Abstract
Background
A recent Cochrane Review found that preoperative biliary drainage (PBD) in patients with resectable pancreatic and periampullary cancer undergoing surgery for obstructive jaundice is associated with similar mortality but increased serious morbidity compared with no PBD. Despite this clinical evidence of its lack of effectiveness, PBD is still in use. We considered the economic implications of PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer.
Materials and methods
Model-based cost-utility analysis estimating mean costs and quality-adjusted life years (QALYs) per patient from the perspective of the UK National Health Service over a 6-month time horizon. A decision tree model was constructed and populated with probabilities, outcomes, and cost data from published sources. One-way and probabilistic sensitivity analyses were undertaken.
Results
PBD was more costly than direct surgery (mean cost per patient £10,775 [$15,616] versus £8221 [$11,914]) and produced fewer QALYs (mean QALYs per patient 0.337 versus 0.343). Not performing PBD would result in cost savings of approximately £2500 ($3623) per patient to the National Health Service. PBD had <10% probability of being cost-effective at a maximum willingness to pay for a QALY of £20,000 ($28,986) to £30,000 ($43,478).
Conclusions
There are significant cost savings to be gained by avoiding routine PBD in patients with resectable pancreatic and periampullary cancer where PBD is still routinely used in this context; this economic evidence should be used to support the clinical argument for a change in practice.
Keywords: Preoperative biliary drainage, Periampullary cancer, Obstructive jaundice, Cost-effectiveness analysis
1. Introduction
Obstructive jaundice is a common symptom in patients with periampullary cancer (located near the ampulla of Vater) or cancer of the pancreatic head. Surgical resection is the only option for cure [1–3]. Because obstructive jaundice is thought to increase the risk of developing postoperative complications, preoperative biliary drainage (PBD) was introduced to improve the postoperative outcome [4]. It has since been incorporated into the standard surgical treatment algorithm of periampullary cancer and cancer of the pancreatic head in the majority of hospitals [4]. Other factors that may influence the use of PBD include temporary contraindications for surgery such as severe malnutrition and other comorbidities that have to be treated before surgery and the interval between diagnosis and treatment. If there is a long waiting time before surgery, PBD may have to be performed [1]. However, the wisdom of delaying surgery in people with an aggressive cancer such as pancreatic cancer is questionable.
In several studies, PBD reduced morbidity and mortality after surgery [4–7]. However, a recent Cochrane Review of the six randomized clinical trials evaluating the safety and effectiveness of PBD versus no PBD found that PBD in patients undergoing surgery for obstructive jaundice is associated with similar mortality but increased serious morbidity compared with no PBD [8,9]. The review concluded that PBD should not be used routinely. Nonetheless, there is evidence that PBD is still commonly used in this context [10] suggesting that clinical considerations alone are not sufficient to change practice. Consideration of the economic implications of carrying out routine PBD to health systems may be needed.
A review of the National Health Service (NHS) Economic Evaluations Database [11] using the search term “biliary drainage” identified eight studies, but none of these evaluated PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer. Therefore, this study investigates the cost-effectiveness of PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer.
2. Materials and methods
This is a model-based cost-utility analysis to estimate the mean cost per patient and the mean outcome per patient associated with PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer. The outcome measure is quality-adjusted life years (QALYs), which combine length of life and quality of life [12]. QALYs are the recommended outcome for use in economic evaluations in the United Kingdom as they are a common unit that allow for comparable decisions about resource allocation across different health conditions.
The analysis is undertaken from the perspective of the UK NHS. Costs are calculated in 2011–2012 UK£ with US$ given in parentheses (UK1 = US$1.449). Since treatment for an acute condition is being investigated and the Cochrane Review found that PBD had no impact on mortality, a time horizon of 6 mo for costs and outcomes was considered to be appropriate and discounting of costs and benefits was unnecessary.
