Abstract
Objective
This study aims to assess the endometrial safety of ospemifene based on phase 2/3 clinical trials of postmenopausal women with up to 52 weeks of exposure to ospemifene 60 mg/day versus placebo.
Methods
Endometrial safety was evaluated in a development program of six randomized, double-blind, placebo-controlled, parallel-group studies of postmenopausal women aged between 40 and 80 years who had vulvar and vaginal atrophy. Participants were randomized 1:1 to ospemifene 60 mg/day or placebo in one 6-week trial and three 12-week trials; one of the 12-week trials had a 40-week extension study. In a separate 52-week trial, women were randomized 6:1 to ospemifene 60 mg/day or placebo. Endometrial safety was assessed by endometrial histology (biopsy), transvaginal ultrasound, and gynecologic examination.
Results
In these trials, 1,242 women who received ospemifene 60 mg/day and 924 women who received placebo were evaluable for safety. Endometrial hyperplasia occurred in less than 1% of women treated with ospemifene; no endometrial cancer was reported. The mean (SD) increase in endometrial thickness among women treated with ospemifene was 0.51 (1.54) mm at 12 weeks, 0.56 (1.61) mm at 6 months, and 0.81 (1.54) mm at 12 months. Women who received placebo had a mean (SD) increase of 0.07 (1.23) mm at 12 months.
Conclusions
These clinical trial data indicate that up to 52 weeks of treatment with oral ospemifene 60 mg/day was safe for the endometrium. There was no increase in the incidence of endometrial cancer or hyperplasia among postmenopausal women treated with ospemifene compared with placebo.
Key Words: Endometrium, Endometrial safety, Estrogen receptor agonist/antagonist, Lubricant, Ospemifene, Vaginal examination, Vulvar and vaginal atrophy
Ospemifene, an estrogen receptor agonist/antagonist with tissue-selective effects that is sometimes referred to as a selective estrogen receptor modulator (SERM), was recently approved for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA) due to menopause.1 Although SERMs lack the steroidal structure of estrogens, certain SERMs have been reported to elicit tissue-specific responses, such as positive effects on the vaginal epithelium, prevention of breast cancer, and treatment and prevention of osteoporosis with an acceptable benefit/risk profile.2 However, SERMs have been reported to have a spectrum of effects on the endometrium. Tamoxifen, a first-generation SERM, increases the risk of endometrial cancer; this finding is not associated with raloxifene, a second-generation SERM.3,4 Clinical development of other SERMs, such as levormeloxifene and idoxifene, was discontinued because of unacceptable safety profiles, where adverse effects on gynecologic tissues and elsewhere outweighed the benefits. Thus, gynecologic evaluation of a SERM is an essential component for establishing its overall benefit/risk profile in postmenopausal women.5 Ospemifene has been shown in preclinical and clinical studies to exert positive effects on the vaginal epithelium and minimal effects on the endometrium.6-8
The only other approved prescription medications for the treatment of VVA include systemic estrogens, estrogen plus progestogen, and local vaginal estrogens.1 The endometrial effects of unopposed systemic estrogens have long been known.9 The Postmenopausal Estrogen/Progestin Interventions trial found that, after 12 months, 25 of 119 women (21.0%) receiving conjugated equine estrogens (CEE) alone had endometrial hyperplasia, 12 women (10.1%) had complex hyperplasia, and 3 women (2.5%) had hyperplasia with atypia. Three women treated with CEE/medroxyprogesterone acetate (MPA) were reported to have simple hyperplasia (n = 2) or complex hyperplasia (n = 1) at 12 months; there were no reports of hyperplasia with atypia.10
Progestin has been added to oral estrogens to protect against endometrial proliferation. Endometrial outcomes in the Women’s Health, Osteoporosis, Progestin, Estrogen trial were reported for women receiving CEE alone (0.3-0.625mg/d) or a CEE/MPA combination (0.3/1.5 to 0.625/2.5 mg/d). Thirty-two of 2,153 women (1.5%) predominantly in the CEE-alone 0.45- and 0.625-mg treatment groups, developed endometrial hyperplasia by the 12-month evaluation. The incidence of hyperplasia was low (≤0.4%) in all CEE/MPA groups. One case of endometrial hyperplasia was identified in each of the CEE 0.3 mg/MPA 1.5 mg and CEE 0.45 mg/MPA 1.5 mg groups. Groups treated with CEE/MPA had a significantly lower (P ≤ 0.05) incidence of endometrial hyperplasia than the groups treated with corresponding doses of CEE alone, with the exception of the lowest dose (CEE 0.3 mg/MPA 1.5 mg and CEE 0.3 mg), where there was one case of hyperplasia in each group.11
Vaginally administered estrogens have been shown to be effective and well-tolerated for the treatment of VVA. Symptom relief is achieved with low doses of estrogen; however, systemic effects have been reported.12 In a recent study of 10-μg estradiol vaginal tablets,13 one case of endometrial hyperplasia (without atypia) and one case of endometrioid carcinoma were reported among women who were treated for up to 52 weeks, resulting in an incidence rate of 0.52% among 386 women who were reported to have undergone an endometrial biopsy. The mean endometrial thickness was not reported to have increased with treatment in this study.
