Abstract
Objective
Sleep disturbances are common among women in midlife; prevalence increases among perimenopausal/postmenopausal women with vasomotor symptoms. Paroxetine 7.5 mg is the only nonhormonal treatment that has been approved in the United States for moderate to severe vasomotor symptoms associated with menopause. In two pivotal phase 3 studies evaluating its efficacy and safety, improvements in sleep disturbances were also prospectively evaluated.
Methods
Postmenopausal women with moderate to severe vasomotor symptoms were randomly assigned to paroxetine 7.5 mg (n = 591) or placebo (n = 593) once daily for 12 weeks (both studies) or 24 weeks (24-wk study). Predefined assessments on weeks 4, 12, and 24 included number of nighttime awakenings attributed to vasomotor symptoms, sleep-onset latency, sleep duration, and sleep-related adverse events. The two studies’ data for weeks 1 to 12 were pooled.
Results
At baseline, participants reported a mean of 3.6 awakenings/night attributed to vasomotor symptoms. Nighttime awakenings attributed to vasomotor symptoms were significantly reduced within 4 weeks of initiating paroxetine 7.5 mg treatment (39% reduction vs 28% for placebo; P = 0.0049), and reductions were sustained through 12 or 24 weeks of treatment. Paroxetine 7.5 mg treatment also significantly increased nighttime sleep duration (week 4, +31 vs +16 min for placebo; P = 0.0075), but no significant between-group differences in sleep-onset latency or sleep-related adverse events such as sedation were observed.
Conclusions
In postmenopausal women treated for menopausal vasomotor symptoms, paroxetine 7.5 mg significantly reduces the number of nighttime awakenings attributed to vasomotor symptoms and increases sleep duration without differentially affecting sleep-onset latency or sedation.
Key Words: Hot flashes, Menopause, Nighttime awakening, Paroxetine 7.5 mg, Sleep duration, Vasomotor symptoms
Disturbances in sleep (eg, sleep induction, sleep maintenance, nighttime awakenings, and early morning wakefulness) are common among women during midlife.1,2 The prevalence of sleep disturbances increases with age and menopause status, with up to half of women aged 40 to 59 years reporting poor sleep quality.2,3 Targeting the specific causal factors/conditions of sleep disturbances, rather than treating the sleep problem nonspecifically with sedative or hypnotic medications, is important given that these medications are recommended for short-term situational sleep problems rather than for long-term disorders because of their addictive potential.4
Hot flashes and night sweats, collectively termed vasomotor symptoms (VMS), occur in up to 80% of perimenopausal and postmenopausal women.5,6 VMS can be bothersome and may negatively impact functioning7 and activities of daily living, including work, social, and leisure activities.8 Furthermore, VMS have been linked to increased prevalence of sleep disturbances and nighttime awakenings during menopause.9-13 Because sleep disturbance is a risk factor for impaired daytime functioning, development of medical and affective disorders, and increased health care costs,14,15 sleep disturbances associated with menopause may negatively affect the health and safety of postmenopausal women and persons with whom they interact.
Hormone therapy is effective for treating VMS associated with menopause16,17 and improves sleep disturbances related to VMS in perimenopausal and early postmenopausal women.1,4,18 However, hormone therapy is contraindicated in, or is not acceptable for use by, some postmenopausal women19-21; this has led researchers to evaluate the efficacy of nonhormonal treatments of VMS.22-29
Paroxetine 7.5 mg capsules (Brisdelle; formerly low-dose mesylate salt of paroxetine), which consist of a low dose of the mesylate salt of paroxetine, were specifically developed for the treatment of women with moderate to severe VMS associated with menopause. In two multicenter, randomized, double-blind, placebo-controlled phase 3 studies,30 paroxetine 7.5 mg once daily significantly reduced the mean weekly frequency (P < 0.0001) and severity (P ≤ 0.0110) of VMS on weeks 4 and 12 in postmenopausal women compared with placebo. Paroxetine 7.5 mg was generally well tolerated; most treatment-emergent adverse events (TEAEs) were mild or moderate in severity.30 Unlike higher doses of paroxetine that have been approved for the treatment of psychiatric conditions (which require gradual dose reduction before drug cessation to reduce the risk of discontinuation syndrome),31,32 paroxetine 7.5 mg could be stopped without the need for tapering; minimal acute discontinuation symptoms were reported upon stopping study drug in phase 3 studies.30 Furthermore, treatment with paroxetine 7.5 mg did not result in adverse events (AEs) such as weight gain and sexual dysfunction, which are commonly associated with higher-dose selective serotonin reuptake inhibitors (SSRIs).33
Although some SSRIs (and serotonin-norepinephrine reuptake inhibitors) are known to worsen sleep in individuals with depression,34,35 recent data suggest that the SSRI escitalopram may improve nighttime sleep disturbances among nondepressed women receiving treatment of VMS.36 The underlying cause of sleep disturbances (whether psychiatric in origin or attributed to VMS) and the population type treated may be factors in how individuals’ sleep outcomes are affected by treatment with SSRIs. The impact of higher (psychiatric) doses of paroxetine on sleep has been inconsistent across studies37-39; therefore, improvements in sleep disturbances with paroxetine 7.5 mg were evaluated a priori in the two phase 3 studies in women with menopausal VMS. Sleep parameters were assessed using predefined sleep diary measures for nighttime awakenings, sleep-onset latency and duration, interference of symptoms with daily living, and difficulty sleeping. AEs related to sleep during the treatment period and after discontinuation of treatment were also evaluated. We now report the impact of paroxetine 7.5 mg/day on these self-reported sleep parameters in an analysis of data from the two pivotal phase 3 studies.
METHODS
Participants and study design
Detailed descriptions of the two multicenter, randomized, double-blind, placebo-controlled phase 3 studies, including inclusion and exclusion criteria and VMS efficacy endpoints, have been published previously.30 The treatment period was 12 weeks in the first study (ClinicalTrials.gov identifier NCT01361308) and 24 weeks in the second study (ClinicalTrials.gov identifier NCT01101841). Otherwise, the two studies were similar in screening, placebo run-in period, population enrolled, and treatment regimens.