2.1. Model structure
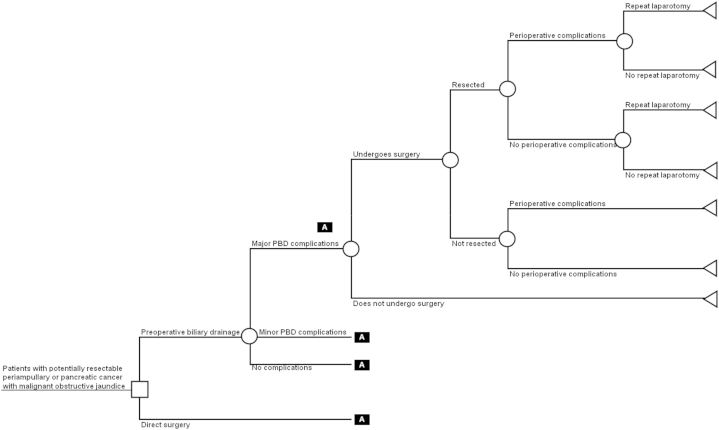
The analysis uses a decision tree to describe the options being compared and the possible pathways following them (Fig. 1). This is a commonly used approach in cost-effectiveness studies of health care programs [12]. The nodes of a decision tree are points where more than one event is possible. The branches are mutually exclusive events following each node. Decision nodes, represented by squares, show the different options that might be chosen by decision makers based on the costs and benefits they produce (e.g., to perform PBD or not). Chance nodes, represented by circles, show uncertain events, each of which is associated with a probability that it will occur (e.g., whether or not PBD will have major, mild, or no complications). Terminal nodes, represented by triangles, are the endpoints of a decision tree, beyond which no further pathways are available. Each terminal node has costs and QALYs associated with it, summarizing the sequence of decisions and events on a unique path leading from the initial decision node to that terminal node. These costs and QALYs are expected values based on the probability of each event on the pathway occurring up to that point, and the costs and QALYs associated with each event.
Fig. 1.
Decision tree model structure.
Patients enter the model with potentially resectable periampullary or pancreatic cancer with malignant obstructive jaundice. If they undergo PBD, the procedure may have major, minor, or no complications. In any case, patients may or may not undergo surgery subsequently because of the complications of PBD or the underlying cancer, and a proportion of patients undergoing surgery will be resected. Those who undergo surgery may experience perioperative complications, and a proportion of those who are resected may require a repeat laparotomy for recurrence or long-term complications such as adhesions.
For patients undergoing surgery directly, without PBD, it was assumed that the treatment pathway is the same as the one subsequent to PBD, but the probabilities, costs, and QALYs associated with each pathway may be different.
2.2. Probabilities
The probabilities associated with mutually exclusive events at each chance node were obtained from published sources (Tables 1 and 2) [13–18]. Additional data were extracted from the six randomized clinical trials included in the Cochrane Review on the probability of major and minor complications related to PBD and to surgery. The probabilities for patients in each group undergoing surgery, being resected if they did undergo surgery, and requiring a repeat laparotomy if they were resected were taken from a single large trial included in the Cochrane Review [17].
Table 1.
Additional data extracted from randomized controlled trials included in the Cochrane Review [8].
| Study | PBD |
Direct surgery |
||||
|---|---|---|---|---|---|---|
| Total number of patients | Number with minor complications related to PBD | Number with serious complications related to PBD | Complications of surgery (after PBD) | Total number of patients | Complications of surgery | |
| Hatfield et al.[13] | 29 | NA | 4 | 4 | 28 | 4 |
| Lai et al.[14] | 43 | NA | 12 | 16 | 44 | 18 |
| McPherson et al.[15] | 34 | NA | 8 | 9 | 31 | 13 |
| Pitt et al.[16] | 37 | NA | 4 | 16 | 38 | 20 |
| van der Gaag et al.[17] | 102 | 20 | 27 | 48 | 94 | 35 |
| Wig et al.[18] | 20 | 2 | 4 | 5 | 20 | 11 |
| Total | 265 | 22 | 59 | 98 | 255 | 101 |
NA = data not available.
Table 2.
Model parameters for decision tree model and range of values used in univariate sensitivity analysis.
