Amaro A., Esposito A.I., Orengo A.M., Capitanio S., Emionite L., Riondato M., Bottoni G., Massara C., Boccardo S., Fabbi M., Campi C., Ravera S., Angelini G., Morbelli S., Cilli M., Cordera R., Truini M., Maggi D., Pfeffer U., Sambuceti G. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12:3490–3499. doi: 10.4161/cc.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salani B., Marini C., Rio A.D., Ravera S., Massollo M., Orengo A.M., Amaro A., Passalacqua M., Maffioli S., Pfeffer U., Cordera R., Maggi D., Sambuceti G. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci. Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rasola A., Neckers L., Picard D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014;24:455–463. doi: 10.1016/j.tcb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hua G., Zhang Q., Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J. Biol. Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- 144.Montesano Gesualdi N., Chirico G., Pirozzi G., Costantino E., Landriscina M., Esposito F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress. 2007;10:342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- 145.Pridgeon J.W., Olzmann J.A., Chin L.S., Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu L., Voloboueva L.A., Ouyang Y., Emery J.F., Giffard R.G. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J. Cereb. Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xiang F., Huang Y.S., Shi X.H., Zhang Q. Mitochondrial chaperone tumour necrosis factor receptor-associated protein 1 protects cardiomyocytes from hypoxic injury by regulating mitochondrial permeability transition pore opening. FEBS J. 2010;277:1929–1938. doi: 10.1111/j.1742-4658.2010.07615.x. [DOI] [PubMed] [Google Scholar]
- 148.Gao J.Y., Song B.R., Peng J.J., Lu Y.M. Correlation between mitochondrial TRAP-1 expression and lymph node metastasis in colorectal cancer. World J. Gastroenterol. 2012;18:5965–5971. doi: 10.3748/wjg.v18.i41.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leav I., Plescia J., Goel H.L., Li J., Jiang Z., Cohen R.J., Languino L.R., Altieri D.C. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am. J. Pathol. 2010;176:393–401. doi: 10.2353/ajpath.2010.090521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Costantino E., Maddalena F., Calise S., Piscazzi A., Tirino V., Fersini A., Ambrosi A., Neri V., Esposito F., Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 151.Masuda Y., Shima G., Aiuchi T., Horie M., Hori K., Nakajo S., Kajimoto S., Shibayama-Imazu T., Nakaya K. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J. Biol. Chem. 2004;279:42503–42515. doi: 10.1074/jbc.M404256200. [DOI] [PubMed] [Google Scholar]
- 152.Sciacovelli M., Guzzo G., Morello V., Frezza C., Zheng L., Nannini N., Calabrese F., Laudiero G., Esposito F., Landriscina M., Defilippi P., Bernardi P., Rasola A. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013;17:988–999. doi: 10.1016/j.cmet.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yoshida S., Tsutsumi S., Muhlebach G., Sourbier C., Lee M.J., Lee S., Vartholomaiou E., Tatokoro M., Beebe K., Miyajima N., Mohney R.P., Chen Y., Hasumi H., Xu W., Fukushima H., Nakamura K., Koga F., Kihara K., Trepel J., Picard D., Neckers L. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1604–E1612. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drose S. Differential effects of complex II on mitochondrial ROS production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta. 2013;1827:578–587. doi: 10.1016/j.bbabio.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 155.Grimm S. Respiratory chain complex II as general sensor for apoptosis. Biochim. Biophys. Acta. 2013;1827:565–572. doi: 10.1016/j.bbabio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 156.Lemarie A., Grimm S. Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer? Oncogene. 2011;30:3985–4003. doi: 10.1038/onc.2011.167. [DOI] [PubMed] [Google Scholar]
- 157.Guzzo G., Sciacovelli M., Bernardi P., Rasola A. Inhibition of succinate dehydrogenase by the mitochondrial chaperone TRAP1 has anti-oxidant and anti-apoptotic effects on tumor cells. Oncotarget. 2014 doi: 10.18632/oncotarget.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]