In brief, participants in both studies were postmenopausal women aged at least 40 years at screening who met one of the following criteria for menopause: spontaneous amenorrhea for 12 consecutive months or more; amenorrhea for 6 months or more, with follicle-stimulating hormone levels higher than 40 mIU/mL; or bilateral salpingo-oophorectomy, with or without hysterectomy, 6 weeks or more before screening. A key inclusion criterion was an average of more than 7 to 8 moderate to severe hot flashes/day or 50 to 60 moderate to severe hot flashes/week reported for 30 days or more before screening. Psychotropic drugs, including all sedative and hypnotic medications (with the exception of zolpidem, zaleplon, eszopiclone, and diphenhydramine), were prohibited during the study. Use of nightly zolpidem, zaleplon, eszopiclone, and diphenhydramine was minimal, and no analysis was performed with respect to the use of these medications. Participants taking psychotropic drugs or estrogen/progestin-containing products were required to undergo prespecified washout periods before the run-in visit. Key exclusion criteria were as follows: known nonresponse of VMS to previous SSRI or serotonin-norepinephrine reuptake inhibitor treatment, untreated hypertension, impaired liver or kidney function, unstable cardiac disease, pregnancy, history of self-injurious behavior, history of clinical diagnosis or treatment of depression or any other psychiatric disorder (including substance abuse or alcohol disorders), and any other ongoing medical condition. No sleep-specific inclusion or exclusion criteria (such as presence of sleep apnea or restless legs) were applied.
Each study began with a single-blind, placebo run-in period of up to 12 days, during which eligible participants received placebo once daily at bedtime and used electronic daily diaries to record the number and severity of VMS and the number of nighttime awakenings that they attributed to VMS. A double-blind treatment period followed, during which participants were randomly assigned 1:1 to receive paroxetine 7.5 mg or an identical capsule of placebo once daily at bedtime for 12 or 24 weeks. Randomization was applied centrally across all sites using an interactive voice response system, and all personnel were blinded to study medication until study completion and database lock.
Study assessments
Participants recorded VMS daily using a real-time interactive voice or Web response system that was accessible 24 hours/day; primary VMS efficacy endpoints have been reported elsewhere.30 Sleep parameters included change from baseline in the total number of nighttime awakenings attributed to VMS (where participants were asked to self-record all nighttime awakenings that they attributed to VMS in the electronic sleep diary the following morning between 6 am and 11 am; Appendix) and other sleep-related measurements (sleep-onset latency and hours of sleep per night; Appendix). Data were recorded throughout the study period and were collected for analysis in both studies on day 1 (study start), day 28 (week 4), and day 85 (week 12; end of 12-wk study) and for the 24-week study on day 169 (week 24; end of 24-wk study).
In addition, the extent to which VMS interfered with sleep was measured on weeks 4, 12, and 24 using the sleep interference item from the 10-item validated Hot Flash–Related Daily Interference Scale (HFRDIS),40 with scores ranging from 0 (no interference with sleep associated with hot flashes) to 10 (interference with sleep associated with hot flashes to the worst possible extent). This HFRDIS item was defined as a secondary endpoint a priori. The proportion of women with moderate to severe difficulty sleeping was also assessed using the validated 21-item Greene Climacteric Scale (GCS),41 which includes a question on “difficulty in sleeping” (0, none; 1, mild; 2, moderate; 3, severe).
Safety was assessed by evaluating TEAEs and potential discontinuation AEs. The Discontinuation-Emergent Signs and Symptoms scale, which includes the symptoms “trouble sleeping/insomnia” and “increased dreaming or nightmares,” was administered within a mean (SD) of 7 (3) days of the last dose of study medication, regardless of when the participant exited the study. Sleep-related TEAEs and discontinuation AEs are presented herein.
Statistical analyses
The principal population for analysis of secondary endpoints was the modified intent-to-treat population (all consenting and randomly assigned participants with valid baseline daily VMS diary data who had taken one or more doses of study medication and had one or more days of on-treatment daily VMS diary data). The safety population comprised all participants who received one or more doses of study medication and who had one or more postdose safety measurements.
In this prospective analysis of sleep-related predefined secondary endpoints, data from the two phase 3 studies were pooled for weeks 1 to 12. Data for weeks 13 to 24 represent the 24-week study only. The two studies were similarly designed and involved comparable populations, allowing pooling of data, which enables evaluation of information from more than 1,100 participants and makes the data set and analysis more robust.
Prespecified analyses were used to assess the impact of paroxetine 7.5 mg on the number of nighttime awakenings attributed to VMS, sleep-onset latency, sleep duration, and sleep scores (HFRDIS and GCS). For each participant, total nighttime awakenings attributed to VMS at baseline were calculated as the average during the run-in period before randomization. Nighttime awakenings during the double-blind treatment period were calculated as the average for each study week. Sleep-onset latency and duration of sleep were calculated as change from baseline in number of minutes at each postbaseline time point evaluated.