| Base case value | Distribution | Alpha | Beta | Sources | Range | |
|---|---|---|---|---|---|---|
| Probabilities | ||||||
| PBD | ||||||
| Pr(major complications with PBD) | 0.223 | Dirichlet (59) | [13–18] | 0–1 | ||
| Pr(minor complications with PBD) | 0.083 | Dirichlet (22) | [13–18] | 0–1 | ||
| Pr(no complications with PBD) | 0.694 | Dirichlet (184) | [13–18] | 0–1 | ||
| Pr(undergoes surgery) | 0.931 | Beta | 95 | 7 | [17] | 0–1 |
| Pr(resected) | 0.600 | Beta | 57 | 38 | [17] | 0–1 |
| Pr(complications if resected) | 0.370 | Beta | 98 | 167 | [13–18] | 0–1 |
| Pr(complications if not resected) | 0.370 | Beta | 98 | 167 | [13–18] | 0–1 |
| Pr(repeat laparotomy) | 0.211 | Beta | 12 | 45 | [17] | 0–1 |
| Direct surgery | 0–1 | |||||
| Pr(undergoes surgery) | 0.979 | Beta | 92 | 2 | [17] | 0–1 |
| Pr(resected) | 0.685 | Beta | 63 | 29 | [17] | 0–1 |
| Pr(complications if resected) | 0.396 | Beta | 101 | 154 | [13–18] | 0–1 |
| Pr(complications if not resected) | 0.396 | Beta | 101 | 154 | [13–18] | 0–1 |
| Pr(repeat laparotomy) | 0.206 | Beta | 13 | 50 | [17] | 0–1 |
| Unit costs | ||||||
| PBD with major complications | 4036 (5849) | Gamma | 1 | 4036 | [27] | 2000–6500 |
| PBD with minor complications | 2846 (4125) | Gamma | 1 | 2846 | [27] | 1700–3300 |
| PBD without complications | 2897 (4199) | Gamma | 1 | 2897 | [27] | 1000–4000 |
| Resection with complications | 9209 (13,346) | Gamma | 1 | 9209 | [27] | 6000–11,000 |
| Resection without complications | 7711 (11,175) | Gamma | 1 | 7711 | [27] | 5000–10,000 |
| Palliative surgery with complications | 5378 (7794) | Gamma | 1 | 5378 | [27] | 3500–6500 |
| Palliative surgery without complications | 4487 (6503) | Gamma | 1 | 4487 | [27] | 2000–6000 |
| Do not undergo surgery (palliative treatment only) | 4487 (6503) | Gamma | 1 | 4487 | [27] | 2000–6000 |
| Repeat laparotomy | 7711 (11,175) | Gamma | 1 | 7711 | [27] | 5000–10,000 |
| Utilities | ||||||
| PBD with complications, undergoes surgery, resected, no complications | ||||||
| 6 wk | 0.54 | Beta | 52.38 | 44.62 | [22] | 0–1 |
| 3 mo | 0.74 | Beta | 71.78 | 25.22 | [22] | 0–1 |
| 6 mo | 0.80 | Beta | 77.60 | 19.40 | [22] | 0–1 |
| PBD with complications, undergoes surgery, resected, with complications | ||||||
| 6 wk | 0.54 | Beta | 52.38 | 44.62 | [22] | 0–1 |
| 3 mo | 0.71 | Beta | 68.87 | 28.13 | [22] | 0–1 |
| 6 mo | 0.78 | Beta | 75.66 | 21.34 | [22] | 0–1 |
| PBD with complications, undergoes surgery, not resected | ||||||
| 6 wk | 0.54 | Beta | 52.38 | 44.62 | [22] | 0–1 |
| 3 mo | 0.67 | Beta | 64.99 | 32.01 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| PBD with complications, does not undergo surgery | ||||||
| 6 wk | 0.54 | Beta | 52.38 | 44.62 | [22] | 0–1 |
| 3 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| PBD no complications, undergoes surgery, resected, no complications | ||||||
| 2 wk | 0.60 | Beta | 58.20 | 38.80 | [22] | 0–1 |
| 3 mo | 0.74 | Beta | 71.78 | 25.22 | [22] | 0–1 |
| 6 mo | 0.80 | Beta | 77.60 | 19.40 | [22] | 0–1 |
| PBD no complications, undergoes surgery, resected, with complications | ||||||
| 2 wk | 0.60 | Beta | 58.20 | 38.80 | [22] | 0–1 |
| 3 mo | 0.71 | Beta | 68.87 | 28.13 | [22] | 0–1 |
| 6 mo | 0.78 | Beta | 75.66 | 21.34 | [22] | 0–1 |
| PBD no complications, undergoes surgery, not resected | ||||||
| 2 wk | 0.60 | Beta | 58.20 | 38.80 | [22] | 0–1 |
| 3 mo | 0.67 | Beta | 64.99 | 32.01 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| PBD no complications, does not undergo surgery | ||||||
| 2 wk | 0.60 | Beta | 58.20 | 38.80 | [22] | 0–1 |
| 3 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| Direct surgery, undergoes surgery, resected, no complications | ||||||
| 2 wk | 0.60 | Beta | 58.20 | 38.80 | [22] | 0–1 |
| 3 mo | 0.74 | Beta | 71.78 | 25.22 | [22] | 0–1 |
| 6 mo | 0.80 | Beta | 77.60 | 19.40 | [22] | 0–1 |
| Direct surgery, undergoes surgery, resected, with complications | ||||||
| 2 wk | 0.57 | Beta | 55.29 | 41.71 | [22] | 0–1 |
| 3 mo | 0.71 | Beta | 68.87 | 28.13 | [22] | 0–1 |
| 6 mo | 0.78 | Beta | 75.66 | 21.34 | [22] | 0–1 |
| Direct surgery, undergoes surgery, not resected | ||||||
| 2 wk | 0.54 | Beta | 52.38 | 44.62 | [22] | 0–1 |
