Abstract
Septic shock due to Salmonella and other gram-negative enteric pathogens is a leading cause of death worldwide. The role of lipopolysaccharide in sepsis is well studied; however, the contribution of other bacterial outer membrane components, such as Braun (murein) lipoprotein (Lpp), is not well defined. The genome of Salmonella enterica serovar Typhimurium harbors two copies of the lipoprotein (lpp) gene. We constructed a serovar Typhimurium strain with deletions in both copies of the lpp gene (lpp1 and lpp2) by marker exchange mutagenesis. The integrity of the cell membrane and the secretion of the effector proteins through the type III secretion system were not affected in the lpp double-knockout mutant. Subsequently, the virulence potential of this mutant was examined in a cell culture system using T84 intestinal epithelial and RAW264.7 macrophage cell lines and a mouse model of salmonellosis. The lpp double-knockout mutant was defective in invading and inducing cytotoxic effects in T84 and RAW264.7 cells, although binding of the mutant to the host cell was not affected when compared to the wild-type (WT) serovar Typhimurium. The motility of the mutant was impaired, despite the finding that the number of flagella was similar in the lpp double knockout mutant and the WT serovar Typhimurium. Deletion in the lpp genes did not affect the intracellular survival and replication of Salmonella in macrophages and T84 cells. Induction of the proinflammatory cytokines tumor necrosis factor alpha and interleukin-8 (IL-8) was significantly reduced in macrophages and T84 cells infected with the lpp double-knockout mutant. The levels of IL-8 remained unaffected in T84 cells when infected with either live or heat-killed WT and lpp mutant, indicating that invasion was not required for IL-8 production and that Toll-like receptor 2 signaling might be affected in the Lpp double-knockout mutant. These effects of the Lpp protein could be restored by complementation of the isogenic mutant. The lpp double-knockout mutant was avirulent in mice, and animals infected with this mutant were protected from a lethal challenge dose of WT serovar Typhimurium. The severe combined immunodeficient mice, on the other hand, were susceptible to infection by the lpp double-knockout mutant. The serovar Typhimurium mutants from which only one of the lpp (lpp1 or lpp2) genes was deleted were also avirulent in mice. Taken together, our data indicated that Lpp specifically contributed to the virulence of the organism.
Salmonella enterica serovar Typhimurium is frequently implicated in causing outbreaks of enteric disease due to the consumption of contaminated food (58). In mice, serovar Typhimurium elicits systemic infection, providing an experimental model of typhoid fever (23). In the United States, the number of prevalent cases of salmonellosis has been estimated to range from 80,000 to 3,700,000 annually (12, 69). An important feature of pathogenesis of Salmonella infections is the ability of the organism to attach to and invade host epithelial cells (74). Serovar Typhimurium colonizes intestinal mucosa and transcytosis M cells and then is phagocytized by resident macrophages, where they multiply and further invade internal organs, such as the liver and spleen (32, 35). Invasion of host cells by serovar Typhimurium induces cytoskeletal rearrangement accompanied by membrane ruffling and cytotoxicity (32, 50).
One of the most serious complications of serovar Typhimurium infection is septic shock, which is mediated in part by lipopolysaccharide (LPS). It has been estimated that more than 600,000 cases of sepsis and septic shock occur annually in the United States, resulting in more than 100,000 deaths (66). LPS exerts its biological effect via lipid A-dependent induction of cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and IL-6 from macrophages and neutrophils; activation of the complement cascade; and by the induction of other inflammatory mediators (36, 60, 79). Recently, we reported that the Braun (murein) lipoprotein (Lpp) (8, 9), a major bacterial outer membrane component of gram-negative bacteria in the family Enterobacteriaceae, also contributed significantly to the development of septic shock (85). In that study, Lpp purified from Escherichia coli was shown to induce the in vivo production of TNF-α and IL-6 in LPS-nonresponsive mice (85). Furthermore, Lpp synergized with E. coli LPS to produce proinflammatory cytokines leading to septic shock, indicating that Lpp and LPS activate cells through different mechanisms (85). LPS activates cellular responses via both Toll-like receptor 4 (TLR4) and CD14 and LPS-binding protein (77). In contrast to LPS, Lpp purified from E. coli activated cells via toll-like receptor 2 (TLR2) (1). The lipid moiety, attached at the N-terminal end of the protein backbone of Lpp, is essential for its immune and inflammatory activities, as shown for LPS (66).
Various functionally distinct species of lipoproteins exist in gram-negative bacteria (3). These lipoproteins are either structural proteins, e.g., Lpp, peptidoglycan-associated lipoprotein (designated Pal), and outer membrane lipoprotein LolB precursor, enzymes, receptors, invasion-associated type III secretion system (TTSS) (e.g., InvH), or transporters performing crucial functions in the bacterial cell envelope. Organisms such as Borrelia burgdorferi and Treponema pallidum, which lack LPS, induce inflammatory mediators through lipoprotein, which indicates that it plays an important role in bacterial pathogenesis (85).
As with LPS, murein Lpp is synthesized in the cytoplasm as a precursor and undergoes sequential posttranslational modifications (lipolization) catalyzed by glyceryl transferase (Lgt), O-acyl transferase, prolipoprotein signal peptidase II, and N-acyl transferase (Lnt) to form mature Lpp (38). Mature Lpp is then transported across the cytoplasmic membrane by protein translocation machinery (55). Recent studies indicated that mutation in genes coding for Lgt and Lnt reduced the growth and motility of serovar Typhimurium (19, 31). Likewise, Neilsen et al. (63) reported that a naturally occurring Lpp− mutant of E. coli JE5505 (44) was less inflammatory than its parental strain. However, it is not known whether this E. coli mutant had alterations in other genes as well, which could affect its virulence. Therefore, targeted mutants for Lpp needed to be developed to precisely define its role in pathogenesis, and they formed the basis of this study.
We showed previously that Lpp and LPS from E. coli and Yersinia enterocolitica were equally potent with regard to host cellular responses (84, 85). Further, the release of LPS and Lpp from bacteria and their synergistic effect (84, 85) warrant further investigation of the critical role Lpp plays in bacterial virulence and induction of the proinflammatory cytokines that lead to septic shock.
In this study, we report, for the first time, the construction of a serovar Typhimurium strain in which two highly homologous murein-lipoprotein genes (lpp1 and lpp2) located in tandem in the Salmonella genome were deleted. Subsequently, the lpp double-knockout mutant was used to study the role of Lpp in Salmonella virulence using both in vitro and in vivo models. Compared to the wild-type (WT) and complemented strains of serovar Typhimurium, the lpp double-knockout mutant was defective in invasion and less cytotoxic and produced reduced levels of proinflammatory cytokines, possibly by altering TLR2 signaling. Importantly, the Lpp− mutant was avirulent in mice, and mice infected with this mutant were resistant to a lethal challenge of WT serovar Typhimurium. The severe combined immunodeficient (SCID) mice lacking immune cells, on the other hand, died after infection with the Lpp mutant of serovar Typhimurium. To define the role of each of the lpp genes in serovar Typhimurium virulence, we constructed mutants of Salmonella in which the lpp1 and lpp2 genes were individually inactivated or deleted by marker exchange mutagenesis. The lpp1 and lpp2 isogenic mutants were similarly avirulent in mice, as noted with the lpp double-knockout serovar Typhimurium mutant.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Serovar Typhimurium strain 14028 was described elsewhere (50), and a spontaneous nalidixic acid resistance strain of serovar Typhimurium was generated in our laboratory. The E. coli strain JE5505 was a naturally occurring Lpp− mutant. This E. coli strain grew and divided normally (44). The suicide vector pDMS197 had a conditional R6K origin of replication (ori), a levansucrase gene (sacB) from Bacillus subtilis, and a tetracycline resistance (Tcr) gene (21). The suicide vector pJQ200SK (67) contained P15A ori, a sacB gene, and a gentamicin resistance (Gmr) gene. The E. coli and Salmonella strains were grown either in Luria-Bertani (LB) medium (2) or on Salmonella-Shigella (SS) agar plates (Difco, Detroit, Mich.). A list of bacterial strains and plasmids used in this study is provided in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Serovar Typhimurium strain 14028 | Salmonella enterica serovar Typhimurium | 50 |
| STM-N | Nalidixic acid resistance (Nalr) strain of serovar Typhimurium 14028 | This study |
| Mutant 66 | Isogenic mutant of serovar Typhimurium in which two copies of the lipoprotein gene (lpp) were deleted; Nalr Knr | This study |
| Mutant 67 | Isogenic mutant of serovar Typhimurium in which two copies of the lpp gene were deleted; Nalr Knr | This study |
| lpp1 mutant | Isogenic mutant of serovar Typhimurium in which only one copy of the lpp gene (lpp1) was truncated by using suicide vector pDMS197; Nalr Knr | This study |
| lpp2 mutant | Isogenic mutant of serovar Typhimurium in which only one copy of the lpp gene (lpp2) was deleted by using suicide vector pDMS197; Nalr Knr | This study |
| lpp1R mutant | Isogenic mutant of serovar Typhimurium in which only one copy of the lpp gene (lpp1) was deleted using λ Red system; Nalr Knr | This study |
| lpp2R mutant | Isogenic mutant of serovar Typhimurium in which only one copy of the lpp gene (lpp2) was deleted using λ Red system; Nalr Knr | This study |
| Mutant 67/pBRlpp | Mutant 67 complemented with two copies of the lpp gene via pBR322 vector; Nalr Knr Tcr | This study |
| E. coli | ||
| DH5α | recA gyrA | Laboratory stock |
| S17-1 | Streptomycin and trimethoprim resistance; λpir | Laboratory stock |
| SM10 | Knr λpir | 21 |
| JE5505 | Naturally occurring Lpp-minus mutant of E. coli | 44 |
| Plasmids | ||
| pBR322 | Apr Tcr | Amersham |
| pBluescript-SK | Apr | Stratagene |
| pUC-4K | Contains a 1.2-kb kanamycinr gene cassette | Amersham |
| pJQ200SK | A suicide vector; P15A ori sacB, Gmr | 67 |
| pDMS197 | A suicide vector; R6K ori sacB, Tcr | 21 |
| pBRlpp | STM lpp genes with its putative promoter region, cloned in pBR322 at the ScaI site | This study |
| pBluelpp | pBluescript vector containing up- and downstream flanking DNA sequences to the lpp genes of STM | This study |
| pBluelppK | Knr gene cassette was inserted at the XhoI site in plasmid pBluelpp that contained 5′- and 3′-flanking sequences to the lpp genes of serovar Typhimurium | This study |
| pJQ200lppK | Suicide vector pJQ200SK containing the Knr gene cassette which was flanked by the up- and downstream flanking DNA sequences to the lpp genes of serovar Typhimurium; it was used to generate lpp double-knockout mutant of serovar Typhimurium | This study |
| pBluelpp1 | pBluescript vector containing lpp1 coding region of serovar Typhimurium | This study |
| pBluelpp1K | Knr gene cassette truncating the serovar Typhimurium lpp1 gene at the HindII site in plasmid pBluelpp1 | This study |
| pDMS197lpp1K | Suicide vector pDMS197 containing the Knr gene cassette that interrupted serovar Typhimurium lpp1 gene for generating the lpp1 single-knockout mutant | This study |
| pBluelpp2 | pBluescript vector containing up- and downstream flanking DNA sequences to the lpp2 gene of serovar Typhimurium | This study |
| pBluelpp2K | Knr gene cassette was inserted at the XhoI site in plasmid pBluelpp2 that contained 5′- and 3′-flanking sequences to the lpp2 gene of serovar Typhimurium | This study |
| pDMS197lpp2K | Suicide vector pDMS197 containing the Knr gene cassette which was flanked by the the up- and downstream flanking DNA sequences to the lpp2 gene of serovar Typhimurium; it was used to generate lpp2 single-knockout mutant of serovar Typhimurium | This study |
| pKD46 | Temperature-sensitive plasmid expressing λ Red recombinase under control of arabinose; Apr | 20 |
| pKD4 | Template plasmid in the λ Red system for Knr gene cassette; Knr Apr | 20 |
Cell culture.
RAW264.7 murine macrophage and T84 intestinal epithelial cell lines were obtained from American Type Culture Collection (Manassas, Va.). Macrophages were maintained in Dulbecco's modified eagle medium with 10% fetal bovine serum supplemented with penicillin-streptomycin (Invitrogen, Carlsbad, Calif.). T84 cells were grown in Dulbecco's modified eagle medium-F-12 medium with 5% fetal bovine serum supplemented with penicillin-streptomycin. Both types of cells were incubated at 37°C with 5% CO2.
Construction of serovar Typhimurium lpp isogenic mutants by using suicide vectors.