RESULTS
Participant disposition and baseline characteristics
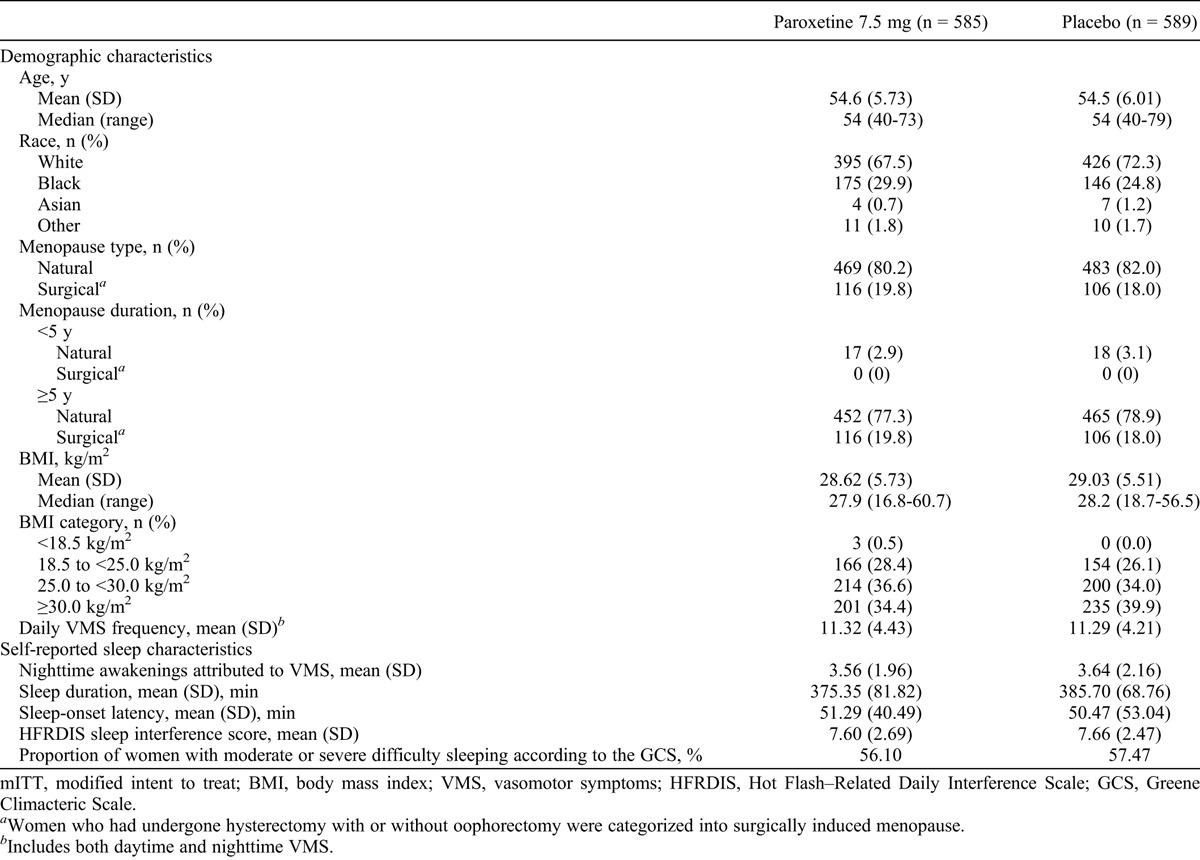
In total, 614 participants were randomly assigned to the 12-week study, and 570 participants were randomly assigned to the 24-week study; overall, more than 80% of women completed the studies, and most were at least 80% compliant with study drug treatment.30 For the pooled analysis, the modified intent-to-treat population comprised 1,174 women (paroxetine 7.5 mg, n = 585; placebo, n = 589), and the safety population comprised 1,175 women (paroxetine 7.5 mg, n = 586; placebo, n = 589; Fig. 1). Demographic characteristics were not notably different between treatment arms at baseline, although statistical analyses were not performed on baseline demographic variables (Table 1). At baseline, the mean (SD) daily frequency of VMS (daytime plus nighttime) was 11.3 (4) in both groups.30 Sleep-related characteristics at baseline were also similar between treatment arms: participants experienced a mean (SD) of 3.6 (2) nighttime awakenings attributed to VMS (or 25 per week) and indicated that hot flashes interfered with sleep to a large extent, with an overall mean HFRDIS score of 7.6 (Table 1).
FIG. 1.
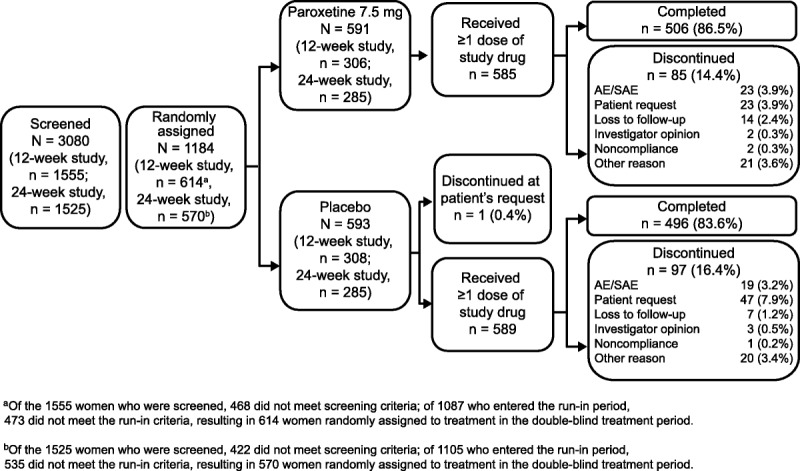
Participant disposition (all randomly assigned populations). AE, adverse event; SAE, serious adverse event.
TABLE 1.
Demographics and baseline characteristics (pooled data; mITT population)
Impact of treatment on self-reported sleep measures
In the pooled analysis of data through week 12, the reduction in the number of nighttime awakenings attributed to VMS was significantly greater among participants receiving paroxetine 7.5 mg than among participants receiving placebo on weeks 4 and 12 (Fig. 2A). On week 4 (pooled data), the reduction from baseline in nighttime awakenings attributed to VMS was 39% (from 3.56 to 2.17) in the paroxetine 7.5 mg treatment arm compared with 28% (from 3.64 to 2.63) in the placebo arm (P = 0.0049). On week 12 (pooled data), reductions from baseline were 54% (from 3.56 to 1.65) in the paroxetine 7.5 mg arm and 43% (from 3.64 to 2.08) in the placebo arm (P = 0.0001). In the 24-week study, this effect was sustained for the entire treatment period, with participants in the paroxetine 7.5 mg arm experiencing a 62% reduction from baseline in nighttime awakenings attributed to VMS (from 3.58 to 1.36) compared with 43% (from 3.56 to 2.02) in the placebo arm (P < 0.0001).
FIG. 2.