| 3 mo | 0.67 | Beta | 64.99 | 32.01 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| Direct surgery, does not undergo surgery | ||||||
| 2 wk | 0.76 | Beta | 73.72 | 23.28 | [22] | 0–1 |
| 3 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
| 6 mo | 0.72 | Beta | 69.84 | 27.16 | [22] | 0–1 |
Unit costs are in 2011–2012 UK£ (US$). The base case values are used to produce the deterministic results. The distributions are used to undertake the probabilistic sensitivity analysis, to produce the probabilistic results, and construct the cost-effectiveness acceptability curves.
2.3. Outcomes
The quality of life component of QALYs is measured by utility scores. A utility score of 1 represents full health and a utility of 0 death; negative values represent states worse than death. A review of utility weights in the cost-effectiveness analysis registry [11] was undertaken using the search terms “pancreas,” “pancreatic,” “ampullary,” and “periampullary.” After reviewing the reference lists of the identified studies and removing duplicates, five studies containing potentially relevant utility data were identified [19–23]. The utility scores used in the model were from one study [22], selected because values were presented for different points over time and utility scores for all the health states in the model were included, thus enabling better comparability between values, and the values reported also reflected trends in disease-specific quality of life measures found in other studies (Table 2) [24–26]. Utility scores were measured at 6 wk, 3 mo, and 6 mo. QALYs were estimated using the trapezium rule for calculating the area under a curve. Because they did not measure directly the utility among patients undergoing PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer, the utility scores were judged to be weak and so were tested comprehensively in sensitivity analyses.
2.4. Costs
The costs of PBD with major, minor, and no complications were assumed to be £4036, £2846, and £2897($5849, $4125, and $4199), respectively (Table 2), based on national mean costs of major endoscopic or percutaneous, hepatobiliary or pancreatic procedures provided on an elective inpatient basis [27]. Surgical resection with and without complications was assumed to cost £9209 ($13,346) and £7711 ($11,175), respectively [27]. Patients who undergo surgery but are not resected receive palliative surgery and this was assumed to cost £5378 ($7794) with complications and £4487 ($6503) without complications [27]. Patients who do not undergo surgery receive palliative treatment only, at an assumed cost of £4487 ($6503) [26]. The cost of repeat laparotomy in those who underwent surgical resection was assumed to be £7711 ($11,175) [27].
2.5. Measuring cost-effectiveness
Cost-effectiveness was measured using monetary net benefits (MNBs). For each treatment, the MNB was calculated as the mean QALYs per patient accruing to that treatment multiplied by decision makers' maximum willingness to pay for a QALY (also referred to as the cost-effectiveness threshold, which in the United Kingdom is approximately £20,000 [$28,986] to £30,000 [$43,478] per QALY gained) [28], minus the mean cost per patient for the treatment. This approach converts the outcomes from each treatment into monetary terms and then subtracts the costs of each treatment from the monetized benefits, calculating the net benefit of each treatment in monetary terms. MNBs were calculated using the base case parameter values shown in Table 2; these are referred to as deterministic results because they do not depend on chance. The treatment with the highest MNB represents the best value for money and is preferred on cost-effectiveness grounds.
2.6. Sensitivity analyses
One-way sensitivity analysis was undertaken, varying the probabilities, outcomes, and costs one at a time within the ranges listed in Table 2. The aim was to identify the threshold value for each parameter, where one exists, where the treatment with the highest MNB changed (e.g., the value at which PBD was the most cost-effective option). An analysis was also undertaken that based on the probabilities of complications with PBD and with complications of surgery on a single large trial [17] rather than all six studies included in the Cochrane Review.