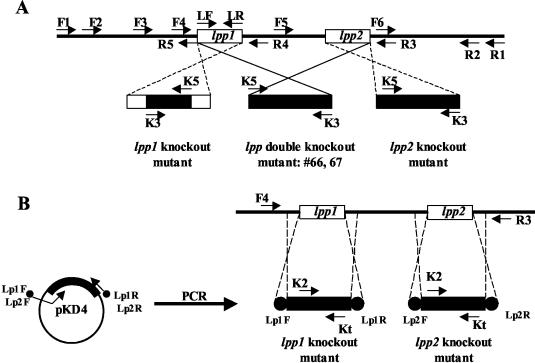
Based on our studies and subsequently on the basis of the genomic sequence of serovar Typhimurium, two copies of the lpp gene (designated lpp1 and lpp2), separated by 82 bp of DNA, are present in the chromosome (Fig. 1) (56). A double-knockout mutant, in which both copies of the lpp gene from strain 14028 were deleted, was first generated by homologous recombination using the suicide vector pJQ200SK. Briefly, the 5′- and 3′-flanking DNA sequences to the lpp genes were amplified from the chromosomal DNA of serovar Typhimurium by the PCR, utilizing two pairs of specific primers (F2/R5 and F6/R2) with appropriate restriction enzyme sites (Fig. 1A and Table 2). A common XhoI restriction enzyme site was introduced into flanking DNA fragments of the lpp genes during PCR. The resulting flanking DNA fragments were ligated through the XhoI site and cloned into a pBluescript vector at the BamHI/ApaI sites to produce a recombinant plasmid, pBluelpp. Subsequently, a kanamycin resistance (Knr) gene cassette (obtained from the plasmid pUC4K; Amersham Pharmacia, Piscataway, N.J.) was inserted at the XhoI restriction enzyme site to generate a recombinant plasmid, pBluelppK. The plasmid, pBluelppK, was digested with BamHI/ApaI restriction enzymes and ligated into a suicide vector, pJQ200SK, to produce a recombinant plasmid, pJQ200lppK, which was then transformed into E. coli S17-1. This E. coli strain contains a chromosomal λpir gene required for the replication of the suicide vector pJQ200SK. Since serovar Typhimurium does not have the λpir gene, the recombinant suicide plasmid could not replicate in serovar Typhimurium. The suicide vector that contained a sacB gene caused lethality in serovar Typhimurium and E. coli when induced with 5% sucrose.
FIG. 1.
Schematic diagram showing construction of lpp isogenic mutants of serovar Typhimurium via homologous recombination. In panel A, the Knr gene cassette-interrupted lpp1 gene replaced the original lpp1 gene on the genome of serovar Typhimurium and generated the lpp1 mutant, while for the lpp2 single-knockout mutant, the Knr gene cassette replaced the whole coding sequence of the lpp2 gene. To generate the lpp double-knockout mutant, both copies of the lpp gene including the sequence between them were replaced by a Knr gene cassette. In panel B, the λ Red system was used, and the Knr gene cassette replaced the corresponding lpp copy on the genome of the single lpp knockout mutants of serovar Typhimurium. Arrows indicate the primers' position used for the generation and identification of the lpp knockout mutants. The exact DNA sequences of the primers were also shown in Table 2. The open bars indicate the copies of the lpp gene on the genome, and the solid bars indicate the Knr gene cassette used to replace or interrupt the lpp genes on the genome of serovar Typhimurium. The solid circles represent the flank DNA sequence to the lpp genes of serovar Typhimurium for homologous recombination in the λ Red system.
TABLE 2.
Sequences of the primers used in this study for preparing serovar Typhimurium isogenic mutants
| Primer position | Primer name and sequenceab | Reference | Purpose |
|---|---|---|---|
| 5′ | F1: 5′ GATGCGTCTGAACTTCTCTC-3′ | This study | PCR for identifing lpp knockout mutants of serovar Typhimurium |
| 3′ | R1: 5′ GCAATGCCGCTCATGGCCTG 3′ | This study | |
| 5′ | F4: 5′ GCTACATGGAGATTAACTCA 3′ | This study | |
| 5′ | F5: 5′ TGGCGCACATCGTGCGCCAT 3′ | This study | |
| 5′ | F2: 5′ GTGGATCCTATGCAGAACACGGTCAGCG 3′ BamHI | This study | PCR amplification of 5′ flanking sequence of lpp gene |
| 3′ | R5: 5′ AGCTCGAGCTAGATTGAGTTAATCTCCA 3′ XhoI | This study | |
| 3′ | R4: 5′ CACTCGAGACGCAGGTACTATTACTTAC 3′ XhoI | This study | |
| 5′ | F6: 5′ ATCTCGAGTACTGCGAAGGCTACTGCGTCG 3′ XhoI | This study | PCR amplification of 3′ flanking sequence of lpp gene |
| 3′ | R2: 5′ AAGGGCCCTTCGCTGGCGATGTATAAC 3′ ApaI | This study | |
| 5′ | F3: 5′ ACAAGCTTGCCGCTGATCGTGGTAGCGAC 3′ HindIII | This study | PCR amplification of lpp genes for complementation |
| 3′ | R3: 5′ AGAAGCTTCGACGCAGTAGCCTTCGCAGTA 3′ HindIII | This study | |
| 5′ | LF: 5′ CACTCGAGATGAAAGCTACTAAACTGGTAC 3′ XhoI | This study | PCR amplification of lpp1 coding region to generate lpp1 single-knockout mutant of serovar Typhimurium |
| 3′ | LR: 5′ CCGAATTCTTACTTGCGGTATTTAGTAGCC 3′ EcoRI | This study | |
| 5′ | K5: 5′ CGCTGAGGTCTGCCTCGTGAAGAAGGTGTT 3′ | 72 | Primers that specifically bind to the knr gene cassette, used to identify lpp knockout mutants of serovar Typhimurium |
| 3′ | K3: 5′ AAAGCCACGTTGTGTCTAAAATCTCTGATGT 3′ | 72 | |
| 5′ | K2: 5′ CGGTGCCCTGAATGAACTGC 3′ | 20 | |
| 3′ | Kt: 5′ CGGCCACAGTCGATGAATCC 3′ | 20 | |
| 5′ | Lp1F: 5′ CTTGTAACGCTACATGGAGATTAACTCAATCTAGA GGGTATTAATATGTGTAGGCTGGAGCTGCTT CG 3′ | This study | Primer set used to make lpp1 single knockout mutant in the λ Red system |
| 3′ | Lp1R: 5′ ATGGCGCACGATGTGCGCCATTTTTATTACGCAGGTACTATTACTTACCATATGAATATCCTCCTTAG 3′ | This study | |
| 3′ | Lp2F: 5′ CCATTTTTTTTACCTATATAACCACACAAAATATAAGGTTATTGTTTGTGTAGGCTGGAGCTGCTTCG 3′ | This study | Primer set used to make lpp2 single-knockout mutant in the λ Red system |
| 5′ | Lp2R: 5′ TGGCGCACGATGTGCGCCATTTTATATCATGCGTCAAATCATTTACAGCATATGAATATCCTCCTTAG 3′ | This study |
Underlining indicates restriction enzyme sites in the primer.
Italic sequence indicates DNA sequence that binds to plasmid pKD4 in the λ Red system.
We used both a suicide plasmid and a lambda (λ) Red system for constructing lpp1 and lpp2 single-knockout mutants. To specifically truncate the lpp1 gene of serovar Typhimurium, the coding region of the lpp1 gene was PCR amplified by using primers LF and LR (Fig. 1A and Table 2). It was subsequently cloned into a pBluescript vector at the compatible sites. A knr gene cassette from the pUC4K plasmid was first digested with the PstI restriction enzyme, and its ends were made blunt by using a PCR polishing kit (Stratagene, La Jolla, Calif.). The knr gene cassette was then inserted into the lpp1-coding region at the HindII restriction enzyme site. This truncated version of the lpp1 gene was subsequently cloned into a suicide vector, pDMS197, at the KpnI/XbaI restriction enzyme sites, generating a recombinant plasmid, pDMS197lpp1K.
To delete the lpp2 gene from serovar Typhimurium, we used a modified strategy similar to one employed in generating an lpp double-knockout mutant (Fig. 1A). The first of two modifications to the strategy included the use of a new 5′-flanking DNA fragment, containing the entire coding region of the lpp1 gene that was generated by using a primer pair, F2 and R4. The pair of primers (F6 and R2) used to amplify the 3′-flanking DNA sequence was the same that was used for generating the lpp double-knockout mutant (Fig. 1A and Table 2). Secondly, we used suicide vector pDMS197 in E. coli strain SM10 (20) instead of pJQ200SK. The newly generated recombinant plasmid was designated pDMS197lpp2K. All of the DNA manipulations were performed as described previously (2).
The recombinant E. coli carrying pJQ200lppK, pDMS197lpp1K, or pDMS197lpp2K was conjugated with serovar Typhimurium (nalidixic acid) resistance [Nalr] (72). The Nalr, Knr, Gms, and sucrose resistance transconjugants were selected as candidates for the lpp double-knockout mutants of serovar Typhimurium, while Nalr, Knr, Tcs, and sucrose resistance transconjugants were designated the candidates for lpp (lpp1 or lpp2) single-knockout mutants of serovar Typhimurium. These selected transconjugants should represent genuine, double-crossover mutants (Fig. 1A). In such mutants, the Knr gene cassette or a truncated version of the lpp gene contained on the suicide vectors must have replaced the native lpp gene(s) on the chromosome of serovar Typhimurium by homologous recombination, with concomitant loss of the remaining suicide vectors containing the sacB and antibiotic resistance genes (Gm for pJQ200SK and Tc for PDMS197) (Fig. 1A).
The double-crossover mutants were identified as serovar Typhimurium by growth on MacConkey's and SS agar media to differentiate them from E. coli used for conjugation. The cultures were further identified as Salmonella by an automated identification system used in the Clinical Microbiology Laboratory, University of Texas Medical Branch (UTMB) (Galveston, Tex.). To demonstrate genuine, double-crossover events, the selected lpp isogenic mutants and the WT serovar Typhimurium were subjected to PCR analysis with different primer sets and/or Southern blot hybridization (as specifically performed for the lpp double-knockout mutants), using the lpp gene, Knr gene cassette, and pJQ200SK plasmid as probes.
Complementation of the serovar Typhimurium lpp double-knockout isogenic mutants.
A DNA fragment containing both the lpp1 and lpp2 genes from WT serovar Typhimurium was PCR amplified by using a primer set, F3 and R3 (Table 2 and Fig. 1A). Subsequently, the ends of the fragment were made blunt with a PCR polishing kit and ligated into the blunt-ended ScaI-digested pBR322 vector. The recombinant plasmid then was transformed into lpp double-knockout mutants by electroporation, following the manufacturer's instructions (Invitrogen).
Construction of lpp1 and lpp2 mutants using a λ Red system.
A λ Red system, as described by Datsenko and Wanner (20), was also used to generate lpp single-knockout (lpp1 or lpp2) mutants of serovar Typhimurium. Two specific primer sets (Lp1F, Lp1R and Lp2F, Lp2R) were synthesized and used with the λ Red system (Fig. 1B and Table 2). Each primer was 68 bp in length, and at the 5′ end of each, there was a flanking DNA sequence (46 to 48 bp in size; shown in nonitalic letters in Table 2) to the specific lpp copy that was targeted to be deleted from the genome of WT serovar Typhimurium. The DNA at the 3′ end of each of the primers had a sequence (shown in italic letters in Table 2) that bordered the Knr gene cassette on the plasmid pKD4 (20). By using each of the specific primer sets, a 1.3-kb DNA fragment could be amplified from the plasmid pKD4. These amplified DNA fragments contained a Knr gene cassette in the middle and a short flanking DNA sequence (46 to 48 bp on each side) to the specific lpp copy for homologous recombination. To increase the frequency of homologous recombination, an ampicillin resistance (Apr) plasmid, pKD46, which contained a phage λ Red recombinase gene, was first transformed into WT serovar Typhimurium. The λ Red recombinase gene could be hyperexpressed after induction of the culture with 1 mM arabinose. The presence of plasmid pKD46 in serovar Typhimurium also provided a temperature-sensitive phenotype, allowing bacteria to replicate normally at 30°C but not at 37°C due to curing of the plasmid with concomitant loss of the Apr phenotype.