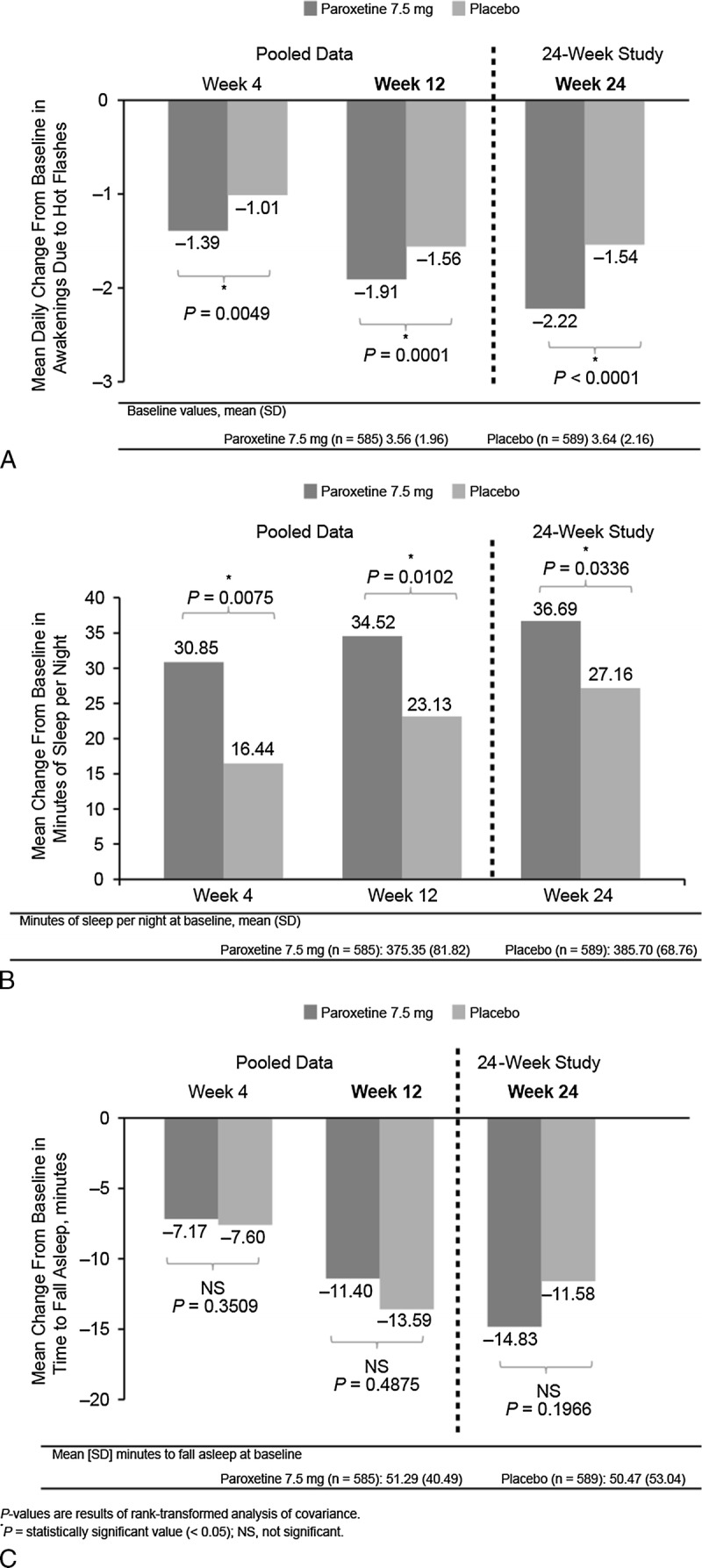
Mean change from baseline in daily nighttime awakenings attributed to vasomotor symptoms (A), duration of sleep (B), and sleep-onset latency (C).
The duration of sleep per night increased significantly more among participants receiving paroxetine 7.5 mg than among those receiving placebo at all postbaseline time points (Fig. 2B). Participants receiving paroxetine 7.5 mg had a significant increase in sleep time (+8%, 31 min) compared with those receiving placebo on week 4 (pooled data: +4%, 16 min; P = 0.0075), week 12 (pooled data: paroxetine 7.5 mg, 9%, 35 min; placebo, 6%, 23 min; P = 0.0102), and week 24 (24-wk study: paroxetine 7.5 mg, 10%, 37 min; placebo, 7%, 27 min; P = 0.0336).
No significant differences in sleep-onset latency were noted between the two treatment arms during the course of the study (Fig. 2C). On week 4 (pooled data), sleep-onset latency was reduced by 14% (7 min) in participants receiving paroxetine 7.5 mg and by 15% (8 min) in those receiving placebo (P = 0.3509). On week 12 (pooled data), sleep-onset latency was reduced by 22% (11 min) and 27% (14 min; P = 0.4875), respectively. On week 24 (24-wk study), reductions were 30% (15 min) and 27% (12 min; P = 0.1966), respectively.
Scores for the HFRDIS sleep item (Fig. 3A) were reduced from baseline to a significantly greater extent in the paroxetine 7.5 mg arm than in the placebo arm on week 4 (pooled data: paroxetine 7.5 mg, 32%; placebo, 20%; P = 0.0068), but not on week 12 (pooled data: paroxetine 7.5 mg, 42%; placebo, 35%; P = 0.0663) or on week 24 (24-wk study: paroxetine 7.5 mg, 51%; placebo, 38%; P = 0.2405).
FIG. 3.