A probabilistic sensitivity analysis (PSA) was undertaken, as recommended by the National Institute for Health and Care Excellence (NICE) [28]. Distributions were assigned to parameters (Table 2) to reflect the uncertainty with each parameter value. A random value from the corresponding distribution for each parameter was selected. This generated an estimate of the mean cost and mean QALYs and the MNB associated with each treatment. This was repeated 5000 times, and the results for each simulation were noted. The mean costs, QALYs, and MNBs for each treatment were calculated from the 5000 simulations; these are referred to as probabilistic results because they depend on chance. Using the MNBs for each of the 5000 simulations, the proportion of times each treatment had the highest MNB was calculated for a range of values for the maximum willingness to pay for a QALY. These were summarized graphically using cost-effectiveness acceptability curves [12].
In the PSA, we used beta and Dirichlet distributions to model uncertainty in the probabilities, beta distributions to model uncertainty in utility scores, and gamma distributions to model uncertainty in costs (Table 2) [29]. Dirichlet distributions were fitted using Excel macros developed by the Centre for Bayesian Statistics in Health Economics at the University of Sheffield [30]. In cases where standard errors were required for the PSA and these were not reported in the sources used, it was assumed the standard error was equal to the mean [29]. For the utilities, the variance was calculated assuming a beta distribution based on 97 observations [22,23]. Parameter values used to characterize each distribution are in Table 2. For each of the base case values, 95% confidence intervals (CIs) were derived using standard deviations calculated from the 5000 simulations in the PSA.
3. Results
Using base case values, PBD for obstructive jaundice in patients with pancreatic and periampullary cancer was more costly than direct surgery, with a mean cost per patient £10,775 (95%, CI £10,502 to £11,048, $15 616, 95% CI, $15 220 to $16 012) versus £8221 (95% CI, £7954 to £8487, $11 914, 95% CI, $11528 to $12 300); a significant cost increase of £2554 ($3701) per patient compared with direct surgery (Table 3). The increase in costs was due to the additional cost of the PBD procedure. QALYs up to 6 mo were slightly lower for PBD compared with direct surgery (0.337 [95% CI, 0.337–0.338] versus 0.343 [95% CI, 0.343–0.344]), because of the complications associated with PBD. The MNBs for PBD were significantly lower than those for direct surgery at maximum willingness to pay for a QALY of £20,000 ($28,986) and £30,000 ($43,478), indicating that direct surgery was preferred on cost-effectiveness grounds. As expected, the probabilistic results were numerically similar to the deterministic results (not shown).
Table 3.
Base case results.
| PBD | Direct surgery | |||
|---|---|---|---|---|
| Costs | ||||
| UK£ | 10,775 | (10,502 to 11,048) | 8221 | (7954 to 8487) |
| US$ | 15,616 | (15,220 to 16,012) | 11,914 | (11,528 to 12, 300) |
| QALYs | 0.337 | (0.337 to 0.338) | 0.343 | (0.343 to 0.344) |
| MNB | ||||
| UK£20,000 | −4031 | (−3758 to −4304) | −1359 | (−1092 to −1626) |
| US$28 986 | −5843 | (−6485 to −5685) | −1969 | (−2551 to –1768) |
| UK£30,000 | −659 | (−386 to −933) | 2072 | (1805 to 2340) |
| US$43 478 | −956 | (−1599 to –798) | 3003 | (2424 to 3206) |
Costs are in 2011–2012 UK£ and US$. Figures are expected values per patient with 95% CIs in brackets. The point estimates are calculated using base case values of the model parameters (deterministic results). The 95% CIs are derived using standard deviations calculated from the 5000 simulations in the probabilistic sensitivity analysis. The MNB is calculated at a maximum willingness to pay for a QALY of £20,000 ($28 986) and £30,000 ($43 478). Numbers may not sum because of rounding.
In the one-way sensitivity analysis (Table 2), the results were neither sensitive to changing the values of the parameters within the ranges stated nor were they sensitive to basing the probabilities of complications with PBD and with complications of surgery on a single large trial [17]: in every situation direct surgery was the most cost-effective option.
