Supplemental digital content is available in the text.
Key Words: autism, children, eye examinations, vision acuity, tonometry
ABSTRACT
Purpose
To compare testability of vision and eye tests in an examination protocol of 9- to 17-year-old patients with autism spectrum disorder (ASD) to typically developing (TD) peers.
Methods
In a prospective pilot study, 61 children and adolescents (34 with ASD and 27 who were TD) aged 9 to 17 years completed an eye examination protocol including tests of visual acuity, refraction, convergence (eye teaming), stereoacuity (depth perception), ocular motility, and ocular health. Patients who required new refractive correction were retested after wearing their updated spectacle prescription for 1 month. The specialized protocol incorporated visual, sensory, and communication supports. A psychologist determined group status/eligibility using DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision) criteria by review of previous evaluations and parent responses on the Social Communication Questionnaire. Before the examination, parents provided information regarding patients’ sex, race, ethnicity, and, for ASD patients, verbal communication level (nonverbal, uses short words, verbal). Parents indicated whether the patient wore a refractive correction, whether the patient had ever had an eye examination, and the age at the last examination. Chi-square tests compared testability results for TD and ASD groups.
Results
Typically developing and ASD groups did not differ by age (p = 0.54), sex (p = 0.53), or ethnicity (p = 0.22). Testability was high on most tests (TD, 100%; ASD, 88 to 100%), except for intraocular pressure (IOP), which was reduced for both the ASD (71%) and the TD (89%) patients. Among ASD patients, IOP testability varied greatly with verbal communication level (p < 0.001). Although IOP measurements were completed on all verbal patients, only 37.5% of nonverbal and 44.4% of ASD patients who used short words were successful.
Conclusions
Patients with ASD can complete most vision and eye tests within an examination protocol. Testability of IOPs is reduced, particularly for nonverbal patients and patients who use short words to communicate.
Autism spectrum disorders (ASDs) are developmental disabilities that occur in 1 out of 68 children in the United States.1 Individuals with ASD have difficulties in reciprocal social interaction, verbal and nonverbal communication, and processing sensory stimuli. Because of these difficulties, patients with ASD may be unable to respond to subjective vision testing or to complete medical procedures. For eye care providers seeking to examine patients with ASD, little information is available. Clinicians need information regarding how to select tests or modify procedures to facilitate patients’ ability to complete testing. Clinicians also need evidence on ASD patients’ ability to complete tests within an eye examination.
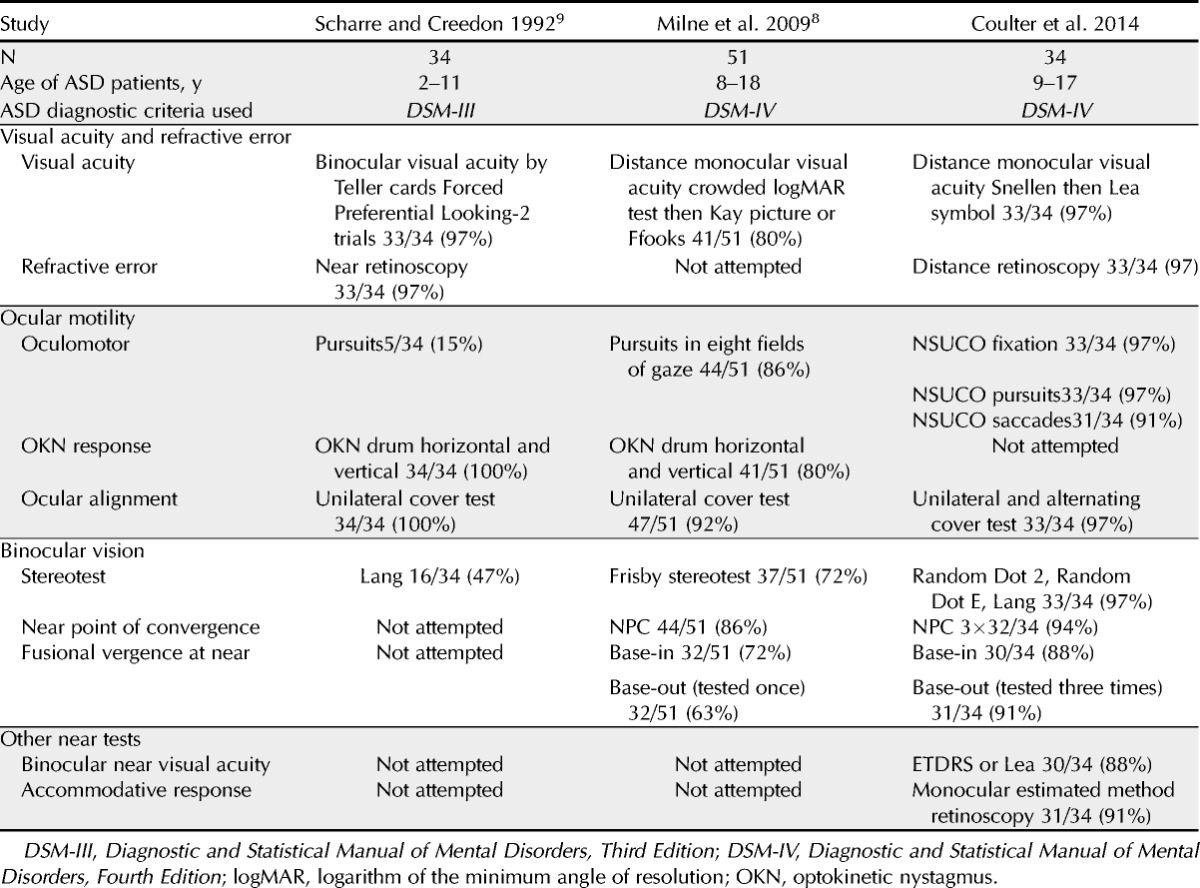
Testability is defined as the ability of a patient to complete a vision or eye test; clinically, it is determined as the proportion of patients within a defined population who are able to complete a test or procedure. For the typically developing (TD) preschool population, studies of vision testing have reported testability of visual acuity,2–5 stereoacuity,2,5,6 and refraction tests.2,7 Few studies have reported testability results for patients with ASD. In 2009, Milne et al.8 investigated vision screening of 51 school-aged patients with ASD and 44 TD patients aged 8 to 18 years using a battery of vision acuity, stereoacuity, cover test, prism fusion range, near point of convergence (NPC), and optokinetic response. Patients with ASD were categorized as high functioning (HF) or low functioning (LF) based on full-scale IQ score on the Weschler Abbreviated Scale of Intelligence test; HF patients had scores greater than or equal to 70, whereas LF patients had scores less than 70 or could not complete the initial test trials. Testability varied by functioning level and ranged from 86 to 100% for HF patients and 40 to 80% for LF patients. In 1992, Scharre and Creedon9 reported outcomes of a visual assessment performed in a school setting of 34 children with autism aged 2 to 11 years. Reported testability was high for most tests; 97% of patients completed Teller card binocular visual acuity, near retinoscopy, unilateral cover test, voluntary smooth pursuit, and optokinetic nystagmus response. Only 47% of patients, however, were able to complete Lang stereoacuity testing.9
To date, no study has prospectively investigated the testability of vision tests and ocular health within a comprehensive eye examination protocol in the ASD population or has compared these results to those of TD peers. This information is needed for eye care providers to select vision tests, diagnose and manage vision problems, and establish a standard of care for ASD patients. The purpose of this study was to determine testability of school-aged patients with ASD on vision and eye tests within an examination protocol and to compare these results to those of TD peers. To promote the ability of patients with ASD to complete testing, an eye examination protocol was designed that incorporated visual, communication, and sensory supports and behavioral strategies.
Creation of an Eye Examination Protocol
Challenges associated with ASD include difficulties with communication, motor planning, sensory processing, transitioning from task to task, maintaining attention, and engagement. A specialized eye examination protocol that targeted these challenges and that could be easily incorporated into clinical practice was designed. This protocol incorporated visual, sensory, and communication supports; included behavioral strategies; and used tests and techniques that minimized tactile sensitivity issues. By minimizing undesirable stimuli, modifying instruction sets, and preparing the patient with ASD, the protocol aimed to maximize the ability of patients with ASD to respond, stay on task, remain calm and regulated, and complete testing procedures.