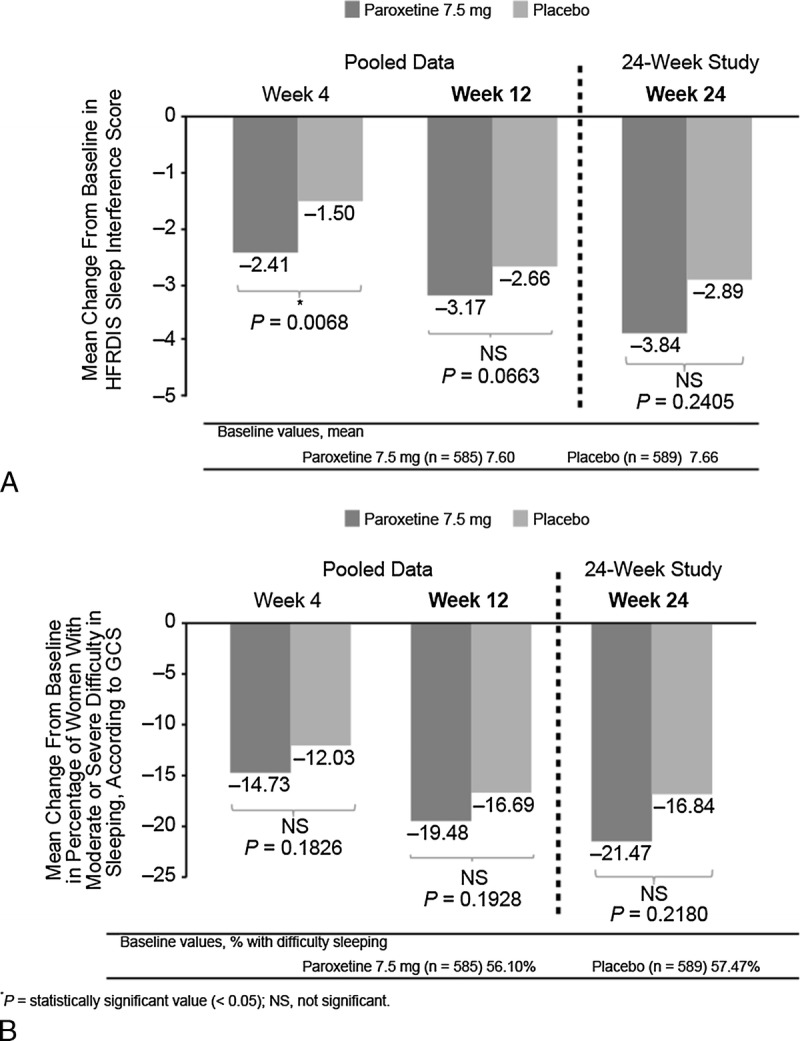
Mean change from baseline in sleep interference scores (A) and in the GCS sleep item “difficulty in sleeping” (B). HFRDIS, Hot Flash–Related Daily Interference Scale; GCS, Greene Climacteric Scale.
On the GCS sleep item “difficulty in sleeping,” the proportions of participants reporting moderate to severe difficulty sleeping decreased from baseline in both treatment arms; however, the differences between treatment groups were not statistically significantly different at any time point (Fig. 3B).
Safety
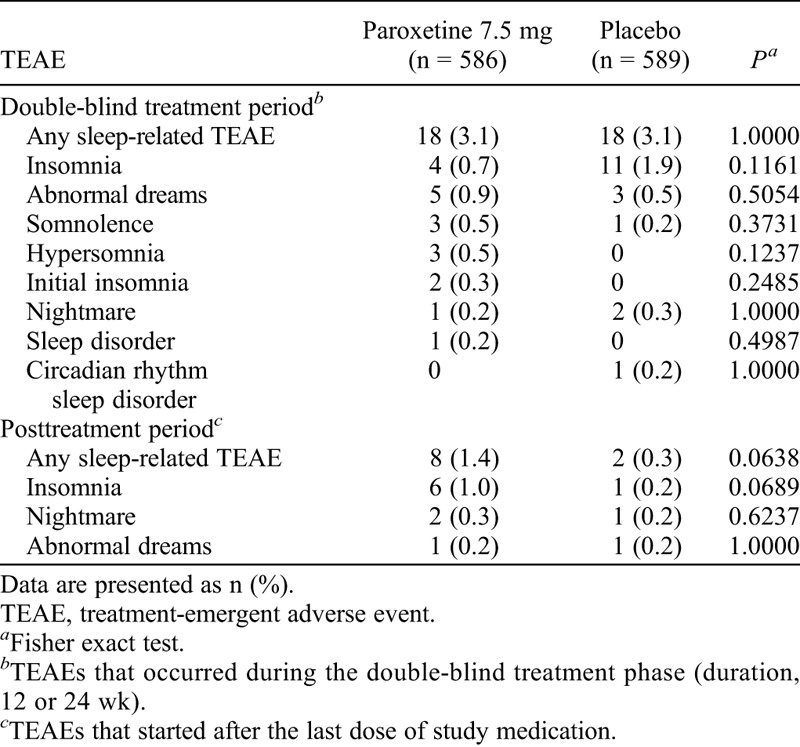
The proportion of participants reporting a sleep-related TEAE was low in both treatment arms. During the treatment period, 3.1% of women in both treatment arms reported at least one sleep-related TEAE; after treatment discontinuation, 1.4% in the paroxetine 7.5 mg arm and 0.3% in the placebo arm reported a sleep-related TEAE. No statistically significant differences in individual sleep-related TEAEs were reported between treatment arms (Table 2). Furthermore, according to the results of the Discontinuation-Emergent Signs and Symptoms scale, no meaningful differences in discontinuation-emergent signs and symptoms were noted between treatment arms after treatment discontinuation without tapering.
TABLE 2.
Sleep-related adverse events reported during the double-blind treatment period and posttreatment period (pooled data; safety population)
DISCUSSION
In this pooled analysis of data from two phase 3 studies, treatment with paroxetine 7.5 mg significantly and sustainably reduced the mean number of nighttime awakenings attributed to VMS and increased the duration of sleep per night compared with placebo without differentially affecting sleep-onset latency or AE reporting of sedation. Although interference of VMS with sleep on the HFRDIS was initially improved to a greater extent with paroxetine 7.5 mg than with placebo, this effect was not sustained. Taken together, these results suggest that paroxetine 7.5 mg had a selective effect on sleep parameters related to VMS rather than a nonspecific effect on sleep parameters (ie, increasing sedation or inducing somnolence).