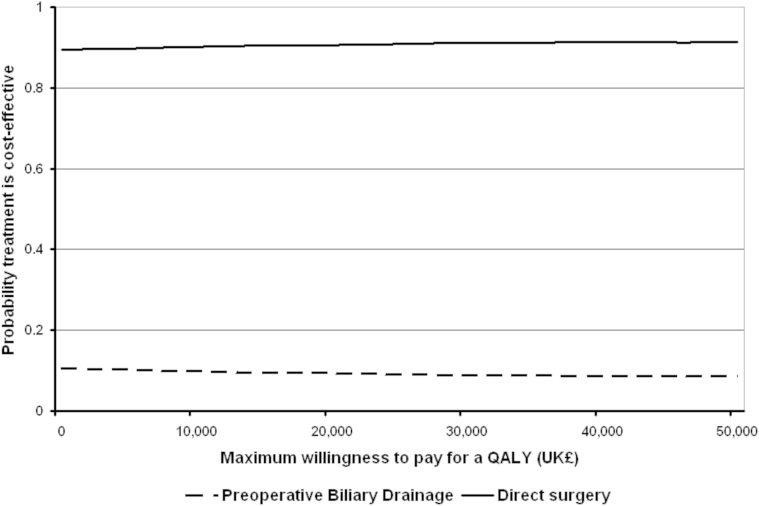
The cost-effectiveness acceptability curves for each treatment show that PBD had a 9.5% probability of being cost-effective at a maximum willingness to pay for a QALY of £20,000 ($28,986) and a 8.9% probability at a value of £30,000 ($43,478; Fig. 2).
Fig. 2.
Cost-effectiveness acceptability curves showing the probability that each option is cost-effective at different values of the maximum willingness to pay for a QALY. In the United Kingdom, the lower and upper limit of the maximum willingness to pay for a QALY are £20,000 ($28 986) and £30,000 ($43 478), respectively.
4. Discussion
4.1. Main findings
This study estimated the expected cost and QALYs of PBD versus direct surgery for obstructive jaundice in patients with pancreatic and periampullary cancer. Routine use of PBD was not cost-effective. It was more costly than direct surgery, with a mean cost per patient £10,775 ($15,616) versus £8221 ($11,914), respectively. It also produced fewer QALYs, with mean QALYs per patient of 0.337 versus 0.343, respectively. The MNB for PBD was lower than for direct surgery at a maximum willingness to pay for a QALY of £20,000 ($28,986) and £30,000 ($43,478). There is little uncertainty with this finding, demonstrated through extensive one-way and probabilistic sensitivity analyses.
4.2. Strengths and weaknesses
The strengths of this study are that it was based on a recently published Cochrane Review that analyzed in detail the available evidence for whether or not routine PBD is beneficial to patients with obstructive jaundice [8,9]. A comprehensive sensitivity analysis has also been performed, showing that although there is some uncertainty in the values used in the base case analysis, the conclusions are not sensitive to changing these values.
There are a number of weaknesses. First, the utility scores on which the QALY estimates were made are weak. However, the results are not sensitive to the values used, as demonstrated in sensitivity analyses. Second, the time horizon of the model over which costs and QALYs are measured is 6 mo. We have ignored differences in costs and QALYs beyond 6 mo. It may be that some of the complications of PBD persist beyond 6 mo, and if so including costs and outcomes beyond 6 mo would favor direct surgery. Third, the analysis was undertaken from the perspective of the UK NHS. A wider perspective, for example, a societal one, would also include impacts on the rest of society, including patients, families, and businesses. Given that PBD is associated with additional morbidity and also involves managing a drain during the period of PBD, it may be that if the costs from these other viewpoints were included, the cost increases attributable to PBD would be greater than shown.
4.3. Comparison with other studies
This is the first study to evaluate the cost-effectiveness of PBD for obstructive jaundice in pancreatic and periampullary cancer. The data on which this cost-effectiveness analysis was based were from a systematic review, which appraised the existing literature in depth [8,9].
4.4. Implications for policy and practice
This study provides a strong economic case to support the clinical evidence that PBD for obstructive jaundice in patients with pancreatic and periampullary cancer should not be used routinely. The findings are equally applicable in patients with distal cholangiocarcinomas and duodenal tumors with obstructive jaundice. This is because although the majority of tumors included in the trials, which provided the data for this cost-effectiveness analysis had pancreatic or ampullary tumors, the underlying reason for performing a PBD is the same in distal cholangiocarcinomas and duodenal tumors. These findings are applicable only in patients eligible for surgical resection with obstructive jaundice. Only about 20% of patients with pancreatic and periampullary cancer are eligible for surgical resection [1]. The findings are also not applicable in patients with cholangitis because of the common bile duct obstruction or in patients undergoing preoperative neoadjuvant chemotherapy. The mean duration of jaundice was stated in three trials and ranged between 28 and 55 d. So, the findings of this review are only applicable when the interval between jaundice and surgery is <2 mo on average [4,15,18]. However, our cost-effectiveness analysis provides a sound basis for avoiding excessive delays to surgery because of administrative reasons.