Briefly, the serovar Typhimurium strain with the pKD46 plasmid was first grown at 30°C in the SOB medium (tryptone [20 g], yeast extract [5 g], and NaCl [0.5 g per liter] [pH 7.5]) in the presence of 1 mM arabinose until an optical density (OD) of 0.6 was reached. The bacteria were harvested, washed twice with ice-cold 10% glycerol, and electroporated with the 1.3-kb DNA fragment which was generated by PCR amplification with the primer sets Lp1F and Lp1R and Lp2F and Lp2R. The transformed cultures were screened on kanamycin (100 μg/ml) containing LB agar plates at 37°C. The Knr colonies were picked up for further PCR analysis with primer sets k2 and F4 and kt and R3 (Fig. 1B, Table 2). The colonies with the correct genotype were then examined for the Apr phenotype. The sensitivity of the culture for Ap would indicate successful curing of plasmid pKD46.
Southern blot and PCR analyses on lpp knockout mutants of serovar Typhimurium.
Chromosomal DNA from the WT and serovar Typhimurium lpp isogenic mutants was isolated using a QIAamp DNA mini kit (Qiagen, Inc., Valencia, Calif.). Southern blot analysis was performed with the lpp double-knockout mutants. Briefly, an aliquot (10 to 15 μg) of the chromosomal DNA from WT and lpp double-knockout mutants was digested with appropriate restriction enzymes (e.g., BglII and MluI) and subjected to 0.8% agarose gel electrophoresis (71). The digested total DNA was transferred to a nylon membrane and baked at 80°C for 2 h. The blots were prehybridized and hybridized using Quickhyb at 68°C, as described by the manufacturer (Stratagene). The Knr gene cassette, the lpp gene, and the suicide vector pJQ200SK labeled with [α32P]dCTP (ICN, Irvine, Calif.) were used as probes. After hybridization, the blots were washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% sodium dodecyl sulfate (SDS) for 20 min, followed by washing with 1× SSC plus 0.1% SDS at 68°C for 20 min, and exposed to X-ray film at room temperature.
Next, PCR analysis was performed to identify the lpp (lpp1 or lpp2) single-knockout mutants generated by a suicide vector, pDMS197. Different primer sets were used to verify the presence of an antibiotic resistance gene cassette (Knr) and junctional sequences in the mutants. These primers were designed either to the Knr gene cassette or to the genome sequence of WT serovar Typhimurium outside the flanking sequences that were used for homologous recombination. This strategy allowed us to correctly predict the genotypes of the mutants. For the lpp1 single-knockout mutant, primer sets K3 and K5, F4 and R3, F4 and K5, and R3 and K3 were used. The primer set F5 and R3 was specifically designed for examining the integrity of the lpp2 gene in the lpp1 single-knockout mutant (Fig. 1A and Table 2). For the lpp2 single-knockout mutant, primer sets K5 and K3, F1 and R1, F1 and K3, and R1 and K5 were employed, and primer set F4 and R4 was used to verify the presence of the lpp1 sequence in the lpp2 single-knockout mutant of serovar Typhimurium (Table 2 and Fig. 1A). We previously described the PCR program used in this study (15). The denaturation temperature varied between 94 and 96°C, and the annealing temperature varied between 55 and 68°C with different sets of primers used in the PCRs.
Preparation of outer membranes of various serovar Typhimurium strains.
Our subsequent detailed studies were performed only with the lpp double-knockout mutants of serovar Typhimurium. The outer membrane proteins from various serovar Typhimurium strains were extracted as previously described (26). Briefly, a 50-ml bacterial culture was centrifuged, and the pellet was resuspended in 9 ml of phosphate-buffered saline (PBS) containing DNase (30 μg/ml) and two proteinase inhibitor tablets (Caltag Laboratories, Burlingame, Calif.). After sonication of the cells, the debris was removed by centrifugation in a Sorvall RC-SB Plus (Kendro Laboratory Products, Ashville, N.C.) at 10,000 rpm for 20 min, and the supernatant was ultracentrifuged in an Optima L-90K (Beckman Coulter, Palo Alto, Calif.) at 35,000 rpm for 1 h. The pellet was resuspended in 5 ml of distilled water with one proteinase inhibitor tablet. Subsequently, sodium sarcosyl was added to a final concentration of 0.5%, and the preparation was incubated at room temperature for 20 min before ultracentrifugation at 35,000 rpm for 45 min. The supernatant contained the inner membrane proteins. The pellet containing the outer membrane proteins was resuspended in 200 μl of distilled water with proteinase inhibitor. The protein concentration was measured at 280 nm.
Western blot analysis.
Western blot analysis was performed to evaluate the presence of Lpp in the WT serovar Typhimurium, its isogenic mutants (double knockout), and the complemented strain. An aliquot (20 to 25 μg) of total proteins from various strains of serovar Typhimurium, as well as their outer membrane protein preparations, was separated by SDS-15% polyacrylamide gel electrophoresis (PAGE) and then transferred to nitrocellulose membranes. Membranes were blocked with 3% gelatin and washed in 1× Tris-buffered saline with 0.05% Tween twice for 10 min each. A primary monoclonal antibody to Y. enterocolitica Lpp (84) in 1% gelatin solution (prepared in 1× Tris-buffered saline with 0.05% Tween) was applied to the membranes, and then they were allowed to incubate for 2 h at room temperature. After washing, appropriate secondary horseradish peroxidase (HRP)-conjugated, antimouse antibodies diluted 1:25,000 in 1% gelatin were applied to the membranes. Subsequently, the membranes were washed, and an enhanced chemiluminescence substrate kit (Pierce, Rockford, Ill.) was used, according to the manufacturer's instructions, for detecting a signal on the X-ray film.
Examination of the cell membrane integrity of the serovar Typhimurium lpp double-knockout isogenic mutant.
The integrity of the cell envelope of various bacterial cultures was examined by methods described below.
(i) Membrane blebbing.
Both the WT and lpp double-knockout mutant were grown to exponential phase (OD at 600 nm of 0.4 to 0.5). The cells were washed and subjected to transmission electron microscopy. Briefly, bacterial culture suspensions were pelleted and fixed in a mixture of 1.25% formaldehyde and 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.3) to which 0.03% CaCl2 and 0.03% trinitrophenol were added. They were postfixed in 1% OsO4 in 0.1 M cacodylate buffer, en bloc stained with 2% aqueous uranyl acetate, dehydrated in ethanol, and embedded in Poly/Bed 812 (Polysciences, Warrington, Pa.). Ultrathin sections were cut on a Reichert-Leica Ultracut S ultramicrotome, stained with lead citrate, and examined in a Philips 201 electron microscope at 60 kV. The membrane blebbing was examined in a minimum of 100 bacterial cells (73).
(ii) Sensitivity to detergents.
To demonstrate sensitivity of the WT serovar Typhimurium and lpp double-knockout mutant to the effect of detergent Triton X-100 (TX-100) and SDS, the bacterial cells were grown to an OD of 0.4 to 0.5 and diluted 50-fold, and then various concentrations of TX-100 (0.5 to 5%) and SDS (0.5 to 2%) were added. The cultures were incubated at 37°C for 3 h with shaking (180 rpm), and the OD was measured. A 50% reduction in the OD in three independent experiments indicated the sensitivity of the culture to the treatment, as previously reported (11). The samples were also plated on LB agar plates to determine numbers of CFU, allowing confirmation of a reduction in the absorbance values.
(iii) Sensitivity to antibiotics.
To determine the effect of antibiotics, the WT and lpp double-knockout mutant of serovar Typhimurium were spread on LB agar plates, and filter paper disks containing 10 to 100 μg of rifampin were placed on the agar plates. The plates were incubated overnight at 37°C, and the zone of inhibition of bacterial growth was measured (78). A 50% increase in sensitivity of the culture to rifampin was considered positive. For the antibiotic vancomycin, various concentrations (100 to 200 μg/ml) were added to the culture, which was originally grown to an OD of 0.4 to 0.5 and then diluted 50-fold. After incubation at 37°C for 3 h, a decrease in the absorbance (by 50%) was recorded as described above.
(iv) Release of β-lactamase.
We also determined outer membrane permeability of the WT and Lpp double-knockout serovar Typhimurium mutant by measuring resident (chromosomally encoded) β-lactamase activity in the total soluble, cell extract fractions and in the supernatant fractions of cells grown to an OD of 0.6 (4). Briefly, after growth, the bacterial cultures were centrifuged, resuspended in the original culture volume of fresh LB medium, and sonicated for 3 min with intermittent cooling on ice. After spinning, the clear cell extracts and the original culture medium were used to measure the enzymatic activity. To a 100-μl sample, we added 900 μl of chromogenic cephalosporin substrate (CENTA; Calbiochem, San Diego, Calif.) at a final concentration of 25 μg/ml and measured the absorbance at 410 nm after incubation at 37°C for 1 to 6 h (5, 11). The percentage of β-lactamase release in the culture supernatant was calculated based on total enzymatic activity in the WT and compared with that of the Lpp mutant of serovar Typhimurium. We used E. coli DH5α and E. coli JE5505 (Lpp− mutant) as controls in these experiments (4).
(v) Secretion of TTSS effector proteins.
To examine TTSS effector proteins, the WT serovar Typhimurium and its lpp double-knockout mutant were grown to an exponential phase (OD 0.4 to 0.5), and the culture supernatants (1 liter each) were precipitated with 10% trichloroacetic acid for 1 h at 4°C (81). The experiment was designed to monitor activity of only Salmonella pathogenicity island 1 (SPI-1)-encoded TTSS. After centrifugation, the trichloroacetic acid precipitates were dissolved in Laemmli's sample buffer (2) and subjected to SDS-12% PAGE. The gels were stained with either Coomassie blue or SYPRO RUBY (Bio-Rad, Hercules, Calif.). Alternatively, the protein bands were transferred to the polyvinylidene difluoride membrane, and the latter was stained and destained (2). The selected bands from the membrane were subjected to NH2-terminal sequence analysis at the Protein Chemistry Core Laboratory, UTMB (Galveston, Tex.).
In vitro binding assay.
The binding assay for various serovar Typhimurium strains was performed as previously described (25). Approximately 5 × 105 T84 intestinal epithelial cells/well were seeded into 24-well tissue culture plates and incubated overnight at 37°C with 5% CO2. Cells were infected with serovar Typhimurium strains (grown to an OD of 0.4 to 0.5) at a multiplicity of infection (MOI) of 10:1 and incubated at 4°C (to inhibit invasion) for 1 h. Binding of the bacteria to the host cell monolayer was facilitated by centrifugation in an IEC Centra-7 (International Equipment Company, Needham Heights, Mass.) at 1,500 rpm for 10 min (22). Unbound bacteria were aspirated, cells were washed four times with PBS and lysed with 0.1% TX-100, and various dilutions of the cell lysates were plated onto SS agar plates for determining numbers of CFU.
Invasion assay.
Invasion of T84 cells by the lpp double-knockout mutant, WT serovar Typhimurium, and the complemented strain was measured as described previously (14). Briefly, cells were seeded and infected as described for the binding assay. After 1 h of incubation at 37°C following infection, cells were washed three times with PBS and incubated at 37°C for an additional hour with gentamicin-containing medium (100 μg/ml) to kill extracellular bacteria. Following incubation, gentamicin-containing medium was removed, and the cells were washed six times with PBS and lysed with 0.1% TX-100. Then, various dilutions of the cell lysates were plated onto SS agar plates for determining numbers of CFU. For both binding and invasion assays, single colonies of various bacterial strains were grown in LB medium overnight at 37°C with shaking (180 rpm) in the presence of appropriate antibiotics. The cultures were diluted (50 to 100-fold) in the fresh medium and grown to an OD of 0.4 to 0.5. We used a well-characterized, nonflagellated and invasive mutant of Salmonella enterica serovar Dublin SL5928 and its respective WT as controls in our invasion assays (64). The mutated strain was nonmotile because of the inactivation of the flagellin gene fliC by transposon insertion (64).
Cytotoxicity and cell death.
The release of lactate dehydrogenase (LDH) enzyme from the host cells was used to measure cytotoxicity of various serovar Typhimurium strains by employing the CytoTox 96 LDH assay kit (Promega, Madison, Wis.). The cell death was examined by following the described method (13). RAW264.7 and T84 cells were seeded into six-well tissue culture plates (4 × 106 cells/well) and incubated overnight at 37°C. Cells were infected with various serovar Typhimurium strains at an MOI of 10:1 and incubated at 37°C for 1 h. After incubation, medium containing unbound bacteria was aspirated, and cells were washed three times with PBS and incubated in gentamicin-containing medium for 1 h at 37°C. Following incubation, cells were washed and incubated in a fresh antibiotic-free medium for 24 h, and their morphology was examined under a Zeiss LSM 510 meta laser scanning confocal microscope (Infectious Disease and Toxicology Optical Imaging Core Facility, UTMB, Galveston, Tex.). The average percentage of dead cells was estimated in 20 microscopic fields. The supernatants from RAW264.7 and T84 cells were examined for the release of LDH at various time points (0, 6, 12, and 24 h) after infection.