Supports and Modifications for Patients with ASD
Communication Supports
To support ASD patients’ receptive and expressive communication, the eye examination protocol used several supports and modifications. A speech and language pathologist reviewed traditional instruction sets. Lengthy and complex statements were eliminated and rephrased to be short, simple, direct, and as exact as possible. Investigators adjusted the rate of their speech to allow the patient additional time to hear, interpret, and respond to directions. Subjects with ASD are more likely to complete a task when the task is presented as a choice of desired positive behaviors.10 In this protocol, this was applied to eye and vision testing; for example, when testing stereoacuity, the investigator asked the patient, “Do you want to put on the Polaroid glasses or do you want me to put them on you?”
For patients with ASD and minimal or limited ability to speak, patients were allowed to respond using augmentative and alternative communication devices.11 These include the patients’ habitual communication boards, electronic devices, speech-generating devices, and tablet computers.11,12 Investigators also used the Answers: YesNo Application (Simplified Touch), presented on a smartphone or tablet computer, to allow nonverbal patients to answer “yes” or “no” to examination-related questions such as “Can you see the letters on the chart?”13
Visual Supports
A variety of visual supports were used to enable patients with ASD to understand test instructions and the sequencing of tasks within the eye examination. With the guidance of a speech and language pathologist, visual representations of instruction sets were created. Verbal directions were paired with pictures and gestures so that patients with ASD could better understand what they were expected to do and what they needed to look for.14,15
Other visual supports, identified from the special education literature including a social story and a visual schedule, were also used.
Social Story
A social story was provided to all families of patients with ASD to ease transitions and avoid behavioral meltdowns (see Fig. 1 and Supplemental Digital Content 1, a pdf file of the complete social story, available at http://links.lww.com/OPX/A189). Written as a short narrative illustrated by photographs, pictures, or symbols, a social story prepares the patient for potentially challenging experiences by concretely identifying strategies, showing positive behaviors, and demonstrating what other people will do to help the patient.16–18 Social stories have been used to prepare patients with ASD for doctor visits, dental visits, allergy tests, blood draws, and electroencephalogram testing.19–22 Social stories must meet specific guidelines for format and language.18,23 When created according to these specifications, social stories decrease tantrums and inappropriate behaviors and increase positive behaviors such as initiating social interactions and responding on task.23
FIGURE 1.

Social story—the first page. For the complete first grade version of social story, see Supplemental Digital Content 1, “A Day at the Eye Doctor!,” available at http://links.lww.com/OPX/A189.
To prepare patients for the eye examination visit, a social story was created that described each step of the examination experience from arriving in the clinic, entering the examination room, meeting the doctor, and preparing for each procedure or test (see Supplemental Digital Content 1, a pdf file of the complete social story, available at http://links.lww.com/OPX/A189). Special education teachers with expertise in social stories for students with ASD wrote the social story according to Gray’s published guidelines. Two versions were created—one at a fourth grade reading level and one at a first grade reading level. The social story was provided a few weeks in advance, so that the patient’s parents or caregivers could read it to the patient several times before the visit.
Visual Schedule
A visual schedule was also used in the eye examination protocol. Visual schedules use photographs or pictures to represent the activities and procedures that will occur and their sequence (see Fig. 2 or Supplemental Digital Content 2, which provides a pdf file of the visual schedule, available at http://links.lww.com/OPX/A190). By informing patients with ASD of what will happen next, visual schedules help patients to make transitions, remember how to respond, and reduce anxiety due to uncertainty.24 Visual schedules are more effective than language-based communication for patients with ASD. They target these patients’ relative strengths in simultaneous processing instead of relying on social or language reasoning abilities.25 Studies have shown that visual schedules enhance on-task behavior and independent functioning and increase the speed of completing transitions.25–27
FIGURE 2.
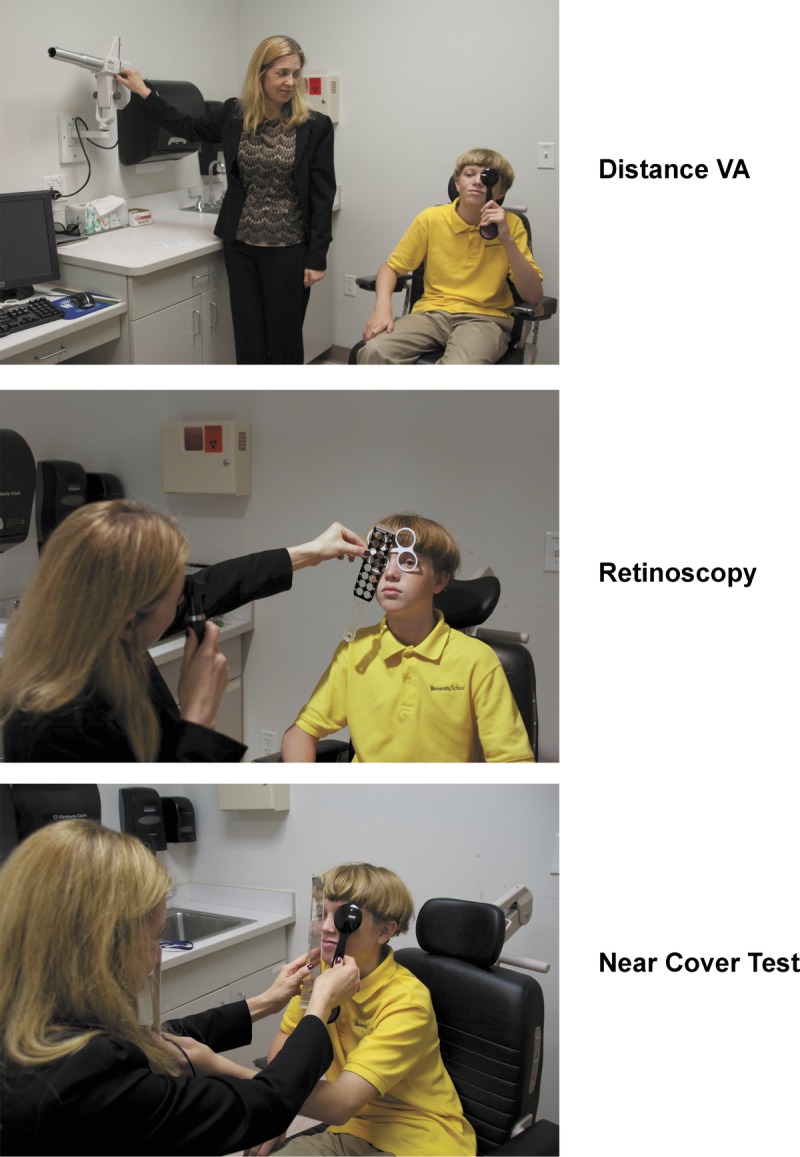
Section of visual schedule. For the entire visual schedule, see Supplemental Digital Content 2, “Visual Schedule!,” available at http://links.lww.com/OPX/A190.
A visual schedule of the activities and eye examination procedures of the eye examination visit was created using a series of photographs (see Fig. 2 or Supplemental Digital Content 2, which provides a pdf file of the visual schedule, available at http://links.lww.com/OPX/A190). The visual schedule displayed the name and a photograph of each procedure in the eye examination protocol and the sequence of the procedures. The visual schedule was available to the patient and the examiner in both a laminated card format and within an iPad/iPhone application called First/Then.28 The photographs used in the visual schedule matched those used in the social story.
Supports and Modifications for Motor Planning Challenges
Patients with ASD often experience motor planning difficulties, that is, the ability to connect the intention of making a motor movement to the execution of the movement itself. To enable nonverbal patients to respond when a response required a point or gesture, motor approximations were accepted. For example, patients responding to visual acuity testing on a picture on a response card were allowed to respond by touching the picture with their whole hand rather than required to point to the picture.