There is no evidence on the extent of use of PBD for obstructive jaundice in patients with pancreatic and periampullary cancer in the United Kingdom, so a national budget impact calculation is not possible. However, based on the findings in the present study, not performing PBD in patients with pancreatic and periampullary cancer would result in cost savings of approximately £2500 ($3623) per patient to the NHS.
4.5. Further research
This study is based on a Cochrane Review of PBD for obstructive jaundice. However, this analysis considered only the cost-effectiveness of PBD use in patients with resectable pancreatic and periampullary cancer who had been considered suitable for inclusion in trials comparing drainage with no drainage before surgery. Further economic evaluation is required to assess the costs and benefits in patients who were excluded from the trials such as those with cholangitis, high bilirubin levels (>250 μL/L), associated renal failure, or those undergoing preoperative neoadjuvant chemotherapy.
The Cochrane Review concluded that further randomized controlled trials, with low risk of bias, including long-term survival and quality of life measures are needed in patients with malignant obstructive jaundice. Such trials should also collect utility and cost data to facilitate cost-effectiveness analyses.
5. Conclusions
Routine PBD for obstructive jaundice in patients with pancreatic and periampullary cancer is not cost-effective.
Acknowledgment
This project was funded by the National Institute for Health Research (National Institute for Health Research Cochrane Programme Grant Scheme; reference number 10/4001/11). The views and opinions expressed are those of the authors and do not necessarily reflect those of the National Institute for Health Research, National Health Service, or the Department of Health.
Authors' contributions: S.M., K.S.G., and B.R.D. conceived and designed the study. S.M. and K.S.G. collected the data. S.M. and K.S.G. analyzed the data. All authors interpreted the data. S.M. wrote the first draft of the manuscript and all authors provided critical revisions that were important for the intellectual content. All authors approved the final version of the manuscript.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
References
- 1.Pancreatic Section of the British Society of Gastroenterology. Pancreatic Society of Great Britain and Ireland. Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland. Royal College of Pathologists. Special Interest Group for Gastro-Intestinal Radiology Guidelines for the management of patients with pancreatic cancer, periampullary and ampullary carcinomas. Gut. 2005;54(Suppl 5):v1. doi: 10.1136/gut.2004.057059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groen P.C., Gores G.J., LaRusso N.F., Gunderson L.L., Nagorney D.M. Biliary tract cancers. N Engl J Med. 1999;341:1368. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Phoa S.S., Reeders J.W., Rauws E.A., De Wit L., Gouma D.J., Laméris J.S. Spiral computed tomography for preoperative staging of potentially resectable carcinoma of the pancreatic head. Br J Surg. 1999;86:789. doi: 10.1046/j.1365-2168.1999.01138.x. [DOI] [PubMed] [Google Scholar]
- 4.van der Gaag N.A., Kloek J.J., de Castro S.M., Busch O.R., van Gulik T.M., Gouma D.J. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13:814. doi: 10.1007/s11605-008-0618-4. [DOI] [PubMed] [Google Scholar]
- 5.Klinkenbijl J.H., Jeekel J., Schmitz P.I. Carcinoma of the pancreas and periampullary region: palliation versus cure. Br J Surg. 1993;80:1575. doi: 10.1002/bjs.1800801227. [DOI] [PubMed] [Google Scholar]
- 6.Trede M., Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39. doi: 10.1097/00000658-198801000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmings A.N., van Deventer S.J., Obertop H., Rauws E.A., Huibregtse K., Gouma D.J. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725. doi: 10.1136/gut.46.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y., Gurusamy K.S., Wang Q. Meta-analysis of randomized clinical trials on safety and efficacy of biliary drainage before surgery for obstructive jaundice. Br J Surg. 2013;100:1589. doi: 10.1002/bjs.9260. [DOI] [PubMed] [Google Scholar]
- 9.Fang Y., Gurusamy K.S., Wang Q. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012;9:CD005444. doi: 10.1002/14651858.CD005444.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daker C., van Someren N., Besherdas K. Surgery for pancreatic cancer without preoperative biliary drainage: fiction in reality? Gut. 2012;61(Suppl 2):A112. [Google Scholar]
- 11.Center for the Evaluation of Value and Risk in Health (CEVR). Cost-effectiveness analysis registry.[Online]. Available from: https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx. [Accessed 3 December 2013].