Intracellular survival.
The assay was performed using RAW264.7 and T84 cells, following the described method (49). Murine macrophages and human T84 cells were seeded in 24-well tissue culture plates (5 × 105 cells/well) and incubated overnight at 37°C with 5% CO2. Cells were infected with the lpp double-knockout mutant and its complemented strain, as well as the WT parental strain, at an MOI of 10:1 and incubated for 1 h. After incubation, the extracellular bacteria were removed by aspiration, and cells were washed three times with PBS and incubated in gentamicin (100 μg/ml)-containing medium for 1 h. The gentamicin-containing medium was aspirated, and cells were washed three times with PBS and then incubated in a fresh medium containing a minimum concentration of gentamicin (5 μg/ml) for 1, 6, 12, and 24 h. After three washes with PBS, cells were lysed by using 0.1% TX-100, cell lysates were plated on SS agar plates, and the survival and growth of the Salmonella strains inside T84 and RAW264.7 cells were assessed.
Motility assay.
LB medium with 0.35% agar was used to characterize the motility phenotype of the lpp double-knockout mutant, along with the WT and the complemented strain of serovar Typhimurium (19). The overnight culture of each Salmonella strain was adjusted to the same optical density, and equal numbers of CFU (106) were stabbed into 0.35% LB agar. Plates were incubated at 37°C overnight, and the motility was assayed by examining the migration of bacteria through the agar from the center towards the periphery of the plate.
In vitro cytokine production.
RAW264.7 and T84 cells were seeded into six-well plates at a concentration of 5 × 106 cells/well. RAW264.7 cells were infected with heat-killed (incubated at 65°C for 30 min) lpp double-knockout mutants or the complemented or WT strain at an MOI of 0.1:1 and incubated for 8 h at 37°C with 5% CO2. After incubation, culture supernatants were collected, centrifuged at 10,000 rpm for 10 min, and saved at −20°C. Heat-killed bacteria were used to prevent invasion-induced cytokine production in RAW264.7 cells. The T84 cells were similarly infected with either live (at an MOI of 10:1) or heat-killed (MOI of 0.1:1) bacteria. Cells infected with live bacteria were incubated for 1 h, washed, and incubated for another hour with medium containing gentamicin (100 μg/ml). After incubation, cells were washed again and incubated with fresh medium without the antibiotic for 12 h. T84 cells treated with killed bacteria were incubated for 8 h at 37°C, as described for the macrophages. Culture supernatants were collected and stored at −20°C. These supernatants were used for measuring TNF-α and IL-8 levels.
ELISA.
Levels of TNF-α and IL-8 were determined in the tissue culture supernatant of RAW264.7 and T84 cells infected with various serovar Typhimurium strains using Enzyme-linked immunosorbent assay (ELISA) (15). Briefly, the purified anticytokine capture antibodies (Pharmingen, San Diego, Calif.) were diluted to 1 to 4 μg/ml in binding buffer (0.1 M sodium bicarbonate buffer [pH 9.0]). The diluted antibodies were added to the wells of ELISA high-binding microtiter plates (Corning Costar, Corning, N.Y.) and incubated overnight at 4°C. Next, the capture antibodies were removed, and any nonspecific binding was blocked by adding 200 μl of blocking buffer (10% fetal bovine serum in PBS) to each well. The plates were incubated at room temperature for 2 h. After incubation, wells were washed three times with PBS-Tween (0.05%) (PBST) buffer.
A 100-μl aliquot of samples or standards was added, and the plates were incubated overnight at 4°C. After incubation, plates were washed six times in PBST. Detection antibodies (biotinylated anticytokine; Pharmingen) were diluted to 0.5 to 2 μg/ml in blocking buffer and added to the wells. Plates were incubated for 45 min at room temperature. After the plates were washed six times in PBST, an enzyme conjugate (streptavidin-conjugated HRP) was diluted to an optimal concentration in blocking buffer and added to the wells. The plates were incubated at room temperature for 30 min and washed six times with PBST. Next, 2,2′-azin-bis-(3-ethylbenzthiazoline-6-sulfonic acid) substrate solution [150 mg of 2,2′-azin-bis-(3-ethylbenzthiazoline-6-sulfonic acid) in 0.1 M anhydrous citric acid, adjusted to pH 4.35 with sodium hydroxide] was mixed, and 100 μl of 3% H2O2 was added to each 11 ml of substrate solution. A 100-μl aliquot was dispensed in each well, and the plates were incubated (for 5 to 80 min) for color development. The color reaction was stopped by adding 50 μl of stopping solution (20% SDS-50% diethyl formamide). Finally, the optical density was read with a microtiter ELISA plate reader (Molecular Devices Corp., Sunnyvale, Calif.) at 405 nm.
Mouse inoculation.
C57/BL6 female mice (6 to 8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, Maine). They were challenged orally or intraperitoneally (i.p.) with various doses either of WT serovar Typhimurium or with the lpp (single- or double-knockout) isogenic mutants. Mice were observed daily for signs of distress and mortality for up to 2 months. BALB/c SCID mice were similarly purchased from Jackson Laboratory and challenged with various doses of the lpp double-knockout mutant by the i.p. route. As a control BALB/c mice were infected with similar doses of either WT or the lpp double-knockout mutant. The animals were observed for mortality for 1 month.
Statistical analysis.
Where appropriate, the data were analyzed using Student's t test, and P values of ≤0.05 were considered significant.
Nucleotide sequence accession number.
The DNA sequence of the lpp genes and the flanking DNA was submitted to GenBank under accession number AY333760.
RESULTS
Cloning and sequence analysis of the serovar Typhimurium lpp gene.
Murein Lpp was shown by us and other investigators to induce in vitro and in vivo cytokine production and toxic shock (63, 84, 85). However, detailed studies in which targeted null mutants were developed using marker exchange mutagenesis have not been performed with any enteric pathogen to demonstrate the role of Lpp in bacterial pathogenesis. It is known that a single copy of the Lpp-encoding gene (lpp) located at 36 min on the chromosome exists in E. coli (62). By using specific primers to the lpp gene of E. coli (7), we amplified the lpp gene from the chromosomal DNA of serovar Typhimurium strain 14028. This PCR fragment was cloned into a TA cloning vector (Stratagene) and subjected to automated DNA sequence analysis in the Protein Chemistry Core Laboratory, UTMB. Our subsequent studies demonstrated that two copies of the lpp gene, separated by 82 bp and designated lpp1 and lpp2, existed in the chromosome of serovar Typhimurium (Fig. 1). The presence of two lpp gene copies was confirmed by the recent genome sequencing of S. enterica serovar Typhi CT18 and of serovar Typhimurium LT2 (56, 65). The availability of the entire genome sequence of Salmonella strains allowed us to amplify the flanking DNA sequences to the lpp genes for preparing isogenic mutants by double-crossover homologous recombination.
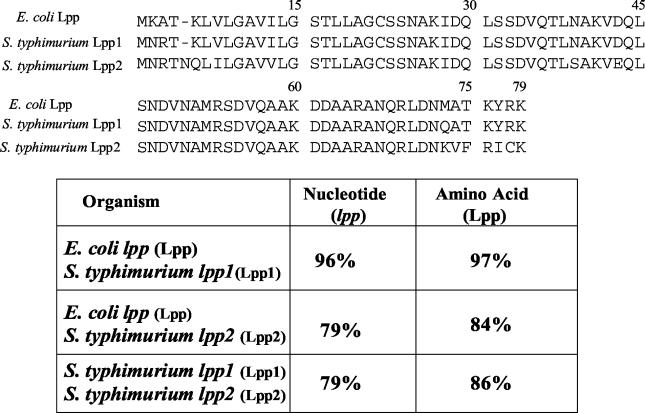
The DNA and amino acid sequence homologies between E. coli Lpp and serovar Typhimurium Lpp1 and Lpp2 are shown in Fig. 2. The homology between the E. coli lpp gene and the serovar Typhimurium lpp1 gene was 96%, whereas the homology between E. coli lpp and serovar Typhimurium lpp2 was 79% at the DNA level. The DNA homology between two copies of the lpp gene (lpp1 and lpp2) of serovar Typhimurium was 79%. At the amino-acid level, E. coli Lpp and serovar Typhimurium Lpp1 exhibited 97% homology. The homology was 84% between E. coli Lpp and serovar Typhimurium Lpp2. The two copies of Lpp (Lpp1 and Lpp2) shared 86% homology (Fig. 2). The Lpp2 copy of serovar Typhimurium contained an additional amino acid residue, asparagine (N), at position 5, which was missing in E. coli Lpp and serovar Typhimurium Lpp1.
FIG. 2.
Nucleotide and amino acid sequence homologies between lipoproteins of E. coli and serovar Typhimurium 14028. lpp, lipoprotein gene; Lpp, lipoprotein. Both E. coli lpp and S. enterica serovar Typhimurium lpp1 genes contained 237 nucleotides, whereas the S. enterica serovar Typhimurium lpp2 gene contained 240 nucleotides.
Analysis of the lpp isogenic mutants of serovar Typhimurium.
As shown in Fig. 1A, two copies of the lpp gene (lpp1 and lpp2) were replaced by a Knr gene cassette in the lpp double-knockout mutant. Deletion of the lpp genes from serovar Typhimurium double-knockout mutants was confirmed by Southern blot analysis using the lpp gene, Knr gene cassette, and pJQ200SK plasmid vector as probes. By using the Knr gene cassette as a probe, the size of the radioactive band in the digested (BglII and MluI enzymes) chromosomal DNA of two selected serovar Typhimurium lpp double-knockout mutants (designated mutants 66 and 67) was 0.6 kb larger (3.0 kb) than that seen in WT serovar Typhimurium (2.4 kb). This increase in size was due to the insertion of the Knr gene cassette (1.2 kb) in the chromosome of the lpp double-knockout mutants. The increase in size was by only 0.6 kb and not 1.2 kb, because 559 bp of the DNA containing two copies of the lpp gene and 82 bp of the DNA fragment between the two copies of the lpp gene were deleted from the lpp double-knockout mutants. As expected, the digested chromosomal DNA from WT serovar Typhimurium did not react with the Knr gene probe (data not shown).
Since both copies of the lpp gene (lpp1 and lpp2) were deleted from serovar Typhimurium mutants 66 and 67, the lpp gene probe did not react with the digested chromosomal DNA from these mutants. Neither digested chromosomal DNA from the double-knockout mutants nor that from WT serovar Typhimurium reacted with the plasmid pJQ200SK when it was used as a probe, indicating the complete loss of the suicide vector from the serovar Typhimurium lpp isogenic mutants 66 and 67 after homologous recombination. As a positive control, we used the pJQ200SK plasmid digested with BamHI, which exhibited a 5.4-kb band (data not shown).
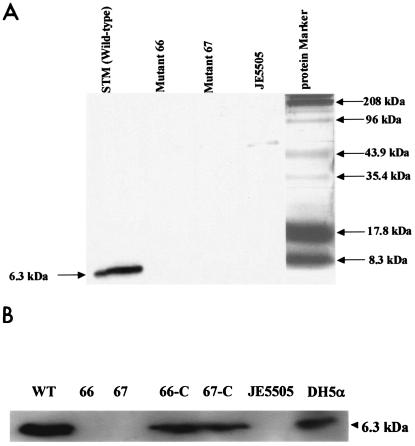
The absence of Lpp protein in the mutant strains was confirmed by Western blot analysis using Y. enterocolitica anti-Lpp monoclonal antibody (85). As is evident from Fig. 3A, a 6.3-kDa band representing Lpp was visualized in the outer membrane preparation of WT serovar Typhimurium. The corresponding band in lpp double-knockout mutants 66 and 67 was missing. The E. coli JE5505 strain lacking Lpp similarly did not exhibit a 6.3-kDa band. These isogenic mutants synthesized similar amounts of LPS, as seen with WT serovar Typhimurium. The LPS levels were determined by the Limulus amebocyte lysate assay (84). We then examined whether the complemented strains of serovar Typhimurium could synthesize Lpp. As is evident from Fig. 3B, both WT serovar Typhimurium and the complemented strains (66-C and 67-C) synthesized Lpp, whereas the lpp double-knockout mutants (66 and 67) were negative for Lpp synthesis. E. coli JE5505 and E. coli DH5α were used as negative and positive controls, respectively. We noted that the lpp double-knockout mutants had the WT growth phenotype when grown in LB and M-9 synthetic medium, indicating no auxotrophy (data not shown).