Supports for Sensory Processing Challenges
Patients with ASD have difficulty modulating or processing visual, tactile, and auditory stimuli.29,30 In an eye examination, tests that are particularly problematic are tests that present bright lights such as binocular indirect ophthalmoscopy (BIO) and tests that involve touching the patient’s face and eye area such as the slit lamp examination and drop instillation. Research shows that patients with ASD are more tolerant to unpleasant stimuli when the stimulus presentation is more predictable and patients perceive they have some control.29,30 Strategies that may increase patient tolerance include distraction techniques such as having the examiner sing, count, or recite the alphabet with patients while performing the procedure and having sensory toys and items available for patients that match their sensory needs.29,30
In creating the eye examination protocol, tests were selected that were less likely to elicit patients’ tactile defensiveness. For example, to obtain intraocular pressures (IOPs), an ICare rebound tonometer was used. This instrument was selected because it was small, did not require anesthetic drops to be administered before obtaining IOPs, and minimally obstructed the visual field. To maximize testability of patients with ASD, all the above supports were incorporated into the eye examination protocol.
Behavioral Strategies
To encourage patients to complete tasks that were particularly challenging, positive behaviors were encouraged through the techniques of shaping and high-probability request/low-probability request. In shaping, a new behavior such as tolerating a bright light is developed through successive reinforcement of attempts that are closer and closer to the desired behavior. For example, a patient tolerates a binocular indirect ophthalmoscope beam, first on his legs, then his shoulders, then his face, and finally his eyes. In high-probability request/low-probability request, requests are first made to do a task that is likely to be done. This is followed by a request that is less likely to be done with the intention of creating momentum. An example would be to first ask a patient to tolerate the ICare tonometer being held by his or her arm before asking him or her to tolerate it against his or her forehead.
METHODS
All investigators followed the tenets of the Declaration of Helsinki throughout the study. The Nova Southeastern University (NSU) Institutional Review Board approved the protocol and informed consent forms. The parent or guardian (subsequently referred to as parent) of each study patient gave written informed consent. Each participating patient completed the assent process according to the protocol approved by the NSU Institutional Review Board. Special procedures, described below, were designed to obtain assent from ASD patients with limited communication skills. Health Insurance Portability and Accountability Act authorization was obtained from parents.
Study Flow
Patient Selection
To participate in the study, patients had to be between 9 and 17 years. A total of 61 patients, 34 with ASD and 27 who were TD, were enrolled. Recruitment fliers were distributed through several venues including the University of Miami-NSU Center for Autism and Related Disabilities e-newsletter, the Broward County Chapter of the Autism Society of America, NSU Health Profession Division Clinics, Denise’s List (a Yahoo listserv for parents of children with autism), Broward County public schools, private schools, local therapy centers, and community health fairs. Subjects were also recruited with the assistance of the Interactive Autism Network Research Database at the Kennedy Krieger Institute and Johns Hopkins Medicine-Baltimore, sponsored by the Autism Speaks Foundation. Parents responded to recruitment fliers by contacting the principal investigator or a coinvestigator who completed a prescreening. If the patient appeared to be eligible, an intake visit was scheduled with the parent only.
Intake Visit
Parents provided information regarding the patient’s sex, race, ethnicity, and medication usage. Parents indicated whether the patient wore a refractive correction, whether the patient had ever had an eye examination, and the age at the last eye examination. Parents of patients with ASD specified the patient’s verbal communication level from three options: (1) nonverbal or minimally verbal; (2) uses short words, can answer questions at least partially; or (3) verbal, speaks fluently, is able to answer questions completely. Parents also indicated how their child preferred to communicate: using an iPad/iPhone, the YesNo application, gestures, some words, or words fluently.
For patients identified as ASD, parents provided documentation of the autism spectrum diagnosis. All parents completed the Social Communication Questionnaire, a standardized instrument used to screen for ASDs. Parents were offered a social story to prepare their child for the eye examination visit.
Confirmation of ASD and TD Group Eligibility
A psychologist with expertise in ASD diagnosis determined eligibility and group status based on the presence or absence of an ASD diagnosis and symptoms consistent with autism spectrum conditions. Children were included in the ASD group if they held community diagnoses and their diagnoses were confirmed using DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision) criteria based on review of previous evaluations combined with parent ratings of symptoms on the Social Communication Questionnaire.31 Children were included in the TD group if they had never received a diagnosis of ASD, and they did not show any research evidence of an ASD-related disorder (i.e., did not exceed the cutoff score of nine on the Social Communication Questionnaire). Of the 64 patients who completed the prescreening and whose parents completed the intake visit, 61 met criteria for the TD or ASD groups.
Eye Examination Visit
All patients who met the criteria of the ASD or TD group were scheduled for an eye examination study visit. Study staff worked with families to schedule appointments for patients with ASD to maximize patient cooperation. The assent process with the child or adolescent was conducted at the time of the eye examination, before any procedures were initiated. The TD children and children with ASD who could read signed an assent form. If the parent or guardian indicated that the child diagnosed with ASD could not read the assent form, it was read to the child and the principal investigator documented the child’s response to the verbal assent process.
Examination Procedures
The following measurements were taken at the eye examination study visit (listed in order of administration): binocular distance visual acuity, monocular distance visual acuity (OD then OS), retinoscopy, cover/uncover (unilateral cover) test and alternate cover test with prism neutralization at distance and near, near point of convergence (NPC), ocular motility testing (fixation, saccades, pursuits), near fusional vergence (break and recovery), stereoacuity, monocular estimation method retinoscopy, binocular near visual acuity, and monocular near visual acuity (OD then OS). All testing at near was performed at 40 cm. Ocular health testing was sequenced as follows: extraocular muscle movement testing, pupils, anterior segment evaluation, tonometry, dilated posterior segment evaluation, and cycloplegic retinoscopy.
Cycloplegic retinoscopy findings were analyzed according to study criteria for prescribing (Table 1). A refractive correction was prescribed for patients if it was determined that there was a significant difference between the patient’s habitual refractive correction and their measured refractive error. Patients requiring a new refractive correction wore their new correction for at least 4 weeks and then returned and completed all vision testing, excluding ocular health tests that had been completed at their initial examination.
TABLE 1.
Refractive error prescribing criteria

Distance Visual Acuity
Patients wore refractive correction for all vision testing except ocular health evaluation. Binocular visual acuity testing began by presenting the Snellen acuity chart to the patient. Patients responded verbally or by typing the letter on a communication device. If the patient was unable to respond to the Snellen acuity chart, visual acuity testing was attempted with the LEA Symbol Crowded Symbol Book (Good-Lite, Elgin, IL, 250700). Patients responded by verbally identifying the symbol or by matching the correct picture using the Response Key Card (Good-Lite, 251700).
To obtain monocular visual acuity, the examiner occluded an eye using an occluder. If the patient showed resistance to the occluder that lasted more than 5 seconds, the patient’s eye was occluded using the palm of the examiner’s or parent’s hand. If the patient still showed resistance, monocular visual acuity was discontinued and only a binocular visual acuity was obtained.
Retinoscopy
The patient viewed a target of interest at 10 feet through +2.00-diopter (D) fogging lenses. Targets used included a parent waving to the patient or a video shown on an iPad or portable DVD player. The examiner neutralized the refractive error for each eye using the Luneau Retinoscopy Rack (Lombart Instruments) supplemented by loose lenses. The examiner determined his or her working distance and recorded the net retinoscopy findings in each meridian for each eye.
Cover Test at Distance and Near—Unilateral and Alternate Cover Test
During the cover test, the patient was presented a fixation target of a 20/30 isolated letter or symbol. The unilateral cover test was administered to determine the presence of strabismus, and if so, whether it was intermittent or constant. To determine the magnitude of the strabismus or phoria, the alternate cover test with prism neutralization procedure was administered. A prism bar (Gulden B-16 horizontal prism bar with prismatic levels from 1 prism diopter (Δ) to 45Δ; Gulden Ophthalmics, Elkins Park, PA) was used to neutralize the deviation using a bracketing method.