- 12.Morris S., Devlin N., Parkin D., Spencer A. 2nd ed. Wiley; London: 2012. Economic analysis in health care. [Google Scholar]
- 13.Hatfield A.R., Tobias R., Terblanche J. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- 14.Lai E.C., Mok F.P., Fan S.T. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 15.McPherson G.A., Benjamin I.S., Hodgson H.J., Bowley N.B., Allison D.J., Blumgart L.H. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- 16.Pitt H.A., Gomes A.S., Lois J.F., Mann L.L., Deutsch L.S., Longmire W.P., Jr. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Gaag N.A., Rauws E.A., van Eijck C.H. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 18.Wig J.D., Kumar H., Suri S., Gupta N.M. Usefulness of percutaneous transhepatic biliary drainage in patients with surgical jaundice. A prospective randomised study. J Assoc Physicians India. 1999;47:271. [PubMed] [Google Scholar]
- 19.Krzyzanowska M.K., Earle C.C., Kuntz K.M., Weeks J.C. Using economic analysis to evaluate the potential of multimodality therapy for elderly patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;67:211. doi: 10.1016/j.ijrobp.2006.07.1390. [DOI] [PubMed] [Google Scholar]
- 20.Murphy J.D., Chang D.T., Abelson J. Cost-effectiveness of modern radiotherapy techniques in locally advanced pancreatic cancer. Cancer. 2012;118:1119. doi: 10.1002/cncr.26365. [DOI] [PubMed] [Google Scholar]
- 21.Glimelius B., Hoffman K., Graf W. Cost-effectiveness of palliative chemotherapy in advanced gastrointestinal cancer. Ann Oncol. 1995;6:267. doi: 10.1093/oxfordjournals.annonc.a059157. [DOI] [PubMed] [Google Scholar]
- 22.Karuna S.T., Thirlby R., Biehl T., Veenstra D. Cost-effectiveness of laparoscopy versus laparotomy for initial surgical evaluation and treatment of potentially resectable Hepatic colorectal metastases: a decision analysis. J Surg Oncol. 2008;97:396. doi: 10.1002/jso.20964. [DOI] [PubMed] [Google Scholar]
- 23.Langenhoff B.S., Krabbe P.F., Peerenboom L., Wobbes T., Ruers T.J. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg. 2006;93:1007. doi: 10.1002/bjs.5387. [DOI] [PubMed] [Google Scholar]
- 24.Nieveen van Dijkum E.J., Kuhlmann K.F., Terwee C.B., Obertop H., de Haes J.C., Gouma D.J. Quality of life after curative or palliative surgical treatment of pancreatic and periampullary carcinoma. Br J Surg. 2005;92:471. doi: 10.1002/bjs.4887. [DOI] [PubMed] [Google Scholar]
- 25.Schniewind B., Bestmann B., Henne-Bruns D., Faendrich F., Kremer B., Kuechler T. Quality of life after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head. Br J Surg. 2006;93:1099. doi: 10.1002/bjs.5371. [DOI] [PubMed] [Google Scholar]
- 26.Chan C., Franssen B., Dominguez I., Ramirez-Del Val A., Uscanga L.F., Campuzano M. Impact on quality of life after pancreatoduodenectomy: a prospective study comparing preoperative and postoperative scores. J Gastrointest Surg. 2012;16:1341. doi: 10.1007/s11605-012-1898-2. [DOI] [PubMed] [Google Scholar]
- 27.Department of Health. National Schedule of Reference Costs - Year 2011-12-NHS trusts and NHS foundation trusts: NHS own costs. [Online]. Available from: https://www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 [Accessed 27 August 2013].
- 28.National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. [Online]. Available from: http://publications.nice.org.uk/pmg9 [Accessed 3 July 2013]. [PubMed]
- 29.Briggs A., Sculpher M., Claxton K. Oxford University Press; Oxford: 2006. Decision modeling for health economic evaluation. [Google Scholar]
- 30.Centre for Bayesian Statistics in Health Economics. Software. [Online]. Available from: http://www.shef.ac.uk/chebs/software [Accessed 27 August 2013].