FIG. 3.
(A) Western blot analysis on the lpp double-knockout mutants of serovar Typhimurium. The outer membranes of serovar Typhimurium WT, lpp double-knockout mutants (mutants 66 and 67), and E. coli JE5505 (Lpp−) were isolated and separated by SDS-15% PAGE. The protein bands were then transferred to a nitrocellulose membrane and probed with Y. enterocolitica Lpp monoclonal antibody (1:1,000 dilution, determined empirically). The secondary antibodies (1:25,000 dilution) were goat antimouse conjugated with HRP. The blots were developed using an enhanced chemiluminescence kit. A band corresponding to the size of approximately 50 kDa in E. coli JE5505 appeared to be nonspecific and reacted with the antibodies. (B) Western blot analysis on the complemented lpp double-knockout mutants of serovar Typhimurium. The whole-cell lysates of serovar Typhimurium, lpp double-knockout mutants (66 and 67), the complemented lpp double-knockout mutants (66-C and 67-C), E. coli JE5505 (Lpp−), and E. coli DH5α (Lpp positive) were isolated and separated by SDS-15% PAGE. The protein bands were then transferred to a nitrocellulose membrane and probed with Y. enterocolitica Lpp monoclonal antibody (1:1,000 dilution). The secondary antibodies (1:25,000 dilution) were goat antimouse conjugated with HRP. The blots were developed by using an enhanced chemiluminescence kit. The arrow indicates the correct size of Lpp.
To characterize the lpp single-knockout mutants of serovar Typhimurium prepared using a suicide vector, pDMS197, a PCR method was used. For the lpp1 single-knockout mutant, the primer sets used were K3 and K5, F4 and R3, F4 and K5, R3 and K3, and F5 and R3 (Fig. 1A and Table 2). These primers amplified DNA fragments of the expected sizes of 1.2, 1.9, 1.3, 1.3, and 0.39 kb, respectively. Similarly, when primer sets K5 and K3, F1 and R1, F1 and K3, R1 and K5, and F4 and R4 were used for the lpp2 single-knockout mutant, fragments of expected sizes of 1.2, 4.8, 3.0, 2.9, and 0.29 kb, respectively, were amplified (Fig. 1A and Table 2). These data indicated insertion of the knr gene cassette at the correct location, as well as the correct conjunctional sequences in the lpp single-knockout mutants of serovar Typhimurium.
Likewise, when primer sets, F4 and kt and R3 and K2, were used with the λ Red system, PCR products of 1.1 and 1.3 kb were amplified from the lpp1 knockout mutant. With primer sets of F4 and kt and R3 and k2, 1.4- and 1.0-kb DNA fragments were amplified from the lpp2-knockout mutant, indicating the correct genotype of the mutant (Fig. 1B and Table 2).
Cell membrane integrity of the lpp double-knockout mutant.
It has been reported that murein Lpp is involved in maintaining the structural integrity of the E. coli cell envelope (73, 83). Therefore, we examined whether the outer membrane integrity of the lpp double-knockout mutant (67) was altered, compared to that of WT serovar Typhimurium, by using different assays, such as analysis of outer membrane blebbing, sensitivity to detergents, permeability of bacterial cells to antibiotics, and the release of β-lactamase from the bacterial cells. We did not observe blebbing of the outer membrane in the mutant strain by transmission electron microscopy. There was no difference in the sensitivity of the lpp double-knockout mutant to the effect of the detergent TX-100 (tested at concentrations of 0.5, 1, 2, and 5%) compared to results with WT Salmonella. The E. coli Lpp mutant JE5505, on the other hand, was sensitive to TX-100 at 5% compared to E. coli DH5α (Table 3). Similarly, both the WT and the lpp double-knockout serovar Typhimurium mutant were resistant to the effect of SDS at concentrations of 0.5, 1, and 2%. E. coli JE5505 was sensitive to SDS even at a concentration of 0.5% (Table 3).
TABLE 3.
Integrity of the membrane in the lpp double-knockout mutant of Salmonella is not affected
| Strain | No. of OMVa | β-Lacb (% release) | Resistance to:
|
|||
|---|---|---|---|---|---|---|
| Triton X-100c (%) | SDSd (%) | Rife (μg) | Vanf (μg/ml) | |||
| WT serovar Typhimurium | 0 | 48.4 ± 7.9 | >5 | >2 | 10 | >200 |
| Salmonella Lpp Mutant (67) | 0 | 51.1 ± 7.3 | >5 | >2 | 10 | >200 |
| E. coli DH5α | 0 | 15.0 ± 3.5 | >5 | >2 | 10 | >200 |
| Lpp mutant of E. coli (JE5505) | ++ | 43.1 ± 6.8* | ≤5 | ≤0.5 | <10 | ≤100 |
Number of outer membrane vesicles observed by electron microscopy in ultra-thin sections of bacteria. 0, no vesicle on cells; ++, many vesicles on all the cells (73).
β-Lactamase activity present in the supernatant was indicated as percentage of the total activity (average values from triplicate experiments ± SD. The asterisk denotes statistically significantly values (P ≤ 0.05) compared to the WT E. coli strain by Student's t test.
Triton X-100 concentration (% vol/vol) leading to a 50% decrease in cell turbidity measured after 3 h of bacterial growth in the presence of TX-100. Three independent experiments were performed.
SDS concentration (% weight/vol) leading to a 50% decrease in cell turbidity measured after 3 h of bacterial growth in the presence of SDS. Three independent experiments were performed.
Rifampin concentration (μg/disk) leading to a more than 50% increase in the diameter of zone of bacterial inhibition with the Lpp mutants compared to their respective parental strains. Three independent experiments were performed.
Vancomyin concentration (μg/ml) leading to a 50% decrease in cell turbidity measured after 3 h of bacterial growth in the presence of the antibiotic. Three independent experiments were performed.
The sensitivity of the lpp double-knockout mutant to the antibiotics rifampin (10, 50, and 100 μg) and vancomycin (100, 150, and 200 μg/ml) was similar to that of the WT serovar Typhimurium. The E. coli Lpp mutant was sensitive to both of these antibiotics (Table 3). Finally, the release of β-lactamase by the WT and the serovar Typhimurium Lpp mutant remained unaltered, while increased release of this enzyme was noted with the Lpp E. coli mutant (Table 3).
We also noted that the secretion of SPI-1-encoded TTSS effector proteins remained unaltered in the lpp double-knockout mutant versus WT serovar Typhimurium. We specifically examined secretion of SipA, SipB, SipC, and invJ in the culture supernatants. Bands of correct size for SipA (89 kDa), SipB (63 kDa), SipC (42 kDa), and invJ (40 kDa) were detected by SDS-PAGE. The identity of SipA and SipC was further confirmed by NH2-terminal sequence analysis of 10 amino acid residues. These data suggested that the integrity of the cell envelope in the serovar Typhimurium lpp double-knockout mutant was not affected.
Invasive ability of various serovar Typhimurium strains.
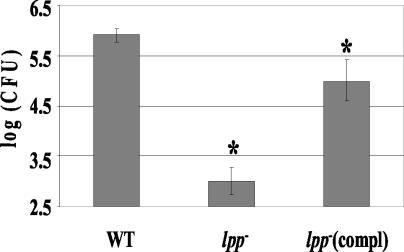
Infection of T84 intestinal epithelial cells with the lpp double-knockout mutant revealed that the deletion of lpp genes rendered Salmonella significantly reduced in invasive activity (Fig. 4). The invasive ability of the selected lpp double-knockout mutant (67) was reduced by 500- to 1,000-fold from that of WT serovar Typhimurium. However, the invasive capacity of the mutant was significantly restored after complementation (Fig. 4). The binding of the lpp isogenic mutant to host cells was minimally affected (data not shown). Studies have shown that Salmonella invades chicken ovarian cells, leading to contamination of egg follicles and vertical transmission of Salmonella (75). Further, Salmonella specifically targets and preferentially replicates within tumor cells (54). We, therefore, used A2780, an ovarian tumor epithelial cell line, to confirm our invasion data obtained using T84 cells. Interestingly, the lpp double-knockout mutant did not invade A2780 cells, compared to the WT and the complemented strain of serovar Typhimurium. Likewise, minimal invasion was seen in HeLa cells infected with the lpp double-knockout mutant (data not shown). As with the T84 cells, we noted no significant effect on binding of the lpp double-knockout mutant to the tumor cell line and the HeLa cells.
FIG. 4.
Invasion of T84 cells by the lpp double-knockout mutant (lpp−) (mutant 67), the WT, and the complemented (compl) strain of the lpp double-knockout mutant. The T84 cells were infected with Salmonella at an MOI of 10:1 for 1 h. The monolayers were washed and incubated with medium containing gentamicin for 1 h. After incubation, cells were washed, lysed with 0.1% TX-100, and plated on SS agar plates. The asterisk denotes statistical significance at P values of ≤0.05 by Student's t test, between the WT and mutant 67 (lpp−) and between 67 (lpp−) and 67-C [lpp−compl]. The values for invasion between the WT and 67-C were not statistically significant. Arithmetic means ± standard deviations were plotted.
We did not observe any difference in the binding of the lpp double-knockout mutant from that of WT serovar Typhimurium at 4°C, and no invasion was seen at 4°C with both the WT and lpp double-knockout mutant. The low invasion rate observed with the lpp double-knockout mutant was not because of lack of motility (see below, motility section), since in our invasion assays bacterial cells were centrifuged onto the monolayers. No statistically significant effect on the invasion rate was noted, whether or not the centrifugation step was included in the invasion assay. These data indicated that the defect in invasion was not the result of lack of motility or because of an inability of the mutant to bind to the host cells, but rather because Lpp had a putative role in cellular invasion. We used a well-characterized invasive and nonflagellated serovar Dublin mutant with its parental strain as controls in this assay. As expected, the WT serovar Dublin was as invasive for the T84 cells whether or not the bacterial cells were centrifuged onto the monolayer. However, with the serovar Dublin fliC mutant, a significant increase in invasion was noted when the bacterial culture was centrifuged onto the monolayer (data not shown).
Cytotoxicity and cell death associated with various serovar Typhimurium strains.
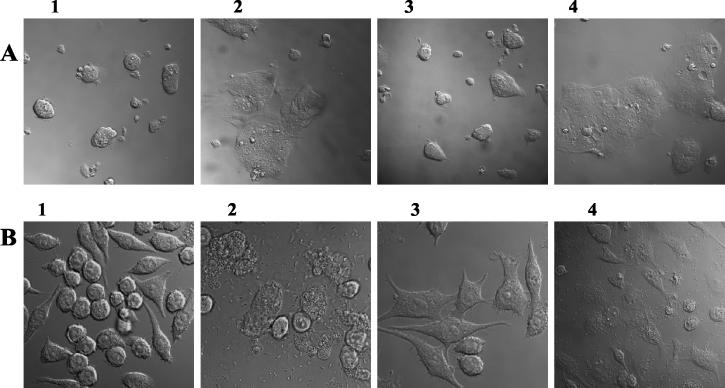
To examine the role of Lpp in inducing cytotoxicity and cell death in host cells, we used RAW264.7 macrophage and T84 intestinal epithelial cell lines. Microscopic examination of T84 (Fig. 5A) and RAW264.7 (Fig. 5B) cells infected with the lpp double-knockout mutant revealed a reduction of about 80% in cell death (Fig. 5, frame 3), compared to cells infected with WT serovar Typhimurium (Fig. 5, frame 2). A large number of RAW264.7 and T84 cells infected with the WT strain exhibited features of apoptosis, such as condensation of chromatin, vacuolation, and membrane blebbing (data not shown). These data were confirmed by the LDH release assay performed on macrophages and T84 using a CytoTox 96 LDH assay kit (data not shown). The lpp double-knockout mutant of serovar Typhimurium caused a minimal release of LDH from the host cells. Salmonella strains complemented with lpp genes restored the WT phenotype (Fig. 5, frame 4), indicating that Salmonella Lpp played a role in the induction of death in the host cells. Frame 1 represented uninfected cells.
FIG. 5.
Cytotoxicity and cell death induced by WT serovar Typhimurium and its various mutants in T84 (A) and RAW264.7 (B) cells. Cells were infected for 1 h with WT serovar Typhimurium, the lpp double-knockout mutant (67), or its complemented strain. Cells were washed and incubated with gentamicin-containing medium for 1 h. After incubation, cells were washed and further incubated with the antibiotic-free fresh medium for 24 h. Cells were gently washed twice in PBS and examined under a confocal microscope. Frame 2, cells infected with WT serovar Typhimurium (note dead cells); frame 3, cells infected with lpp double-knockout mutant (67) (note normal morphology of the cells); frame 4, cells infected with complemented strain (note dead cells); frame 1 represents a noninfected control.