Near Point of Convergence
The examiner held a ruler with the zero point of the ruler parallel and equal to the bridge of the patient’s nose. The examiner showed the patient a detailed fixation stick target of 20/30 (Good-Lite, 542055), either with Sloan letters or Lea symbols, at the midline of the patient’s body. The patient was instructed to “Look here. Tell me when the letters/pictures break into two, but try to keep the target single/one as long as possible. When it breaks try to see one.” To visually show the patient what to look for, while giving the verbal instructions, the examiner also showed the patient the visual demonstration card showing double versus single images.
The examiner moved the fixation target toward the subject at approximately 1 to 2 cm/s until the examiner observed a loss of fusion. This point was considered the NPC break. The distance from the NPC break to the bridge of the patient’s nose was measured to the nearest centimeter with the ruler. The target then was moved away from the subject until the examiner observed a recovery of fusion. Both the NPC break and recovery were measured to the nearest centimeter and the test was performed three times.
Evaluation of Fixation
For fixation assessment and NSUCO (Northeastern State University College of Optometry) oculomotor testing, testing was completed while the patient was standing. Using a nickel-plated ball target (Good-Lite, Wolff Wand kit, 660700), the patient was instructed to “Look here,” while pointing to the target. The examiner determined if the patient was able to maintain fixation for at least 10 seconds.
NSUCO Oculomotor Test
For all NSUCO oculomotor testing, the patient stood in front of the examiner. To determine if the patient understood the words used in the NSUCO saccades and pursuit tests, a pretest for NSUCO was performed. The patient was shown the nickel-plated (silver) and brass-plated (gold) ball targets (Good-Lite, Wolff Wand kit, 660700). The patient was directed to “Touch the gold ball.” The patient was then directed to “Touch the silver ball.” If the patient responded by touching the gold and silver ball after the corresponding instruction, the patient passed the pretest. The examiner then proceeded to administer the NSUCO oculomotor test for saccades and pursuits. If the patient could not pass the pretest, NSUCO saccades and NSUCO pursuit tests were not performed and the patient was recorded as untestable.
NSUCO Oculomotor Test—Saccades
The examiner held the nickel-plated (silver) and brass-plated (gold) ball targets (Good-Lite, Wolff Wand kit, 660700) at Harmon’s distance (the distance from the patient’s elbow to the middle knuckle) from the patient. Each target was held 10 cm from the midline of the patient. The instructions to the patient were “I have two balls. When I say silver, look at the silver ball. When I say gold, look at the gold ball. Remember, don’t look until I tell you.” The examiner then alternately said “gold” and “silver” and repeated these so that the patient had to make 10 saccades (5 to the gold target and 5 to the silver target). The examiner observed the saccadic eye movements and rated the patient’s performance in head movement and body movement, ability, and accuracy using standardized scoring criteria.
NSUCO Oculomotor Test—Pursuit
The examiner held a nickel-plated (silver) ball target at Harmon’s distance (distance from the patient’s elbow to the middle knuckle) or no farther from 40 cm from the patient. The examiner instructed the patient, “Watch the silver ball go around. Don’t move your head. Keep watching the ball.” The examiner moved the fixation target in a path no more than 20 cm in diameter, performed at the midline of the patient.
The examiner observed the pursuit eye movement and rated the performance in the categories of head movement and body movement, ability, and accuracy using standardized scoring criteria.
Negative Fusional Vergence at Near
Negative fusional vergence was measured with a horizontal prism bar (Gulden B-16 horizontal prism bar with prismatic levels from 1Δ to 45Δ; Gulden Ophthalmics, Elkins Park, PA) while the patient fixated a handheld fixation target. The patient looked at a single column of letters or Lea symbols (Good-Lite, 542055) of 20/30 equivalent at a distance of 40 cm. The patient was instructed to “Look here. Tell me when the letters/pictures become blurred or break into two, but try to keep the target single/one as long as possible. When it breaks, try to see one.”
To provide a visual support, while giving the verbal instructions, the examiner also showed the patient the visual demonstration card showing blur and diplopia. The examiner held the prism bar in a base-in direction in front of the right eye and increased the amount of base-in prism in front of the right eye at approximately 2Δ per second, stopping when the patient lost fusion and no longer made a divergence movement in response to increasing prism. This was recorded as the break point. The examiner then increased the base-in demand by 5 more prism diopters and then at a rate of about 2Δ per second reduced the base-in prism until the patient regained fusion as indicated by a divergence movement or the patient stating that he or she saw one.
Positive Fusional Vergence (Convergence Amplitudes) at Near
Positive fusional vergence was measured three times with a horizontal prism bar (Gulden B-16 horizontal prism bar with prismatic levels from 1Δ to 45Δ; Gulden Ophthalmics, Elkins Park, PA) while the patient fixated a handheld fixation target. The target used was a single column of letters or Lea symbols (Good-Lite, 542055) of 20/30 equivalent at a distance of 40 cm. The patient was instructed to “Look here. Tell me when the letters/pictures become blurred or break into two. Try to keep the target single/one as long as possible. When it breaks try to see one.” While giving the verbal instructions, the examiner also showed the patient the visual demonstration card showing blur and diplopia. The examiner held the prism bar in a base-out direction in front of the right eye and increased the amount of base-out prism in front of the right eye at approximately 2Δ per second, stopping when the patient lost fusion and failed to show a responsive convergence movement. This was recorded as the break point. The examiner then increased the base-out demand by 5 more prism diopters and then at a rate of about 2Δ per second reduced the base-out prism until the patient regained single vision.
Monocular Estimation Method Retinoscopy
The patient sat opposite to the examiner. The examiner held the retinoscope with a monocular estimation method reading or picture card attached. The examiner instructed the patient to “Keep your eyes open. Read the words or look at the pictures here.” The examiner briefly (<1 second) held a loose lens in front of one eye at a time to neutralize the motion of the reflex in each eye. The lens that neutralized the reflex was recorded.
Near Visual Acuity
Testing for near visual acuity was performed with letters using the SLOAN ETDRS (Early Treatment Diabetic Retinopathy Study) Format Near Point Vision Test (Precision Vision, 2106). If the patient did not respond to letters, testing was done using the LEA Symbols Near Vision (Good-Lite, 250800) and matching response card (Good-Lite, 251700). The patient’s responses were considered correct if the patient either said the correct letter or picture symbol or matched the correct picture on the LEA Symbols response card.
Stereoacuity
Stereoacuity testing was first attempted with the Random Dot 2 Stereotest (Good-Lite, 100750). If the patient did not respond to the Random Dot 2 Stereotest, testing was attempted with the Random Dot E (Precision Vision, 3700) using a forced-choice presentation. If the patient did not respond to either the Random Dot Stereotest or the Random Dot E test, testing was attempted with the Lang Stereotest 1 (Bernell, Mishawaka, IN). For the Lang Stereotest, the patient was allowed to match presented black and white pictures of the picture targets to the Lang testing plate.
Extraocular Muscle Movements and Pupils
The examiner tested extraocular muscle movements and pupils and recorded any abnormalities.
Anterior Segment Evaluation and Tonometry
The examiner examined the structures of the anterior segment using a slit lamp or an Eidolon Bluminator ophthalmic illuminator (Eidolon Optical, Natick, MA) and/or direct ophthalmoscopy. The examiner noted any disease present.
For tonometry and BIO, the patient was seated watching a DVD video player placed at eye level. While the patient watched a DVD video, tonometry testing was attempted using the ICare Rebound tonometer. Up to three attempts were made to measure the IOP. If a readable finding was not obtained after three attempts, tonometry testing was discontinued and the patient was recorded as untestable.