Paroxetine 7.5 mg was specifically developed to treat moderate to severe VMS associated with menopause.30 SSRIs such as paroxetine at doses prescribed for psychiatric conditions may adversely affect sleep37,42,43; therefore, we examined the effect of this low-dose formulation in a population of women without mental health issues. In this analysis of phase 3 data, treatment with paroxetine 7.5 mg significantly reduced the mean nighttime number of nighttime awakenings attributed to VMS within 1 month of the start of therapy (−1.39) compared with placebo (−1.01; a difference of 0.38 additional awakenings reduced by paroxetine vs placebo). Furthermore, the magnitude of the reduction was sustained, and even increased, after 12 and 24 weeks of ongoing paroxetine 7.5 mg treatment. Although the overall reduction of 0.38 in nighttime awakenings is only a moderate effect, it may be clinically meaningful for some women, potentially resulting in positive benefits for daily functioning and quality of life. Of note, only the number of nighttime awakenings associated with VMS was recorded in these phase 3 studies; there is no available information on whether the number of awakenings unrelated to VMS was altered or whether the duration of nighttime awakenings attributed to VMS changed.
In addition to reducing the number of nighttime awakenings attributed to VMS, paroxetine 7.5 mg significantly increased the duration of sleep per night, indicating a link between reduced nighttime awakenings and improvement in overall sleep duration. The improvement in sleep duration with paroxetine 7.5 mg (an increase of up to 37 min/night from baseline) compares favorably with results obtained with agents used specifically for the treatment of insomnia, such as zolpidem, which has been shown to increase total sleep time by 51 minutes or more per night.44,45 Studies using objective sleep assessments have shown that nighttime VMS do not extensively alter sleep architecture,13 suggesting that reducing VMS may be sufficient to restore normal sleep patterns. Additional studies would allow a direct correlation of sleep improvements with changes in menopause-specific quality-of-life measures.
No significant differences in sleep-onset latency were observed between paroxetine 7.5 mg and placebo. It is notable that baseline levels of sleep-onset latency were higher than expected for a group of women not selected for sleep problems or insomnia. Sleep modeling studies may provide further information on whether sleep effects attributable to paroxetine 7.5 mg treatment are direct or indirect and may define how sleep duration is increased without differential reduction in sleep-onset latency.
The use of the HFRDIS in the present analysis to assess the impact of VMS on sleep indicates that, during the double-blind treatment period, scores in both treatment arms were reduced within 4 weeks of treatment initiation (pooled data: paroxetine 7.5 mg, −2.41; placebo, −1.50) and reductions continued for up to 24 weeks of treatment (24-wk study: paroxetine 7.5 mg, −3.84; placebo, −2.89). Although between-treatment group differences were significant only on week 4, a trend toward greater improvement with paroxetine 7.5 mg was observed on weeks 12 and 24. However, it must be noted that use of a single item from the HFRDIS to evaluate sleep has not been validated. The proportion of participants with moderate to severe difficulty sleeping, according to the GCS, decreased in both groups at all time points, but differences between treatment arms were not statistically significantly different. Future studies would benefit from the inclusion of specific sleep interference scales, such as the Insomnia Severity Index (ISI),46 to identify the importance of sleep improvement for participants.
The frequency of sleep-related AEs, such as somnolence, at doses of paroxetine prescribed for psychiatric conditions is dose-related,42 suggesting that lower doses (such as 7.5 mg) may result in fewer such AEs. Consistent with this hypothesis, somnolence was reported as an AE in only 0.5% of paroxetine 7.5 mg–treated women in the paroxetine 7.5 mg phase 3 studies, further reinforcing the supposition that the effects of paroxetine 7.5 mg on sleep are probably a result of a reduction in VMS rather than sedation. In a phase 2 trial of paroxetine 7.5 mg, sleep parameters were not assessed, but neither insomnia nor somnolence was reported as an AE.47
Previous publications have observed a link between VMS associated with menopause and increased prevalence of sleep disturbances and nighttime awakenings.9 A subcohort single-site study of 51 white and African-American postmenopausal women who participated in the Study of Women’s Health Across the Nation evaluated whether VMS during sleep were associated with poorer sleep. Measures included sternal skin conductance to capture VMS, actigraphy to objectively assess sleep, a VMS diary, and a sleep diary completed before sleep and on awakening.10 For these women, VMS reported on awakening—but not physiologically assessed VMS or VMS reported during sleep—were related to poorer actigraphic sleep.10 A single-cohort university study (Do Stage Transitions Result In Detectable Effects [STRIDE]) evaluated associations among sleep disturbance and the frequency, bothersomeness, and interference of hot flashes in 623 women at various menopause stages. Here, VMS were assessed annually during a 2-week period using the HFRDIS, and women self-reported on sleep. In multivariable models, women reporting bothersome hot flashes were more likely to report sleep disturbances than women who reported no VMS (odds ratio, 2.1; 95% CI, 1.4-3.2).11 In another recent study, estradiol was suppressed by leuprolide in 29 healthy premenopausal volunteers, and VMS were assessed using polysomnography, a sleep diary, and standardized questionnaires.13 Increasing nighttime VMS frequency measured by polysomnography resulted in increased wake after sleep onset time and number of awakenings, validating the subjective experiences of women reporting awakenings in the sleep diary.13
The ISI46 and the Pittsburgh Sleep Quality Index (PSQI)48 were used to evaluate subjective sleep quality in a recent 8-week randomized study of the SSRI escitalopram (10-20 mg/d) versus placebo in 205 perimenopausal/postmenopausal women with VMS.36 Treatment with escitalopram significantly reduced ISI and PSQI scores on week 8 compared with placebo (P < 0.001 for both). Clinical improvements in insomnia symptoms and subjective sleep quality (defined as ≥50% decreases in the ISI and PSQI from baseline) occurred more frequently in escitalopram-treated women than in the placebo group.36 Although these data support the inference from paroxetine 7.5 mg studies that SSRI treatment can improve sleep in postmenopausal women with VMS, a direct comparison of outcomes is not possible because different sleep measures were used in each study.