Intracellular survival of various serovar Typhimurium strains.
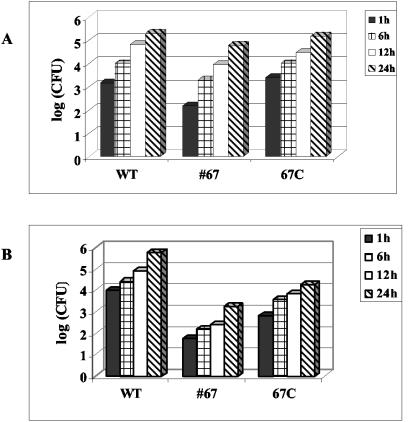
Another virulence mechanism of Salmonella is the ability to survive the hostile environment inside professional phagocytes such as macrophages. Studies have shown that mutants of serovar Typhimurium that failed to replicate in cultured intestinal epithelial cells (e.g., Caco-2 and HeLa) and in macrophages were avirulent in a mouse model (16, 49, 50). To examine this, a RAW264.7 macrophage cell line was infected with the lpp double-knockout mutant, WT serovar Typhimurium, or complemented serovar Typhimurium strain for 24 h. As seen in Fig. 6A, the lpp double-knockout mutant was able to survive and replicate inside macrophages in a manner similar to that of WT serovar Typhimurium. We also examined the intracellular survival of serovar Typhimurium in T84 cells. Although the total number of lpp double-knockout mutants recovered from T84 cells was less than that of the WT, because of the decreased ability of the mutant to invade, the growth rate of the lpp double-knockout mutants was not significantly affected compared to those of the WT and complemented serovar Typhimurium strains in intestinal epithelial cells (Fig. 6B). These data indicated that Lpp was not required for the intracellular survival of Salmonella in macrophages and T84 cells.
FIG. 6.
Intracellular replication of the lpp isogenic mutant inside RAW264.7 cells (A) and T84 cells (B). Cells were infected with the lpp double-knockout mutant (67), the complemented lpp double-knockout mutant (67-C), and WT serovar Typhimurium 14028 for 1 h. Cells were washed and incubated for 1 h with gentamicin-containing (100 μg/ml) medium. After incubation, cells were washed and incubated in fresh medium containing a minimum concentration of gentamicin (5 μg/ml) for different time points (1, 6, 12, and 24 h). Finally, cells were lysed with 0.1% TX-100 and plated on SS agar plates to determine numbers of CFU. Three independent experiments were performed. Data from a representative experiment are shown here.
Motility of various serovar Typhimurium strains.
We performed a motility assay to test whether deletion of both of the lpp genes would have an effect on Salmonella motility. The lpp double-knockout mutant grew at the site of the initial inoculum but showed no motility, compared to the complemented and WT serovar Typhimurium, indicating that Lpp drastically affected Salmonella motility. To test whether this impairment in motility resulted from changes in flagellar production, we performed scanning electron microscopy. However, the electron micrograph (data not shown) indicated no change in the number of flagella per cell in the lpp isogenic mutant from that of the WT serovar Typhimurium.
In vitro cytokine production by various serovar Typhimurium strains.
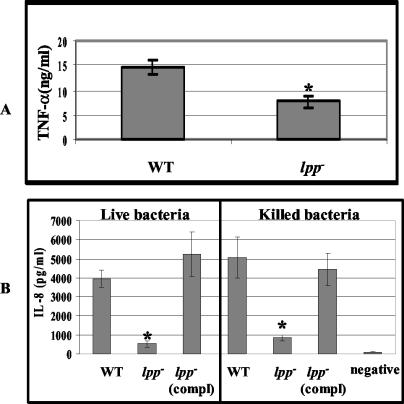
To test whether deletion of both of the lpp genes would affect the overall induction of TNF-α and IL-8, tissue culture supernatants from T84 and RAW264.7 cells stimulated with either heat-killed or live Salmonella were assayed for these cytokines. TNF-α production was significantly reduced in macrophages infected with the heat-killed lpp, double-knockout mutants, compared to results with the WT serovar Typhimurium (Fig. 7A). Neither the lpp double-knockout mutants nor the WT-infected T84 cells produced TNF-α; however, a similar reduction in IL-8 levels was observed in T84 cells infected with either live or heat-killed lpp double-knockout mutant, compared to the WT and complemented strains of serovar Typhimurium (Fig. 7B). These data indicated that Salmonella Lpp significantly contributed to the induction of inflammatory cytokines and that invasion of T84 cells was not required for IL-8 production, since IL-8 was also induced by heat-killed WT serovar Typhimurium. The reduced IL-8 production noted for the live or heat-killed lpp double-knockout mutant suggested a possible lack of signaling through the TLR2 rather than the lack of translocating TTSS-1 effectors.
FIG. 7.
TNF-α induced by lpp double-knockout mutant in RAW264.7 cells (A) and IL-8 production by T84 cells infected with live and heat-killed bacteria (B). Macrophages and T84 cells were stimulated with the heat-killed lpp double-knockout mutant or WT serovar Typhimurium at an MOI of 0.1:1 and incubated for 8 h. T84 cells were infected with live WT, the lpp isogenic mutant (67) (lpp−), or the complemented lpp mutant [lpp− (compl)] at an MOI of 10:1 and incubated for 1 h. After incubation, cells were washed and incubated in gentamicin-containing medium for 1 h, after which cells were washed and incubated in antibiotic-free medium for 12 h. TNF-α and IL-8 levels were determined using ELISA as described in Materials and Methods. An asterisk denotes statistically significant data (P ≤ 0.05) by Student's t test, between the WT and the lpp double-knockout mutant and between the lpp double-knockout mutant and its complemented strain. The values were not significant between WT and the lpp mutant complemented strain. Negative denotes no addition of the bacterial cells. The arithmetic means ± standard deviations were plotted. Compl denotes complemented strain.
Virulence in mice with various serovar Typhimurium strains.
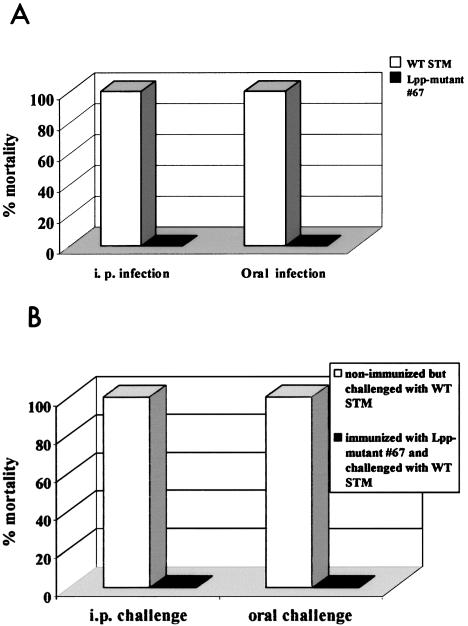
To investigate whether the Lpp deletion mutant (deletion of both Lpp1 and Lpp2) of serovar Typhimurium had altered virulence properties in vivo, we used a well-established mouse model. In initial experiments, we infected mice with various doses of either WT serovar Typhimurium or the Lpp double-knockout mutants to accurately calculate the lethal dose. Mice were challenged via the oral or i.p. routes and followed for 2 months for mortality. We determined that the lethal dose of WT serovar Typhimurium for oral challenge was 3,000 CFU, and that for i.p. challenge was 40 CFU. Results of a representative experiment are presented in Fig. 8A. Lpp-deficient serovar Typhimurium had no adverse health effects on mice following either oral challenge or i.p. challenge. In contrast, mice challenged with WT serovar Typhimurium died within 10 to 12 days following oral or i.p. challenge. More importantly, mice infected with a two-logs-higher number of Lpp-deficient serovar Typhimurium were healthy and showed no signs of disease or discomfort (data not shown).
FIG. 8.
(A) Mortality in mice following infection with the lpp double-knockout mutant (67) and WT serovar Typhimurium. Four groups of mice were used: two groups were infected orally with 3 × 103 CFU of the lpp mutant (67) or WT serovar Typhimurium. The other two groups were infected with 40 CFU of the lpp mutant (67) or WT serovar Typhimurium i.p. The group infected with WT serovar Typhimurium orally or i.p. showed 100% mortality compared to 100% survival in the groups that were infected with the mutant serovar Typhimurium. (B) Mortality in mice challenged with a virulent strain of serovar Typhimurium following immunization with the lpp double-knockout mutant of serovar Typhimurium (67). Out of four groups of mice used for the challenge study, two groups were immunized with mutant strain 67, and one group was orally inoculated (3 × 103 CFU) while the second group received i.p. (40 CFU) inoculation. The other two groups were left unimmunized as controls. After 2 months postimmunization, all groups of mice (immunized and nonimmunized) were challenged with a lethal dose of a virulent strain of serovar Typhimurium, either orally with 3 × 103 CFU or by the i.p. route with 3 × 103 CFU. Then, mice were observed for 4 weeks, and mortality was recorded.
To investigate whether the Lpp-deficient serovar Typhimurium mutant might immunize mice to Salmonella infection, they were infected with the Lpp− mutant (double knockout) either orally (3,000 CFU) or i.p. (40 CFU) and then allowed to rest for 2 months. We noted that the lpp null mutant was cleared from mice within 2 to 3 weeks after infection (data not shown). Control mice were inoculated with saline. These mice were then challenged with WT serovar Typhimurium either orally (3,000 CFU) or i.p. (3000 CFU) and monitored for survival and disease. Mice immunized with Lpp-deficient serovar Typhimurium were completely protected from subsequent WT serovar Typhimurium challenge (Fig. 8B). These mice showed no signs of disease, and no deaths occurred over a 2-month period of observation. The unimmunized group of mice, which was given only saline, died within 10 to 12 days after challenge with WT serovar Typhimurium.
More importantly, 100% of the SCID mice lacking T, B, and NK cells inoculated i.p. with 500 CFU of the Lpp double-knockout mutant died. The death rate was 75% when mice were infected with 100 CFU, and 25% of the mice died at a dose of 10 CFU over a period of 1 month. In a parallel control experiment, in which BALB/c mice were infected with either 100 or 500 CFU of the Lpp mutant, 100% of the mice survived, while the death rate was 100% in mice infected with WT serovar Typhimurium. A total of four to five mice were used per group for these experiments. These data indicated a critical role of the immune system in providing protection against Salmonella infection.
We noted that infection of mice (n = 5 in each group) with the single lpp isogenic mutant (lpp1 or lpp2) developed by using either the suicide vector or the λ Red system at a dose of 105 CFU by the i.p. route was not lethal as noted for the double-knockout mutant (mutant 67). Similarly, no lethality was noted with the mutants in mice when they were fed with 106 bacteria orally. All of the mice died within a week when challenged with WT serovar Typhimurium at these bacterial doses. We noted that the invasive ability of the lpp1 and lpp2 single-knockout mutants in T84 cells was approximately 2.4 logs higher than that of the Lpp double-knockout mutant, 67. The reduction in invasion by the double-knockout mutant from that by WT serovar Typhimurium was approximately 3.3 logs. Compared to the WT serovar Typhimurium, the single-knockout mutants exhibited 1.4 log less invasion in T84 cells. The invasive capacity of both the single-knockout mutants (lpp1 or lpp2) was very similar. The use of this in vitro biological (invasion) assay with Lpp1 and Lpp2 mutants provided evidence that both of the lpp genes were functional. We opted for a biological assay for determining functionality of the lpp1 and lpp2 genes, since it is more accurate than Western blot analysis for evaluation of gene expression and function. As noted above, the invasive potential of Lpp1 and Lpp2 mutants was increased over that of the Lpp double-knockout mutant in T84 cells.
DISCUSSION
Murein Lpp from E. coli and Y. enterocolitica mimics many of the in vitro and in vivo properties associated with LPS (63, 84, 85). In this study, we provide for the first time conclusive evidence that Lpp plays an important role in Salmonella virulence and induction of systemic infections by developing lpp mutants through marker exchange mutagenesis. We used a suicide vector, pJQ200SK, for constructing the lpp double-knockout mutant, while both a suicide vector (e.g., pDMS197) and the λ Red system were employed for developing lpp single-knockout mutants of serovar Typhimurium. The rationale for selecting these two systems was that when the suicide vector pDM197 was employed, the lpp1 gene was truncated with the Knr gene cassette, while the lpp2 gene was deleted and replaced with the Knr gene cassette. Consequently, we were somewhat concerned about direct comparisons of our results obtained with the lpp1, lpp2, and lpp1 lpp2 knockout serovar Typhimurium mutants, because of possible polar effects in the lpp1 mutant.