Instillation of Mydriatic/Cycloplegic Spray
A combination mydriatic/cycloplegic spray of 0.5% tropicamide, 0.5% cyclopentolate, and 2.5% phenylephrine was used. This commonly used combination achieves effective mydriasis and cycloplegia in the pediatric population, while minimizing risks associated with cyclopentolate.32,33 Of specific concern for the ASD population, instillation of cyclopentolate has been reported to elicit seizures in patients with neurological impairment.32
While the patient watched a video, the examiner told the patient that the spray would be instilled and practiced singing and counting with them. The examiner administered a mydriatic, cycloplegic spray of 0.5% tropicamide, 0.5% cyclopentolate, and 2.5% phenylephrine to both eyes simultaneously, a technique called the Fecho double spray instillation (Fig. 3).
FIGURE 3.
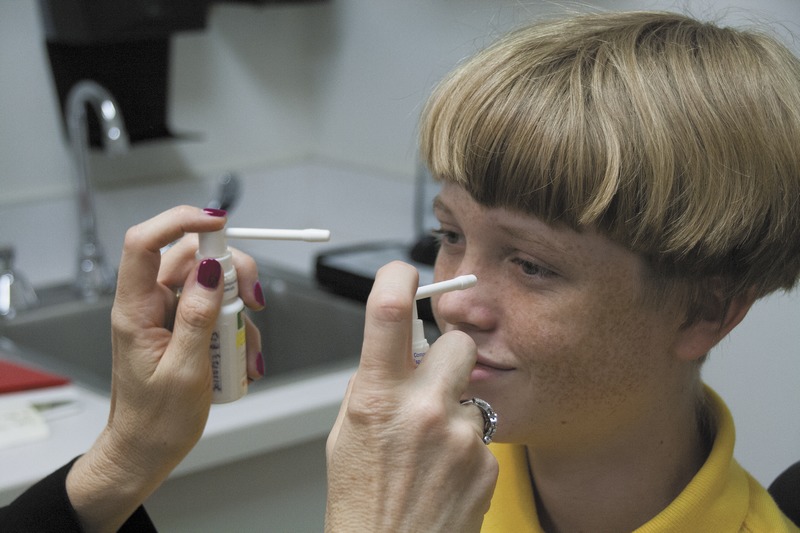
Fecho double spray instillation.
Posterior Segment Evaluation and Cycloplegic Retinoscopy
Thirty minutes after instillation of the combination mydriatic/cycloplegic spray, posterior segment evaluation and cycloplegic retinoscopy were completed.
Binocular Indirect Ophthalmoscopy
To encourage the patient to tolerate the bright beam of the binocular indirect ophthalmoscope, a shaping technique was used. The light beam was presented to the patient in an incremental manner. The examiner presented the light beam to the patient first by shining the beam on the examiner’s hand first and then on the patient’s hand. The examiner then counted for 5 seconds holding the light beam on the patient’s leg and then backed away. This pattern was repeated as the beam was shown the patient’s hand, face, and eye. The examiner then put the BIO on his or her head and shined it into the patient’s eye and counted with the patient for 5 seconds. The examiner used a lighted toy to direct the patient’s direction of gaze during the examination of the peripheral fundus.
Cycloplegic Retinoscopy
The patient viewed video playing on a DVD placed on a table or chair 3 feet away. The patient viewed the target through lens flippers of +2.00. The examiner neutralized the refractive error for each eye using the Luneau Retinoscopy Rack (Lombart Instruments) and/or loose lenses. The examiner determined his or her working distance and recorded the net retinoscopy findings in each meridian for the OD and OS.
Need for Refractive Correction
The patient’s cycloplegic retinoscopy findings were compared with study guidelines for refractive correction to determine if a new refractive correction was indicated (Table 1). For patients whose findings showed a significant difference from their habitual correction and their cycloplegic retinoscopy findings, a new spectacle prescription was provided. The patient wore the appropriate spectacle correction for 4 weeks and returned to the clinic to repeat the following tests: binocular distance visual acuity, monocular distance visual acuity, retinoscopy, cover/uncover (unilateral cover) test and alternate cover test with prism neutralization at distance and near, NPC, ocular motility testing, near fusional vergence (break and recovery), stereoacuity, monocular estimation method retinoscopy, binocular near visual acuity, and monocular near visual acuity. The results from this second testing session were used in data analysis.
Statistical Analysis
All statistical testing was performed using SAS version 9.3. Chi-square tests were used to compare testability of patient groups (TD vs. ASD) and based on communication level reported by the parent (nonverbal, uses short words, verbal/able to speak fluently). Independent sample t tests were used to compare the mean age and time since last eye examination between TD and ASD patients. For comparisons of age and time since last examination between levels of communication, analysis of variance was used with the Tukey method used to control the overall error rate of any post hoc pairwise comparisons.
RESULTS
Between August 2010 and June 2012, 61 patients aged 9 to 17 years were enrolled. The mean age for each group did not differ significantly (p = 0.54): ASD, mean age = 11.65 years; SD = 2.8; and TD, mean age = 11.22 years; SD = 2.5. Of note, given the typical sex distribution for the ASD population of four male subjects to each female subject, the sex distribution for both the ASD and TD groups was similar (p = 0.53): for the ASD group, 59% were male, and for the TD group, 66% were male. Race was also similar: 96% of TD patients and 79% of ASD patients were white (p = 0.067). Among those identified as white, there were no significant differences between groups in the percentage of patients who were Hispanic (p = 0.22). Typically developing patients were more likely to be medication free (p = 0.056).
Among the ASD patients, parental report of verbal communication indicated that 23% (n = 8) were nonverbal, 27% (n = 9) were verbal and able to use short words, and 50% (n = 17) were verbal/spoke fluently. There were no age differences between patients in the three communication levels (p = 0.20). The average age of verbal ASD patients was 11.71 years (SD = 2.7), whereas patients using short words were, on average, 10.44 years (SD = 2.6). Nonverbal ASD patients were, on average, 12.88 years (SD = 3.0).
Parent Report of Prior Eye Examination
Nearly two-thirds (65%) of the ASD patients had a previous eye examination compared with 85% of TD patients (p = 0.071). Among the ASD patients, the percentage who had attended a prior eye examination differed only slightly by the level of reported verbal communication: 50% of the nonverbal patients, 56% of the patients who used short words, and 77% of the verbal patients who spoke fluently (p = 0.35).
Parent Report of Time since Last Eye Examination
Information on the age of the patient at his or her last eye examination and current age was used to calculate the time since last examination for those with a previous examination. The time between the study visit and the last reported eye examination was greater for ASD patients (mean = 2.36 years; SD = 2.5) than TD patients (mean = 0.70 years; SD = 1.1; p = 0.007). Time since last eye examination for verbal patients was similar to that of TD patients (mean = 1.00 years; SD = 0.9; p = 0.94). In contrast, the average time since an eye examination was significantly longer for both the patients using short words to communicate (mean = 4.00 years; SD = 2.7; p < 0.001) and nonverbal patients (mean = 4.75 years; SD = 3.1; p < 0.001).