The endometrial safety of conjugated estrogens vaginal cream 0.3 mg applied once daily or twice weekly was evaluated in 155 participants with endometrial biopsies and revealed no endometrial hyperplasia or carcinoma. Transvaginal ultrasound (TVUS) results obtained at week 52 or at early termination showed endometrial thickness of at least 5 mm in approximately 10% of participants.14
The Food and Drug Administration (FDA) draft guidance for vasomotor symptoms and VVA clinical trials recommends evaluating the incidence rate of endometrial hyperplasia at 12 months: “We recommend that the results from the clinical trial demonstrate a hyperplasia rate that is ≤1% with an upper bound of the one-sided 95 percent confidence interval for that rate that does not exceed 4 percent. The frequency of atypical hyperplasia and cancer are important additional factors to be considered in determining approvability of the drug product.” The FDA guidance also advises that “the incidence of hyperplastic polyps and associated atypia would be considered in the safety review.”15
This report will present and discuss the gynecologic effects of a recently approved nonestrogen oral product, ospemifene 60 mg/day.
METHODS
The phase 2/3 randomized, double-blind, placebo-controlled, parallel-group studies (one 6-wk study, two 12-wk studies, one 12-wk study with a 40-wk extension study, and one 52-wk safety study) compared ospemifene 60 mg/day and placebo in the treatment of postmenopausal women.16-20 Participants were aged 40 to 80 years. Baseline criteria for VVA included 5% or less superficial cells on vaginal smear (maturation index), vaginal pH higher than 5.0, and at least one moderate or severe symptom of VVA. One 12-week study (N = 79), the 40-week long-term extension study (N = 118), and the 52-week long-term safety study (N = 426) required participants to have an intact uterus. The 40-week extension study required women to remain on the same therapy they were randomized to receive during the 12-week study. Only women who completed the 12-week study could qualify for the 40-week extension study. The three 12-week studies and the 6-week study randomized participants 1:1 to receive ospemifene or matching placebo, whereas in the 52-week long-term safety study, women were randomized 6:1 to receive ospemifene or matching placebo. Ospemifene (or matching placebo) was taken orally each morning with food.
Women were excluded if they had abnormal endometrial histology other than atrophy based on baseline biopsy, uterine bleeding of unknown origin, clinically significant abnormal gynecologic findings, endometrial thickness of 4 mm or more on centrally read TVUS, pathologic findings on endometrial biopsy or Papanicolaou test, or clinically significant findings on physical examination. Participants were not permitted to take other hormonal products, including progestins, during the course of the investigation.