Hormone therapy is beneficial for treating VMS associated with menopause16,17 and improves sleep disturbances related to VMS in perimenopausal and early postmenopausal women.1,4,18 In a randomized cross-over study of 63 postmenopausal women, hormone therapy demonstrated significantly improved sleep quality and fewer nighttime awakenings compared with placebo (P < 0.001).49 Furthermore, women treated with hormone therapy experienced a longer duration of rapid eye movement sleep, shortened sleep latency, and improvement in sleep efficiency.50 However, nonhormonal treatments are needed for women for whom hormone therapy is contraindicated and for those unwilling to be treated with hormone therapy.
The major strength of the present analysis is the prospective randomized gathering of sleep information from a large number of study participants, with no exclusions for sleep apnea or restless legs (the most common diagnoses interrupting sleep beyond VMS); thus, the findings are generalizable to the larger population. Use of data from two similarly designed studies with similar patient populations allowed pooling of data and comparison with baseline values, providing a more robust evaluation of outcomes. An additional strength of this analysis is the inclusion of minority and overweight study participants (Table 1). The fact that the instruments used for VMS and sleep analysis were self-reported, rather than objectively measured, is a limitation of this analysis; however, self-perception of VMS bothersomeness and sleep disruption is of particular clinical relevance. That sleep outcomes were secondary, rather than primary, endpoints is another potential limitation of this analysis.
CONCLUSIONS
In the phase 3 studies, paroxetine 7.5 mg once daily for the treatment of VMS in postmenopausal symptomatic women significantly reduces the number of nighttime awakenings attributed to VMS and increases the duration of sleep. Although the overall reduction in nighttime awakenings is moderate, it has been sustained through 12 and 24 weeks of treatment with paroxetine 7.5 mg and may represent a meaningful magnitude of improvement for some women with symptoms attributed to VMS. Improvements in nighttime awakenings and sleep duration occur without increased sedation, reduced sleep-onset latency, or increased sleep-related AEs, suggesting that, in these studies, paroxetine 7.5 mg has a selective therapeutic effect on sleep parameters related to VMS.
Acknowledgments
We thank Maribeth Bogush, PhD, Lynn Brown, PhD, and Sally-Anne Mitchell, PhD (of ApotheCom), for medical writing support and editorial assistance.
APPENDIX
Sleep diary
Footnotes
All authors had access to study data, were involved in all aspects of manuscript writing and editing, and provided final approval to submit the manuscript for publication.
Preliminary findings of these studies were presented in poster format at The North American Menopause Society 2013 Annual Meeting, Dallas, TX, October 9 to 12, 2013.
Funding/support: The studies (ClinicalTrials.gov identifiers NCT01361308 and NCT01101841) were funded by Noven Pharmaceuticals Inc. Medical writing support and editorial assistance were funded by Noven Pharmaceuticals Inc.
Financial disclosure/conflicts of interest: In the past 12 months, J.V.P. has served as a consultant (fees to the University of Virginia) to Pfizer Inc, Noven Pharmaceuticals Inc, Novo Nordisk, DepoMed, and Shionogi; has received grants/research support (fees to the University of Virginia) from Therapeutics, DepoMed, Bionova, and Endoceutics; has received travel funds from Pfizer Inc, Noven Pharmaceuticals Inc, DepoMed, Novo Nordisk, Endoceutics, and Shionogi; and has received editorial writing support from Pfizer Inc, Shionogi, DepoMed, and Noven Pharmaceuticals Inc. In the past 12 months, H.J. has received research grant support from Cephalon (now Teva) and has served as a consultant to Noven Pharmaceuticals Inc. K.K. and H.M. have no conflicts of interest to disclose. S.B. and J.L. are employees of Noven Pharmaceuticals Inc.
REFERENCES
- 1. Guidozzi F. Sleep and sleep disorders in menopausal women. Climacteric 2013; 16: 214- 219. [DOI] [PubMed] [Google Scholar]
- 2. Blumel JE, Cano A, Mezones-Holguin E, et al. A multinational study of sleep disorders during female mid-life. Maturitas 2012; 72: 359- 366. [DOI] [PubMed] [Google Scholar]
- 3. Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause 2010; 17: 1128- 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas 2011; 68: 224- 232. [DOI] [PubMed] [Google Scholar]
- 5. Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 2008; 11: 32- 43. [DOI] [PubMed] [Google Scholar]
- 6.US National Institutes of Health. NIH state-of-the-science conference statement on management of menopause-related symptoms, 2005. Available at: http://consensus.nih.gov/2005/menopausestatement.pdf. Accessed February 14, 2014.