Testability of Vision and Eye Test Measures
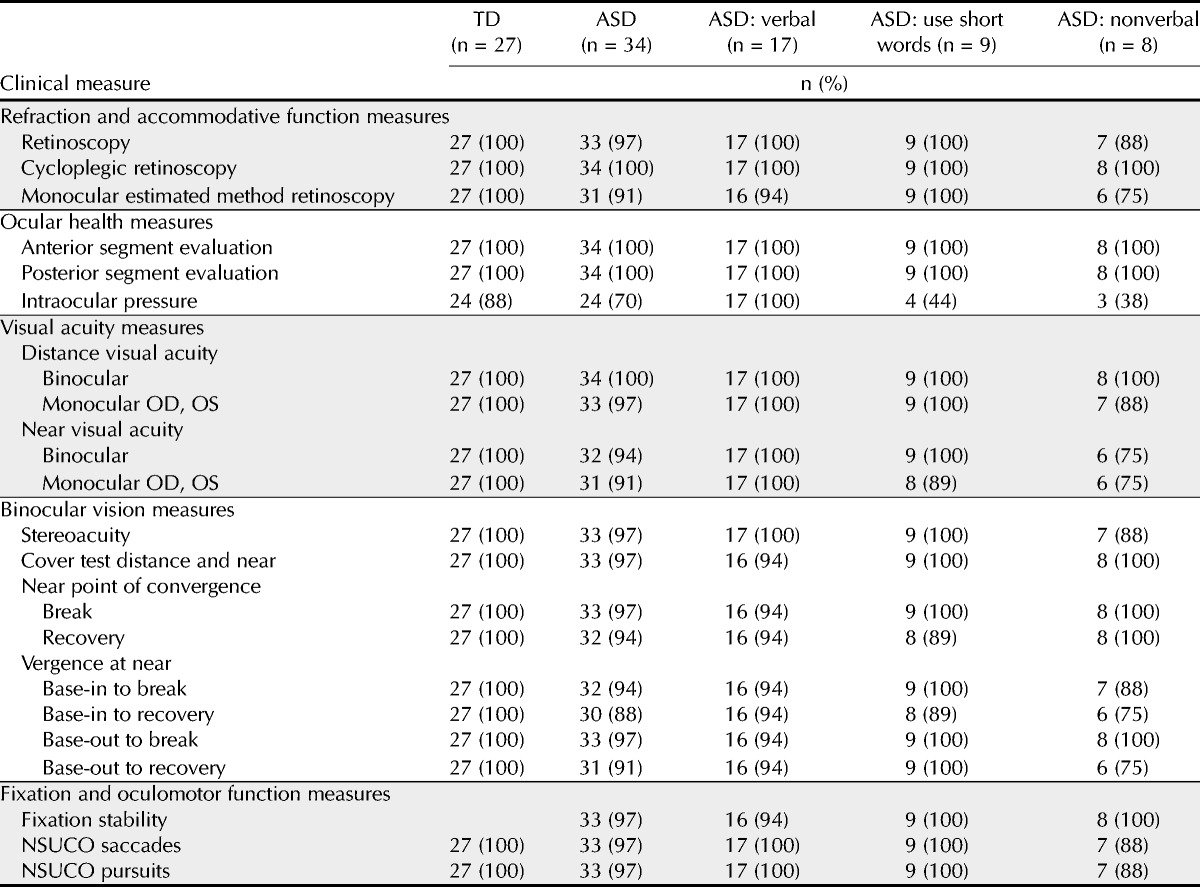
As shown in Table 2, the proportion of ASD patients able to complete testing was high for most vision and eye tests (TD, 100%; ASD, 88 to 100%), except for IOP measurement, which was reduced in both the ASD group (71%) and the TD group (89%; p = 0.083). For all measures except IOP (p < 0.001), there was no association between communication level of ASD patients and testability.
TABLE 2.
Number (and percentage) of patients completing each clinical measure, by patient type and communication level
Testability of Visual Acuity Measures
Binocular distance visual acuity could be obtained for all TD and ASD patients (Table 2). Similarly, monocular distance visual acuity could be obtained for all TD and all but one ASD patient. For both patient groups, visual acuity at distance was primarily obtained using the Snellen letter chart: 100% of TD patients and 71% of ASD patients. Further examination of the ASD patients, however, showed that the specific test used to determine visual acuity at distance was dependent on communication level (p values = 0.01; Table 3). Of the 17 patients with ASD described as verbal by their parents, 16 (94%) were able to complete distance visual acuity testing using the Snellen chart, whereas 1 patient (6%) needed the Lea Crowded Symbol Book and response card (Table 3). Of the 9 patients with ASD described as using short words, distance visual acuity was determined using the Snellen chart for 4 patients (44%), whereas the Crowded Symbol Book and response card was necessary for 5 patients (56%). For the 8 patients with ASD described as nonverbal, 4 (50%) were tested using the Snellen chart and 4 (50%) were tested using the Crowded Symbol Book and response card.
TABLE 3.
Number (and percentage) of patients tested with each specific clinical test for visual acuity at distance and near and stereoacuity, by ASD communication level
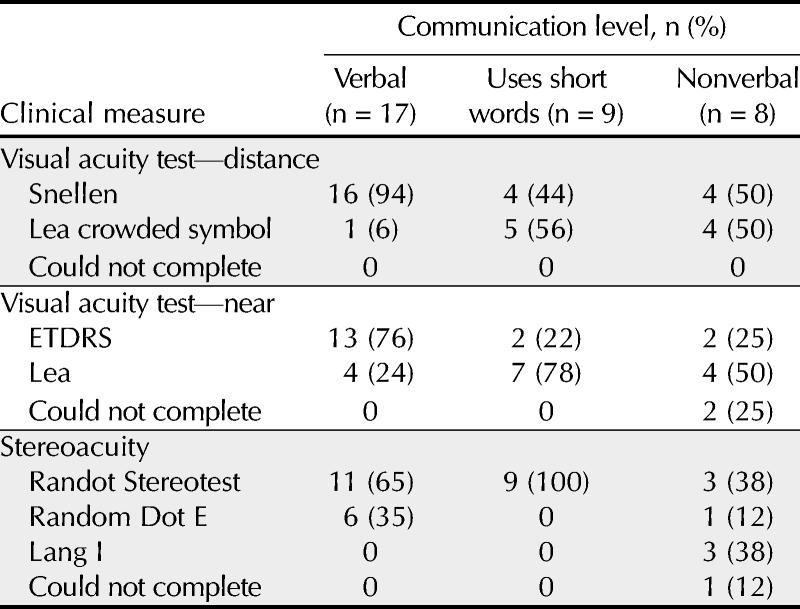
As with distance visual acuity, both binocular and monocular near visual acuity measurements were obtained from all TD patients while testability ranged from 91 to 94% among ASD patients. In addition, testability of binocular near visual acuity was associated with communication level in the group of ASD patients (p = 0.032). Although binocular visual acuity at near could be obtained from all ASD patients who were verbal or used short words, only 75% of the nonverbal patients could complete the test. Intraocular pressure was obtained on all ASD verbal patients, but it was obtained on only 44% of those who communicate using short words and on 38% of the nonverbal patients.
Unlike distance visual acuity, the test required to obtain near visual acuity depended on patient type (p = 0.002). All TD patients were tested using the ETDRS chart, whereas only 24 of 34 (70%) ASD patients were able to respond to that specific test. In addition, the test used for ASD patients was significantly associated with communication level (p = 0.004). Like TD patients, most verbal ASD patients (76%) were tested using the ETDRS compared with only 22% of ASD patients described as using short words and 25% of nonverbal ASD patients.
Testability of Binocular Vision Measures
Testability of both sensory and motor fusion tests was generally good to excellent for both ASD and TD groups. Stereoacuity measurements were obtained from all TD patients and 33 of 34 (97%) ASD patients. The one ASD patient who could not be tested was described as nonverbal. Although testability of ASD patients was not related to communication level, the specific test required was related to method of communication (p = 0.002; Table 3). The Randot Stereotest was used to measure stereoacuity for all 9 patients described as using short words to communicate, 11 of the 17 (65%) verbal ASD patients, and 3 of the 8 (38%) nonverbal ASD patients. The remaining 6 ASD patients described as verbal were tested using the Random Dot E stereotest. Testability for NPC and near fusional vergence again was excellent for TD patients and slightly reduced for ASD patients ranging from 88 to 97%; however, these differences between the TD and ASD groups were not statistically significant (p values > 0.06). In addition, testability for ASD patients for NPC and near fusional vergence was not related to verbal communication level (p values > 0.15).
Testability of Eye Movement Measures
Fixation stability was obtained from all patients except for one ASD patient described as verbal (Table 2). Similarly, NSUCO saccades and NSUCO pursuits were obtained for all except one nonverbal ASD patient. As with tests of binocular vision, testability was not related to communication level.
DISCUSSION
The aim of this study was to design an eye examination protocol that would be successful for patients with ASD and to compare the testability of vision tests and eye tests using this protocol for patients with ASD to that of TD peers. Autism spectrum disorder patients have multiple challenges in social interaction, communication, making transitions, and sensory processing. When vision testing was integrated with communication and sensory processing supports and behavioral strategies, the proportion of ASD patients able to complete vision testing was generally high.