We therefore also used the λ Red system for constructing the lpp single-knockout mutants. With this system, the lpp1 and the lpp2 genes were replaced with the Knr gene cassette as noted for the lpp2 single-knockout and the lpp double-knockout mutants, resulting in direct comparison of the results. However, recently Murphy and Campellone (61) reported that extended expression of the recombination functions by using the λ Red system could induce a 10-fold increase in the rate of spontaneous mutations. We were therefore concerned about whether such mutations in vivo could lead to nonspecific effects rather than specific phenotypes associated with Lpp. Using different strategies to prepare the knockouts provided authenticity to our data, since the single-knockout mutants generated either by using the suicide vector or the λ Red system behaved very similarly in both in vitro and in vivo models of serovar Typhimurium infection.
Murein Lpp has been shown to play a role in stabilization of the bacterial cell envelope. An E. coli strain with a mutation in the lpp gene demonstrated outer membrane blebbing, sensitivity to TX-100, SDS, and antibiotics, and leakage of the enzymes RNase I and β-lactamase (11, 73, 83). Although mutation in the E. coli lpp gene caused the outer membrane to bleb outwards, the cytoplasmic membrane remained intact (83), thereby providing a barrier to some extent to the inner cellular components. Our studies with the lpp double-knockout mutant of serovar Typhimurium indicated no blebbing of the outer membrane, and the sensitivity of the lpp double-knockout mutant to detergent TX-100 and SDS was not altered. Further, the lpp double-knockout mutant was resistant to antibiotics, and the release of β-lactamase remained unaffected in the lpp double-knockout mutant compared to results for WT serovar Typhimurium (Table 3). The mechanism(s) of β-lactamase release in significant and comparable amounts from serovar Typhimurium and its lpp double-knockout mutant without indication of membrane vesicle formation is currently unclear (5).
Recently, several bacterial lipoproteins were identified that play an important role in bacterial pathogenesis (11, 18, 19, 27, 40, 82). Studies by Hazumi et al. (39) and Cascales et al. (11) demonstrated that Pal also was important in maintaining the outer membrane integrity of E. coli and that Pal contributed to bacterial virulence during sepsis. The Pal mutant of E. coli reduced mortality in mice and induced a low level of IL-6 compared to findings with mice infected with WT E. coli (40). Both Pal and murein Lpp are localized in the cell envelope, interact with the peptidoglycan layer, and have a common chemical structure at the amino terminus (47). Overproduction of Pal was shown to restore the outer membrane integrity of an E. coli lpp mutant; however, overproduction of murein Lpp did not complement the Pal mutant (11). Although we have not examined the expression level of gene-encoding Pal with our serovar Typhimurium lpp null mutant (double knockout), it is possible that Pal might compensate for the lack of Lpp, thereby providing stability to the outer membrane, as noted in this study.
Invasion of the host cells is an important virulence feature during systemic infection with Salmonella. This event is mediated by multiple bacterial virulence determinants, and the most important are the TTSS proteins encoded by the SPI-1 (17, 30, 46). The mechanism by which Lpp might affect Salmonella invasion (Fig. 4) is not known. Activation of a signal transduction pathway in the host cells involving Ca2+ and actin rearrangement, leading to membrane ruffling, is a key event in internalization of the bacteria (10, 32, 35, 68). Invasion-defective mutants of Salmonella were unable to induce membrane ruffling. Epidermal growth factor has been shown to rescue the ability of such mutants to invade host cells (28, 29). Therefore, it is possible that Lpp might be involved in triggering such signaling, most likely through TLR2, and this needs further investigation.
Recent studies also indicated that lipoproteins (other than Lpp and Pal) constitute a significant component of TTSS (e.g., PrgH, PrgK, and InvH), TTSS-associated invasion proteins (10, 17, 18), and the flagellar basal body (19). However, these lipoproteins are distinct from murein Lpp and Pal. Since in the serovar Typhimurium lpp null mutant (double knockout) the secretion of TTSS effector proteins encoded on the SPI-1 was not affected, these data suggested that Lpp might have a direct or indirect (regulatory) role in modulating serovar Typhimurium virulence.
Cytotoxicity and the ability to induce cell death are important virulence mechanisms of Salmonella (13, 48, 52, 59). Salmonella-induced cytotoxicity has been shown to be triggered through LPS and components of the TTSS (30, 36, 52, 80). However, whether Lpp leads to cell toxicity and cell death is not known. Data from this study indicated that the ability of the lpp double-knockout mutant to induce cell death in RAW264.7 and T84 cells was significantly reduced (Fig. 5). Although Salmonella-induced cytotoxicity and cell death were shown to be triggered by transmembrane signaling, requiring no bacterial internalization, Salmonella strains incapable of efficiently invading host cells failed to induce cytotoxicity and cell death (59). We similarly observed that the lpp double-knockout mutant and WT serovar Typhimurium entered macrophages in almost similar numbers and both replicated intracellularly; however, significant cytotoxicity and cell death were observed only in macrophages infected with the WT and the lpp-complemented strain.
Studies of Chen et al. (13) and Santos et al. (69) noted that mutations in the invasion-associated type III secretion proteins InvJ, SpaO, SipB, SipC, and SipD encoded by SPI-1 abolished cytotoxicity in macrophages. Although the mechanism by which Lpp induces cytotoxicity is not fully defined, the possible involvement of a TLR-dependent stimulation of macrophages has been a matter of speculation (43). The reduced cytotoxicity induced by the lpp double-knockout mutant did not seem to be the result of a low bacterial load, as observed in T84 cells, since the reduction in cytotoxicity was also observed in RAW264.7 cells, which had an intracellular bacterial load similar to that seen with the WT-infected macrophages (Fig. 6A and B). These results suggested that Lpp directly contributed to the cytotoxicity and death of the host cells.
Survival of Salmonella inside macrophages is important during systemic infection and is dependent on the ability of the bacteria to modulate intracellular trafficking and to neutralize the activity of the antimicrobial effector mechanism (6, 16, 41). Therefore, the ability of the Lpp− mutant (double knockout) to survive and replicate inside macrophages and T84 cells was examined. Effectors of SPI-2 TTSS have been implicated in the modulation of intracellular trafficking and have been shown to be required for intracellular growth and systemic infection (6, 37, 42, 76). Indeed, mutation in SPI-2 TTSS exhibited a defect in the intracellular growth of Salmonella in both macrophages and epithelial cells (16, 41). Our studies indicated that Lpp did not play a role in the intracellular survival and replication of Salmonella, since the Lpp mutant (double knockout) multiplied in a manner similar to that of WT serovar Typhimurium in both macrophages and epithelial cells (Fig. 6A and B).
The lpp double-knockout mutant displayed an impaired motility compared with the WT and the complemented strain, with no change in the number of flagella per cell in the lpp double-knockout mutant compared to that of the WT serovar Typhimurium, as determined by electron micrographs (data not shown). These data suggested that a mutation in Lpp might affect the composition rather than the flagellar number, which could alter flagellar motion. Indeed, mutations in Lnt and Lgt (enzymes required for biosynthesis of Lpp) were shown to affect flagellar assembly and motility (19).
The role of inflammatory cytokines has been established in the pathogenesis of systemic infection and septic shock (36, 60, 77). Studies from our laboratory showed that Lpp from E. coli induced TNF-α and IL-6 in macrophages from LPS-responsive and LPS-nonresponsive mice and acted synergistically with LPS to induce proinflammatory cytokine production (85). Neilsen et al. (63) reported that Lpp from E. coli DH5α caused the up-regulation of the same battery of cytokines induced by LPS. Reduction in the levels of TNF-α and IL-8 induced by the Lpp− mutant (double knockout) indicated that Salmonella Lpp significantly contributed to the induction of inflammatory cytokines (Fig. 7).
Studies have shown that serovar Typhimurium induces the production of IL-8 in T84 cells through the NF-κB pathway (24, 34). Induction of IL-8 and NF-κB activation in intestinal epithelial cells infected with Salmonella was preceded by and required an increase in intracellular Ca2+ (34). Pretreatment of cells with p38 mitogen-activated protein kinase inhibitors prevented Salmonella-induced IL-8 production (45). Therefore, Salmonella induction of IL-8 may be mediated by Lpp activation of the mitogen-activated protein kinase pathway through TLR2 (1, 33, 45, 51, 70) and needs further investigation. This hypothesis appears attractive, since the heat-killed Salmonella WT serovar Typhimurium and complemented strain-infected T84 cells synthesized similar levels of IL-8, while the lpp (double-knockout) null mutants (live or heat-killed) were defective in IL-8 production, clearly establishing the role of Lpp in Salmonella virulence (Fig. 7). These data also suggested that for the lpp null mutant, TLR2 signaling associated with Lpp leading to the IL-8 production was affected.
Our data from in vitro experiments using RAW264.7 and T84 cells indicated that a deletion in the lpp genes rendered Salmonella defective in the invasion, cytotoxicity, and induction of cytokines. The in vivo studies performed with mice confirmed that the Lpp double-knockout mutant was indeed avirulent in mice (Fig. 8A). Further, mice immunized with the lpp null mutant were protected from subsequent challenge with WT serovar Typhimurium after the nonspecific immunity due to macrophage activation was returned to normal (Fig. 8B). Susceptibility of SCID mice to death with the lpp null mutant reflected the contribution of the immune response during salmonellosis.
Further, deletion of either one of the lpp genes was sufficient to render Salmonella avirulent in mice at the tested dose. Interestingly, the single-knockout mutants (Lpp1 and lpp2 mutants) were more invasive in vitro than the lpp double-knockout mutant; however, this difference in invasion did not alter lethality in mice. More detailed studies are needed, using higher bacterial doses and determining the mean time of death, to demonstrate whether Lpp1 or Lpp2 contributes more towards bacterial virulence.
The increased invasiveness of the single-knockout (lpp1 and lpp2) mutants indicated that both copies of the lpp gene were functional. These data also suggested that attenuation of virulence observed in the double-knockout (lpp1 lpp2) mutant could be more related to alteration of TNF-α or IL-8 release than to invasiveness and should be studied in detail in the future.
Interestingly, we determined that the lpp double-knockout mutant was resistant to bacteriophages P22 and ES18. While this work was in progress, Dailey and Macnab (19) reported that transposition within the lpp and ltg genes (encoding an enzyme required for the modification of Lpp) conferred resistance to a P22 phage. In addition, InvG, a component of the TTSS, has been shown to be required for f1 phage assembly (18). Our phage-binding assay (53) indicated a minimal reduction of P22 phage binding to the lpp double-knockout mutant, compared to that to WT serovar Typhimurium (data not shown), suggesting that Lpp might be involved in phage assembly.
In conclusion, the data presented in this study indicated that murein Lpp plays an important role in the virulence of serovar Typhimurium and that the effect of Lpp is not pleiotropic due to alteration in the cell envelope integrity. The ability of the lpp null mutant (double knockout) to survive and replicate inside macrophages and epithelial cells and to secrete SPI-1 TTSS effector proteins further indicated that the integrity of the cell envelope of the Lpp mutant remained intact. Although the precise mechanism of Lpp's action is not known, it is possible that Lpp mediates invasion and other cellular responses through activation of TLR2 or possibly indirectly by regulating serovar Typhimurium virulence factors (11). Our future studies will be targeted at defining the mechanism by which Lpp affects virulence of Salmonella and further exploring bacterium-host cell interaction with the lpp single- and double-knockout mutants. Further, it will be crucial to determine the importance of duplication of the lpp gene in Salmonella. Indeed, there are reports of duplication of the cholera toxin gene during infection through homologous recombination (57). Our future studies also will include delineating the mechanism of duplication of the lpp gene.
Acknowledgments
Jian Sha was supported in part by a J. W. McLaughlin postdoctoral fellowship.
The assistance of Mardelle Susman in editing the manuscript is highly appreciated. We thank Alfredo Torres and T. D. Eaves-Pyles, Department of Microbiology and Immunology, UTMB, Galveston, for providing us with the lambda Red system and S. enterica serovar Dublin strains, respectively.