- 7. Kumari M, Stafford M, Marmot M. The menopausal transition was associated in a prospective study with decreased health functioning in women who report menopausal symptoms. J Clin Epidemiol 2005; 58: 719- 727. [DOI] [PubMed] [Google Scholar]
- 8. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population–based study shows negative impact on health-related quality of life. Maturitas 2009; 62: 153- 159. [DOI] [PubMed] [Google Scholar]
- 9. Ameratunga D, Goldin J, Hickey M. Sleep disturbance in menopause. Intern Med J 2012; 42: 742- 747. [DOI] [PubMed] [Google Scholar]
- 10. Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause 2012; 19: 742- 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Thurston RC, Matthews KA, Bryce CL, Kapoor WN, Hess R. Are hot flashes associated with sleep disturbance during midlife? Results from the STRIDE cohort study. Maturitas 2012; 71: 34- 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joffe H, White DP, Crawford SL, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause 2013; 20: 905- 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep 2013; 36: 1977- 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buysse DJ. Insomnia. JAMA 2013; 309: 706- 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews KA, Everson-Rose SA, Kravitz HM, Lee L, Janssen I, Sutton-Tyrrell K. Do reports of sleep disturbance relate to coronary and aortic calcification in healthy middle-aged women? Study of Women’s Health Across the Nation. Sleep Med 2013; 14: 282- 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The North American Menopause Society. Menopause Practice: A Clinician’s Guide. 4th ed Mayfield Heights, OH: NAMS; 2010. [Google Scholar]
- 17. Ribowsky J. Hormone therapy for menopause: a concise update of the benefits and risks. Adv NPs PAs 2011; 2: 19- 22. [PubMed] [Google Scholar]
- 18. Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med 2010; 28: 404- 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stearns V. Serotonergic agents as an alternative to hormonal therapy for the treatment of menopausal vasomotor symptoms. Treat Endocrinol 2006; 5: 83- 87. [DOI] [PubMed] [Google Scholar]
- 20. Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health 2009; 5: 361- 384. [DOI] [PubMed] [Google Scholar]
- 21.Writing Group on behalf of the Workshop Consensus Group. Aging, menopause, cardiovascular disease and HRT. International Menopause Society Consensus Statement. Climacteric 2009; 12: 368- 377. [DOI] [PubMed] [Google Scholar]
- 22. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295: 2057- 2071. [DOI] [PubMed] [Google Scholar]
- 23. Thacker HL. Assessing risks and benefits of nonhormonal treatments for vasomotor symptoms in perimenopausal and postmenopausal women. J Womens Health (Larchmt ) 2011; 20: 1007- 1016. [DOI] [PubMed] [Google Scholar]
- 24. Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial. Menopause 2008; 15: 310- 318. [DOI] [PubMed] [Google Scholar]
- 25. Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol 2007; 25: 308- 312. [DOI] [PubMed] [Google Scholar]
- 26. Reddy SY, Warner H, Guttuso T, Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol 2006; 108: 41- 48. [DOI] [PubMed] [Google Scholar]
- 27. Pinkerton JV, Constantine G, Hwang E, Cheng RF. Desvenlafaxine compared with placebo for treatment of menopausal vasomotor symptoms: a 12-week, multicenter, parallel-group, randomized, double-blind, placebo-controlled efficacy trial. Menopause 2012; 20: 28- 37. [DOI] [PubMed] [Google Scholar]
- 28. Pinkerton J, Kagan R, Portman D, Sathaynarayana RM, Sweeney M. Efficacy of gabapentin extended release in the treatment of menopausal hot flashes: results of the breeze 3 study. Paper presented at: Annual Meeting of The North American Menopause Society; October 3-6, 2012; Orlando, FL. [Google Scholar]
- 29. Pinkerton JV, Archer DF, Guico-Pabia CJ, Hwang E, Cheng RF. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause 2013; 20: 38- 46. [DOI] [PubMed] [Google Scholar]
- 30. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose mesylate salt of paroxetine (7.5 mg) for menopausal vasomotor symptoms: two randomized controlled trials. Menopause 2013; 20: 1027- 1035. [DOI] [PubMed] [Google Scholar]
- 31. Black K, Shea C, Dursun S, Kutcher S. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci 2000; 25: 255- 261. [PMC free article] [PubMed] [Google Scholar]
- 32. Tint A, Haddad PM, Anderson IM. The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study. J Psychopharmacol (Oxf) 2008; 22: 330- 332. [DOI] [PubMed] [Google Scholar]
- 33. Portman DJ, Kaunitz AM, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Effects of low-dose paroxetine 7.5 mg on weight and sexual function during treatment of vasomotor symptoms associated with menopause [published online ahead of print February 17, 2014] Menopause. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wichniak A, Wierzbicka A, Jernajczyk W. Sleep and antidepressant treatment. Curr Pharm Des 2012; 18: 5802- 5817. [DOI] [PubMed] [Google Scholar]
- 35. Kikuchi T, Suzuki T, Uchida H, Watanabe K, Mimura M. Coping strategies for antidepressant side effects: an Internet survey. J Affect Disord 2012; 143: 89- 94. [DOI] [PubMed] [Google Scholar]
- 36. Ensrud KE, Joffe H, Larson JC, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause 2012; 19: 848- 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Argyropoulos SV, Hicks JA, Nash JR, et al. Redistribution of slow wave activity of sleep during pharmacological treatment of depression with paroxetine but not with nefazodone. J Sleep Res 2009; 18: 342- 348. [DOI] [PubMed] [Google Scholar]
- 38. Stearns V, Isaacs C, Rowland J, et al. A pilot trial assessing the efficacy of paroxetine hydrochloride (Paxil) in controlling hot flashes in breast cancer survivors. Ann Oncol 2000; 11: 17- 22. [DOI] [PubMed] [Google Scholar]
- 39. Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: a trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med 2012; 13: 1184- 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 2001; 22: 979- 989. [DOI] [PubMed] [Google Scholar]
- 41. Greene JG. Constructing a standard climacteric scale. Maturitas 1998; 29: 25- 31. [DOI] [PubMed] [Google Scholar]
- 42. PEXEVA (paroxetine mesylate) tablets. Miami, FL: Noven Therapeutics LLC. [Google Scholar]
- 43. Hicks JA, Argyropoulos SV, Rich AS, et al. Randomised controlled study of sleep after nefazodone or paroxetine treatment in out-patients with depression. Br J Psychiatry 2002; 180: 528- 535. [DOI] [PubMed] [Google Scholar]
- 44. Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: a prospective placebo-controlled study. Sleep 2012; 35: 1551- 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorsey CM, Lee KA, Scharf MB. Effect of zolpidem on sleep in women with perimenopausal and postmenopausal insomnia: a 4-week, randomized, multicenter, double-blind, placebo-controlled study. Clin Ther 2004; 26: 1578- 1586. [DOI] [PubMed] [Google Scholar]
- 46. Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011; 34: 601- 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joffe H. Low-dose mesylate salt of paroxetine (LDMP) in treatment of vasomotor symptoms (VMS) in menopause. Paper presented at: Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; May 5-9, 2012; San Diego, CA. [Google Scholar]
- 48. Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193- 213. [DOI] [PubMed] [Google Scholar]
- 49. Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol 1998; 178: 1002- 1009. [DOI] [PubMed] [Google Scholar]
- 50. Polo-Kantola P, Saaresranta T, Polo O. Aetiology and treatment of sleep disturbances during perimenopause and postmenopause. CNS Drugs 2001; 15: 445- 452. [DOI] [PubMed] [Google Scholar]