The results of this study were compared with those reported in other prospective studies of vision assessment in ASD patients (Table 4). Using the enhanced protocol designed for this study, the proportion of ASD patients able to complete testing generally exceeded that reported by other investigators. This difference is particularly true for stereoacuity, oculomotor testing, and near fusional vergence testing. The testability of visual acuity testing in this study is also higher than Ikeda et al.’s report34 by retrospective record where only 40% of ASD patients completed recognition visual acuity. Testability is an important aspect of clinical and research data collection. Future clinical investigators may consider the supports and strategies used and the interdisciplinary collaboration of the study team in creating research protocols.
TABLE 4.
Comparison of testability in prospective studies of children and/or adolescents with ASD
Testing a patient’s vision and eye health cannot be performed without considering a patient’s development, abilities, and challenges in other areas. Understanding the patient’s ability to communicate, attend to, and respond to testing is important in selecting and administering tests in an eye examination. If the purpose of the vision testing is to gain information from patients with ASD, testing needs to be tailored to these patients’ differences and then evaluated for its usefulness. Testing that excludes significant numbers of patients of this population or subgroups within it, because they cannot complete the tests, yields data that are incomplete and skewed and may not accurately reflect the patient population’s visual status. Before investigators or clinicians can determine the frequency and nature of vision problems found in patients with ASD, they need to consider how these patients’ challenges in communication, sensory processing, and motor planning impact the testing being administered.
Using strategies and protocols that target the developmental level of a specific population is not new to vision testing. Infant vision testing became more productive when Teller35 developed infant vision testing by forced-choice preferential looking and grating patterns using the observation that TD infants preferred to look at patterned stimuli over homogenous gray stimuli. Fulton et al.36 demonstrated that as testability of infants increased with the use of Forced Preferential Looking, the identification of patients who had decreased vision as a result of strabismus or cataracts improved. By considering ASD patients’ developmental level and abilities, investigators will be better able to select tests, design eye examination protocols, and diagnose and manage patients with vision and eye health problems.
Good clinicians may intuitively use some of the strategies identified such as selecting tests to avoid tactile defensiveness, shaping a desired response, following a high probability request with a low one, presenting desired behaviors framed as choices, or using distraction techniques to increase tolerance to unpleasant stimuli. Other tools, such as the use of a visual schedule, social story, or the yes/no application, may be relatively unknown. In either case, working toward a more systematic approach to examining ASD patients may be helpful for those investigating aspects of vision or those who provide comprehensive vision and eye care. Understanding why certain approaches work may also be useful in the training of current and future eye care providers. Our results show that these strategies and tools are not difficult for clinicians to implement.
Incorporation of these strategies and tools offers advantages to patients as well. By increasing patients’ understanding of the testing process and how they can participate in it, clinicians and investigators can decrease patient and parent anxiety and lay the groundwork for future visits. This may ultimately increase their access to quality vision and eye care.
Which Test Worked and for Whom
Testability for vision and eye tests varied greatly for ASD patients by the level of verbal communication reported by the parents. Although the parent-reported verbal communication level is not a standardized measure of language, it quickly provides needed information to the eye care provider. All the ASD and TD patients were able to do binocular distance visual acuity testing; however, the tests that the patients responded to differed by ASD subgroup (p = 0.0021). For ASD patients who were described as verbal, testability results were very similar to the TD population. In testing visual acuity, almost all patients who were fluent were able to complete the Snellen acuity chart. In comparison, only half of the patients who used short words or who were nonverbal were able complete the Snellen acuity chart. In testing stereoacuity, most patients who were fluent or had some words could complete the Random Dot 2 test. Nonverbal patients were generally able to complete a stereotest, if it was presented in a forced-choice presentation or did not require wearing Polaroid goggles. In this study, testability varied most for tonometry. Tonometry was particularly challenging for nonverbal patients. As these patients mature into adulthood and their risk for glaucoma increases, there will be a need for alternative ways to measure IOP or other types of testing and/or protocols that can detect early glaucomatous changes.
Few studies have reported the findings of their ASD patients by functional subgroups. Milne et al.8 reported the vision screening results by subgroups based on IQ testing results. High-functioning subjects were defined as those who had IQ test results higher than or equal to 100, whereas LF patients were those with IQ lower than or equal to 100 or whose IQ was untestable. In this study, testability for HF subjects was generally high: visual acuity, 97%; stereoacuity, 86%; prism fusional vergence, 66%; and NPC, 100%. Testability for LF subjects was marked lower: visual acuity, 60%; stereoacuity, 40%; prism fusional vergence, 27%; and NPC, 80%.
Our ASD patient subgroups were based on parent report of verbal communication levels and not measured IQ test results. This required the parent to make a judgment about his or her child’s ability. Although this determination is a practical and easy method for eye care providers, it is not based on a standardized questionnaire or clinical assessment.
Our testability results, particularly those from less verbal patients, compare favorably with those reported by Milne et al.8 We suggest that consideration of test selection, examination strategies, and supports are particularly important for ASD patients who are less verbal or less able to respond to conventional testing techniques, as their findings may be most likely to be eliminated from scientific study otherwise.
Eye care providers need to determine guidelines for eye examination testing for patients with ASD protocols. In future studies and investigations, testability of the vision and eye tests used needs to be considered. Vision testing, screening, and eye examination studies that eliminate up to 30 to 60% of the patients with ASD tested do not accurately reflect visual status or functional abilities of the ASD population.
It is also important to acknowledge the diversity of the ASD population. The wide range of abilities found in this population may mean that multiple protocols are needed to accommodate patients of differing abilities. Current investigation of the ASD population aims to identify phenotypes of patients who are more similar. In the meantime, clinicians need clinical classifications that communicate a patient’s profile quickly and do not require access to additional testing or documentation. Parental report of verbal communication may be a reasonable starting point as long as clinicians understand the limitations of this designation.
Limitations
It is important to note the limitations of this study when considering its application to clinical practice. The study sample of patients with ASD reflected an atypical sex distribution. The ratio of male to female typically found in the ASD population is 4:1; in this study, it was 10:7. This may reflect a bias on the part of the parents who chose to enroll their child and to participate. Sex was not related to the testability of any of our eye examination procedures (p values > 0.05; data not shown).
Another study limitation is the older age range of patients who were 9 to 17 years at the time of testing. Patients with ASD whose parents reported their verbal communication level as verbal showed vision and eye test completion at rates similar to those of TD patients. Patients with ASD who are younger than 9 years and who have not yet completed years of early intervention, speech, and occupational therapy or educational programs may show lower testability rates on vision and eye tests. Finally, our study administered cycloplegic and mydriatic agents in a spray administration. It is possible that eye drop administration of these same agents to obtain a more controlled cycloplegia might negatively impact testability for ocular health examination and cycloplegic refraction.
CONCLUSIONS
In summary, the results of this study are as follows:
Most patients with ASD including those who are nonverbal can complete most vision tests within an eye examination using a protocol that incorporates visual, communication, and sensory supports.
It is not that difficult to incorporate the appropriate protocols to allow for successful testing of patients with ASD. Most can be implemented with relatively little additional time or resources by the examiner.
Testability of near binocular visual acuity and IOPs varies for ASD patients by the level of verbal communication reported by the parent.
Testability of IOPs is reduced, particularly for nonverbal patients and patients who used short words. Future research is needed to refine examination procedures and investigate treatment implementation in this patient population.
Supplementary Material
Rachel Anastasia Coulter
College of Optometry
Nova Southeastern University
3200 S University Dr
Fort Lauderdale, FL 33328
e-mail: staceyco@nova.edu
ACKNOWLEDGMENTS
We are grateful to all participants and their parents and caregivers. We thank Michael Alessandri, PhD, and the NSU-CARD staff, Broward County Public Schools, Westlake Academy, CasaBlanca Academy, and Tania Diaz-Fernandez, MOT, for their assistance in patient recruitment. We thank Darryl M. Horn, PhD, for comments that greatly improved this article. This work was supported by a Nova Southeastern University Chancellor’s Faculty Research & Development Grant and a Nova Southeastern University Health Professions Division Grant. This work has been presented in part at the American Academy of Optometry (Phoenix, AZ, 2012) and the International Meeting for Autism Research (San Sebastian, Spain, 2013).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.optvissci.com).