Editor: F. C. Fang
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 3.Babu, M. M., and K. Sankaran. 2002. DOLOP—database of bacterial lipoproteins. Bioinformatics 18:641-643. [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom, S., O. Olsson, and S. Normark. 1982. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J. Bacteriol. 150:528-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubes. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuzon, C. R., S. Meresse, K. E. Unsworth, J. Ruiz-Albert, S. Garvis, S. R. Waterman, T. A. Ryder, E. Boucrot, and D. W. Holden. 2000. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 19:3235-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Berna, V. Burland, M. Rily, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of E. coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V., and K. Rehn. 1969. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of E. coli cell wall: the specific effect of trypsin on the membrane structure. Eur. J. Biochem. 10:426-438. [DOI] [PubMed] [Google Scholar]
- 9.Braun, V., and K. Hantke. 1974. Biochemistry of bacteria cell envelopes. Annu. Rev. Biochem. 43:89-121. [DOI] [PubMed] [Google Scholar]
- 10.Brumell, J. H., O. Steel-Mortimer, B. B. Finlay. 1999. Bacterial invasion: force feeding by Salmonella. Curr. Biol. 9:R277-R280. [DOI] [PubMed] [Google Scholar]
- 11.Cascales, E., A. Bernadac, M. Gavioli, J. C. Lazzaroni, and R. Lioubes. 2002. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol. 184:754-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalker, R. B., and M. J. Blaser. 1988. Review of human salmonellosis III. Magnitude of Salmonella infection in the United States. Rev. Infect. Dis. 10:111-124. [DOI] [PubMed] [Google Scholar]
- 13.Chen, L., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 14.Chopra, A. K., J. H. Huang, X. J. Xu, K. Burden, D. W. Niesel, M. W. Rosenbaum, V. L. Popov, and J. W. Peterson. 1999. Role of Salmonella enterotoxin in overall virulence of the organism. Microb. Pathog. 27:155-177. [DOI] [PubMed] [Google Scholar]
- 15.Chopra, A. K., X, J. Xu, D. Ribardo, M. Gonzalez, K. Kuhl, J. W. Peterson, and C. W. Houston. 2000. The cytotoxic enterotoxin of Aeromonas hydrophila induces proinflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect. Immun. 68:2808-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 17.Collazo, C. M., and J. E. Galan. 1997. The invasion-associated type-III secretion system in Salmonella—a review. Gene 192:51-59. [DOI] [PubMed] [Google Scholar]
- 18.Daeffer, S., and M. Russel. 1998. The Salmonella typhimurium InvH protein is an outer membrane lipoprotein required for the proper localization of InvG. Mol. Microbiol. 28:1367-1380. [DOI] [PubMed] [Google Scholar]
- 19.Dailey, F. E., and R. M. Macnab. 2002. Effects of lipoprotein biogenesis mutations on flagellar assembly in Salmonella. J. Bacteriol. 184:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards, R. A., H. K. Linda, and D. M. Schifferli. 1998. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149-157. [DOI] [PubMed] [Google Scholar]
- 22.Eichelberg, K., and J. E. Galan. 2000. The flagellar sigma factor FliA (σ28) regulates the expression of Salmonella genes associated with the centisome 63 type III secretion system. Infect. Immun. 68:2735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekperigin, H. E., and K. V. Nagaraja. 1998. Salmonella. Vet. Clin. N. Am. Food Anim. Pract. 14:17-29. [PubMed] [Google Scholar]
- 24.Elewaut, D., J. A. DiDonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with entroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 25.Fadl, A. A., K. S. Venkitanarayanan, and M. I. Khan. 2002. Identification of Salmonella enteritidis outer membrane proteins expressed during attachment to human intestinal epithelial cells. J. Appl. Microbiol. 92:173-179. [DOI] [PubMed] [Google Scholar]
- 26.Filip, C., G. Fletcher, J. L. Wulff, and C. F. Earthhart. 1973. Solubilization of the cytoplasmic membrane of E. coli by ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortney, K. R., R. S. Young, M. E. Bauer, B. P. Katz, A. F. Hood, R. S. Munson, and S. M. Spinola. 2000. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 68:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis, C. L., T. A. Raya, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct micropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 29.Galan, J. E., J. Pace, and M. J. Hayman. 1992. Involvement of epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature 357:588-589. [DOI] [PubMed] [Google Scholar]
- 30.Galan, J. E. 2001. Salmonella interaction with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 31.Gan, K., S. D. Gupta, K. Sankaran, M. B. Schmid, and H. C. Wu. 1993. Isolation and characterization of a temperature sensitive mutant of Salmonella typhimurium defective in prolipoprotein modification. J. Biol. Chem. 268:16544-16550. [PubMed] [Google Scholar]
- 32.Garcia-del Portillo, F., M. G. Pucciarelli, W. A. Jeffries, and B. Finlay. 1994. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J. Cell Sci. 107:2005-2020 [DOI] [PubMed] [Google Scholar]
- 33.Gewirtz, A. T., A. M. Siber, J. L. Madara, and B. McCormick. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gewirtz, A. T., A. S. Rao, P. O. Simon, D. Merlin, D. Carnes, J. L. Madara, and A. S. Neish. 2004. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-κB pathway. J. Clin. Investig. 105:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginocchio, C., J. Pace, and J. Galan. 1992. Identification and molecular characterization of Salmonella typhimurium gene involved in triggering the internalization of Salmonella into cultured epithelial cells. Proc. Natl. Acad. Sci. USA 89:5976-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glauser, M. P., G. Zanetti, J. D. Baumgartner, and J. Cohen. 1991. Septic shock: pathogenesis. Lancet 338:732-739. [DOI] [PubMed] [Google Scholar]
- 37.Guy, R. L., L. A. Gonias, and M. A. Stein. 2000. Aggregation of the host endosomes by Salmonella requires SPI2 translocation of SseFG and involves SpvR and the fms-aroE intragenic region. Mol. Microbiol. 37:1417-3145. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi, S., and H. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 39.Hazumi, N., H. Yamada, and S. Mizushima. 1978. Two new peptidoglycan-associated proteins in the outer membrane of E. coli K-12. FEMS Microbiol. Lett. 4:275-277. [Google Scholar]
- 40.Hellman, J., J. D. Roberts, M. M. Tehan, and J. E. Allaire. 2002. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J. Biol. Chem. 277:14274-14280. [DOI] [PubMed] [Google Scholar]
- 41.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vasquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Gene encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 42.Hensel, M. 2000. Salmonella pathogenicity island 2. Mol. Microbiol. 36:1015-1023. [DOI] [PubMed] [Google Scholar]
- 43.Hersh, D., D. Monack, M. R. Smith, N. Ghori, and S. Falkow. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirota, Y., H. Suzuki, Y. Nishimura, and S. Yasuda. 1977. On the process of cellular division of E. coli: a mutant of E. coli lacking a murein-lipoprotein. Proc. Natl. Acad. Sci. USA 74:1417-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 46.Hueck, C. J. 1998. Type III protein secretion system in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichihara, S., M. Hussain, and S. Mizushima. 1981. Characterization of new membrane lipoproteins and their precursors of E. coli. J. Biol. Chem. 256:3125-3129. [PubMed] [Google Scholar]
- 48.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoff, and M. F. Kagnoff. 1998. Apoptosis of intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leung, K. Y., and B. B. Finlay. 1991. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 88:11470-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. Fang, D. Guiney, G. Songer, and F. Haffron. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 91:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carrol, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 52.Lindgren, S. W., J. Stojiljkovie, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu, M., R. Deora, S. R. Doulatov, M. Gingery, F. A. Eiserling, A. Preston, D. J. Maskell, R. W. Simons, P. A. Cotter, J. Parkhill, and J. F. Miller. 2002. Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 295:2091-2094. [DOI] [PubMed] [Google Scholar]
- 54.Low, K. B., M. Ittensohn, T. Le, J. Platt, S. Sodi, M. Amoss, O. Ash, E. Carmichael, A. Chakraborty, J. Fischer, S. Lin, X. Luo, S. I. Miller, L. Zheng, I. King, J. M. Pawelek, and D. Bermudes. 1999. Lipid A mutant Salmonella with suppressed virulence and TNF-α induction retain tumor-targeting in vivo. Nat. Biotechnol. 17:37-41. [DOI] [PubMed] [Google Scholar]
- 55.Matsuyama, S., T. Tajima, and H. Tokuda. 1995. A novel periplasmic carrier protein involved in the sorting and transport of E. coli lipoproteins destined for the outer membrane. EMBO J. 14:3365-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Laterille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 57.Mekalanos, J. 1983. Duplication and amplification of toxin genes in Vibrio cholera. Cell 35:253-263. [DOI] [PubMed] [Google Scholar]
- 58.Mishu, B., J. Koehler, L. A. Lee, D. Rodrigue, F. H. Brenner, P. Blake, and R. V. Tauxe. 1994. Outbreaks of Salmonella enteritidis in the United States, 1985-1991. J. Infect. Dis. 169:547-552. [DOI] [PubMed] [Google Scholar]
- 59.Monack, D. M., B. Raupach, A. E. Kromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison, D. C., and J. L. Ryan. 1987. Endotoxins and disease mechanisms. Annu. Rev. Med. 38:417-432. [DOI] [PubMed] [Google Scholar]
- 61.Murphy, K. C., and K. G. Campellone. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. http://www.biomedcentral.com/1471-2199/4/11. [DOI] [PMC free article] [PubMed]
- 62.Neidhardt, F., R. Curtiss III, J. L. Ingraham, E. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger. 1996. Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 63.Neilsen, P. O., G. A. Zimmerman, and T. M. McIntyre. 2001. E. coli Braun lipoprotein induces a lipopolysaccharide-like endotoxic response from primary human endothelial cells. J. Immunol. 167:5231-5239. [DOI] [PubMed] [Google Scholar]
- 64.Newton, S. C., M. Kotb, T. M. Poirier, B. A. Stocker, and E. H. Beachey. 1991. Expression and immunogenicity of a streptococcal M protein epitope inserted in Salmonella flagellin. Infect. Immun. 59:2158-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrel. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 66.Parrillo, J. E. 1993. Pathogenic mechanisms of septic shock. N. Engl. J. Med. 328:1471-1477. [DOI] [PubMed] [Google Scholar]
- 67.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 68.Ruschkowski, S., L. Rosenshine, and S. Falkow. 1992. Salmonella typhimurium induces an inositol phosphate flux in infected epithelial cells. FEMS Microbiol Lett. 74:121-126. [DOI] [PubMed] [Google Scholar]
- 69.Santos, R. L., R. M. Tsolis, S. Zhang, T. A. Fight, A. J. Baumler, and L. G. Adams. 2001. Salmonella-induced cell death is not required for enteritis in calves. Infect. Immun. 69:4610-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savkovic, S. D., A. Koustsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 71.Sha, J., M. Lu, and A. K. Chopra. 2001. Regulation of cytotoxic enterotoxin gene in Aeromonas hydrophila: characterization of an iron uptake regulator. Infect. Immun. 69:6370-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sha, J., E. V. Kozlova, and A. K. Chopra. 2002. Role of various enterotoxins in Aeromonas-induced gastroenteritis: generation of enterotoxin gene-deficient mutants and evaluation of their enterotoxic activity. Infect. Immun. 70:1924-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki, H., Y. Nishimura, S. Yasuda, A. Nishimura, M. Yamada, and Y. Hirota. 1978. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol. Gen. Genet. 167:1-9. [DOI] [PubMed] [Google Scholar]
- 74.Taheuchi, A. 1967. Electron microscopic studies on experimental Salmonella infection. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 75.Thiagarajan, D., M. Saeed, J. Turek, and E. Asem. 1996. In vitro attachment and invasion of chicken ovarian granulose cells by Salmonella enteritidis phage type 8. Infect. Immun. 64:5015-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uchiya, K., M. A. Barberi, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ulevitch, R. J., and P. S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437-457. [DOI] [PubMed] [Google Scholar]
- 78.Vaara, M., and T. Vaara. 1994. Ability of Cecropin B to penetrate the enterobacterial outer membrane. Antimicrob. Agents Chemother. 38:2498-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van der Poll, T., and S. F. Lowry. 1995. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of the host defense. Shock 3:1-12. [DOI] [PubMed] [Google Scholar]
- 80.Van Der Velden, A. W., S. W. Lindgren, M. J. Worly, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophage by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watson, P., A. Benmore, S. Khan, P. Jones, D. Maskell, and T. Wallis. 2000. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect. Immun. 68:3768-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson, P. R., E. E. Galyon, S. M. Paulin, P. W. Jones, and T. S. Wallis. 1998. Mutation of InvH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun. 66:1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yem, D., and H. C. Wu. 1978. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 133:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang, H., D. W. Niesel, J. W. Peterson, and Gary R. Klimpel. 1998. Lipoprotein released from bacteria: potential factor in bacterial pathogenesis. Infect. Immun. 66:5196-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang, H., J. W. Peterson, D. W. Niesel, and G. R. Klimpel. 1997. Bacterial lipoprotein and LPS act synergistically to induce lethal shock and proinflammatory cytokine production. J. Immunol. 159:4868-4878. [PubMed] [Google Scholar]