REFERENCES
- 1.Autism Spectrum Disorders: Data and statistics. Atlanta: Division of Birth Defects, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 2014. Available at: http://www.cdc.gov/ncbddd/autism/data.html Accessed March 27, 2014.
- 2. Trager MJ, Dirani M, Fan Q, Gazzard G, Selvaraj P, Chia A, Wong TY, Young TL, Varma R, Saw SM. Testability of vision and refraction in preschoolers: the strabismus, amblyopia, and refractive error study in Singaporean children. Am J Ophthalmol 2009; 148: 235– 41 e6. [DOI] [PubMed] [Google Scholar]
- 3. Kulp MT, Dobson V, Peskin E, Quinn G, Schmidt P. The electronic visual acuity tester: testability in preschool children. The Vision in Preschoolers (VIP) Study Group. Optom Vis Sci 2004; 81: 238– 44. [DOI] [PubMed] [Google Scholar]
- 4. Cotter SA, Tarczy-Hornoch K, Wang Y, Azen SP, Dilauro A, Borchert M, Varma R. Visual acuity testability in African-American and Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Am J Ophthalmol 2007; 144: 663– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt PP, Maguire MG, Moore B, Cyert L. Testability of preschoolers on stereotests used to screen vision disorders. Vision in Preschoolers Study Group. Optom Vis Sci 2003; 80: 753– 7. [DOI] [PubMed] [Google Scholar]
- 6. Tarczy-Hornoch K, Lin J, Deneen J, Cotter SA, Azen SP, Borchert MS, Wang Y, Varma R. Stereoacuity testability in African-American and Hispanic pre-school children. Optom Vis Sci 2008; 85: 158– 63. [DOI] [PubMed] [Google Scholar]
- 7. Pai AS, Rose KA, Samarawickrama C, Fotedar R, Burlutsky G, Varma R, Mitchell P. Testability of refraction, stereopsis, and other ocular measures in preschool children: the Sydney Paediatric Eye Disease Study. J AAPOS 2012; 16: 185– 92. [DOI] [PubMed] [Google Scholar]
- 8. Milne E, Griffiths H, Buckley D, Scope A. Vision in children and adolescents with autistic spectrum disorder: evidence for reduced convergence. J Autism Dev Disord 2009; 39: 965– 75. [DOI] [PubMed] [Google Scholar]
- 9. Scharre JE, Creedon MP. Assessment of visual function in autistic children. Optom Vis Sci 1992; 69: 433– 9. [DOI] [PubMed] [Google Scholar]
- 10. Sterling-Turner HE, Jordan SS. Interventions addressing transition difficulties for individuals with autism. Psychol Schools 2007; 44: 681– 90. [Google Scholar]
- 11. Cafiero JM, Meyer A. Your child with autism: when is augmentative and alternative communication (AAC) an appropriate option? Except Parent 2008; 38: 28– 30. [Google Scholar]
- 12. Sughrue K. (Producer). Apps for Autism. 60 Minutes [television broadcast]. Washington, DC: CBS News; Oct. 23, 2011. [Google Scholar]
- 13.Answers:YesNo [iOS app]. Simplified Touch; 2011.
- 14. Cohen M, Sloan DL. Visual Supports for People with Autism: A Guide for Parents and Professionals. Bethesda, MD: Woodbine House; 2007. [Google Scholar]
- 15. Ingersoll B, Dvortcsak A. Teaching Social Communication to Children with Autism. New York: The Guilford Press; 2010. [Google Scholar]
- 16. Gray C. The New Social Story Book: Illustrated Edition, 2nd ed Arlington, TX: Future Horizons Inc.; 2000. [Google Scholar]
- 17. Test DW, Richter S, Knight V, Spooner F. A comprehensive review and meta-analysis of the social stories literature. Focus Autism Other Dev Disabl 2011; 26: 49– 62. [Google Scholar]
- 18. Briody J, McGarry K. Using social stories to ease children’s transitions. Young Children 2005; 60: 38– 42. [Google Scholar]
- 19. Backman B, Pilebro C. Augmentative communication in dental treatment of a nine-year-old boy with Asperger syndrome. ASDC J Dent Child 1999; 66: 419– 20. [PubMed] [Google Scholar]
- 20. Daly DA, Gill JM, Cullinane CG, Hourihane JO. Using social stories to prepare children with autism for allergy testing. J Allergy Clin Immunol 2010; 125: AB51. [Google Scholar]
- 21.Visiting Nate and Franc. Chapel Hill, NC: University of North Carolina School of Medicine, Division of Occupational Science and Occupational Therapy, 2014. Available at: http://www.med.unc.edu/ahs/ocsci/sep/sep-image-and-files/EEGSocialStory.pdf Accessed May 27, 2014.
- 22.ATN/AIR-P Blood Draw Tool Kit. Nashville, TN: Autism Speaks Autism Treatment Network, 2011. Available at: http://www.autismspeaks.org/science/resources-programs/autism-treatment-network/tools-you-can-use/blood-draw-toolkits Accessed May 27, 2014.
- 23. Scattone D, Wilczynski SM, Edwards RP, Rabian B. Decreasing disruptive behaviors of children with autism using social stories. J Autism Dev Disord 2002; 32: 535– 43. [DOI] [PubMed] [Google Scholar]
- 24. Banda DR, Grimmett E, Hart SL. Activity schedules: helping students with autism spectrum disorders in general education classes manage transition issues. Teach Except Child 2009; 41: 16– 21. [Google Scholar]
- 25. Quill KA. Instructional considerations for young children with autism: the rationale for visually cued instruction. J Autism Dev Disord 1997; 27: 697– 714. [DOI] [PubMed] [Google Scholar]
- 26. Myles BS, Grossman BG, Aspy R, Henry SA, Coffin AB. Planning a comprehensive program for students with autism spectrum disorders using evidence-based practices. Educ Train Dev Disab 2007; 42: 398– 409. [Google Scholar]
- 27. Schall CM, McDonough JT. Autism spectrum disorders in adolescence and early adulthood: characteristics and issues. J Vocat Rehab 2010; 32: 81– 8. [Google Scholar]
- 28.First Then Visual Schedule [app]. Good Karma Applications; 2011.
- 29. Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res 2009; 49: 2705– 39. [DOI] [PubMed] [Google Scholar]
- 30. Robertson AE, Simmons DR. The relationship between sensory sensitivity and autistic traits in the general population. J Autism Dev Disord 2013; 43: 775– 84. [DOI] [PubMed] [Google Scholar]
- 31. Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry 1999; 175: 444– 51. [DOI] [PubMed] [Google Scholar]
- 32. Bartlett JD, Jaanus SD. (Eds). Clinical Ocular Pharmacology, 5th ed St. Louis, MO: Butterworth-Heinemann Elsevier; 2008. [Google Scholar]
- 33. Wong CY, Fan DS, Yu CB, Lam DS. Topical mydriatic and cycloplegic spray for Chinese children. J Pediatr Ophthalmol Strabismus 2003; 40: 349– 52. [DOI] [PubMed] [Google Scholar]
- 34. Ikeda J, Davitt BV, Ultmann M, Maxim R, Cruz OA. Brief report: incidence of ophthalmologic disorders in children with autism. J Autism Dev Disord 2013; 43: 1447– 51. [DOI] [PubMed] [Google Scholar]
- 35. Teller DY. The forced-choice preferential looking procedure: a psychophysical technique for use with human infants. Infant Behav Dev 1979; 2: 135– 53. [Google Scholar]
- 36. Fulton AB, Manning KA, Dobson V. Infant vision testing by a behavioral method. Ophthalmology 1979; 86: 431– 9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