Endometrial thickness was measured on TVUS at baseline (screening/visit 1); on weeks 12, 26, and 52; or at the end of therapy. For consistency of data, TVUS images and videos were read at a central laboratory. Endometrial biopsy data were only reported from studies of 12 weeks’ duration or longer. Endometrial biopsies in the 52-week trial were obtained at baseline and on week 52, and those in the 12-week studies were obtained at baseline and on week 12. Endometrial evaluation of biopsy-obtained tissues was performed on weeks 12 and 26 if TVUS assessment demonstrated an endometrial thickness of 4 mm or more. Endometrial biopsy samples were collected using a suction curette and analyzed by two independent pathologists in a central laboratory. Pathologists were blinded to study treatment and to each other’s readings of the histology slides. If there was disagreement over the endometrial histology, a third pathologist evaluated the samples. The final diagnosis was determined by concurrence between two of the three independent pathologists; if there was no agreement among the three pathologists, the most severe histopathologic diagnosis was reported. Histology was summarized as number (percentage) in the following categories: no tissue, tissue insufficient for diagnosis, atrophic, inactive, proliferative (weakly proliferative, actively proliferative, disordered proliferative), secretory pattern (cyclic type, progestational type including stromal decidualization), menstrual type, simple hyperplasia without atypia, simple hyperplasia with atypia, complex hyperplasia without atypia, complex hyperplasia with atypia, and carcinoma. Blaustein’s criteria were used to classify endometrial hyperplasia.21
Endometrial histopathologic characterization of endometrial polyps was performed after identification with TVUS based on regulatory guidance. Investigative sites were asked to locally confirm the polyps identified on TVUS because views of the suspected polyps identified on TVUS that were sent for central reading were limited compared with real-time dynamic views available locally. If the local site confirmed the central reader’s finding of a polyp, the participant was discontinued from the study, and hysteroscopy was performed to obtain tissue for histologic diagnosis. The tissue—when available and believed to represent a uterine polyp—was then sent for external expert review.
All studies were carried out in accordance with the Declaration of Helsinki (2000) and current Good Clinical Practice outlined in the International Conference on Harmonisation for Good Clinical Practice (E6) and in compliance with local regulatory requirements. Before study initiation, all participants provided written informed consent forms using forms approved by the independent ethics committee. The protocols, amendments, and informed consent forms were reviewed and approved by the independent ethics committee before study initiation.
Statistical methods
We reported safety data on the intent-to-treat population, which included all participants who had taken at least one dose of the study drug. Treatment-emergent adverse events (TEAEs) were tabulated by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA, version 10), causality, and severity of event (mild, moderate, or severe). Baseline and time point assessments for clinical chemistry and laboratory safety variables were summarized by descriptive statistics. All statistical tests were two-sided tests; P < 0.05 was considered statistically significant. One-sided 95% CI (upper limit) was calculated to assess serious endometrial outcomes (endometrial hyperplasia, cancer, or both). McNemar’s test for correlated proportions was used to examine changes in endometrial thickness at two different time points. Changes in endometrial thickness were converted from continuous data into a binary categorical variable using the following definitions: “increase” was defined as a change of more than 1 mm, whereas “unchanged” or “decrease” was defined as a change of less than 1 mm. Differences in categorical variables between ospemifene and placebo were tested using the Fisher exact test. Incidence rates and 95% CIs were based on the Poisson distribution, with the comparison of incidence rates based on the maximal likelihood ratio test. Hazard ratios were also computed. For certain parameters, data from both the 12-week study and its 40-week extension study were used. For example, for women in both the 12-week study and its corresponding 40-week study, duration of therapy was the combined sum of therapy (range, 12-52 wk) and completion of therapy was determined by the 40-week extension, as all women in the 40-week extension were required to complete the 12-week study. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Vaginal bleeding
Participants with any of the following terms were included in the statistical analysis for vaginal bleeding: coital bleeding, postmenopausal hemorrhage, genital hemorrhage, vaginal hemorrhage, uterine hemorrhage, metrorrhagia, dysfunctional uterine bleeding, bleeding anovulatory, menorrhagia, and polymenorrhagia. Cases having a higher-level group term of “menstrual cycle and uterine bleeding disorders” were also included.
For all adverse events (AEs), if a woman had more than one TEAE that coded to the same preferred term, the woman was counted only once for that preferred term. Preferred terms are sorted in descending frequency in the ospemifene group. Post–endometrial biopsy bleeding/spotting cases (with the preferred term “postprocedural hemorrhage”) were not included.
RESULTS
A total of 2,166 women were randomized to ospemifene 60 mg/day or placebo in the phase 2/3 double-blind, placebo-controlled, clinical trials: 1,242 women were randomized to the ospemifene 60 mg/day treatment group, and 924 women were randomized to the placebo group. Of the 1,394 randomized women with an intact uterus, 851 received ospemifene and 543 received placebo. One 6-week study, two 12-week studies, one 12-week study with a 40-week extension study, and one 52-week study with a specific focus on endometrial safety were included in this analysis (Fig.). All participants were required to remain on the randomized treatment throughout each of the studies, including the 40-week extension study; no participants were rerandomized.
FIG.
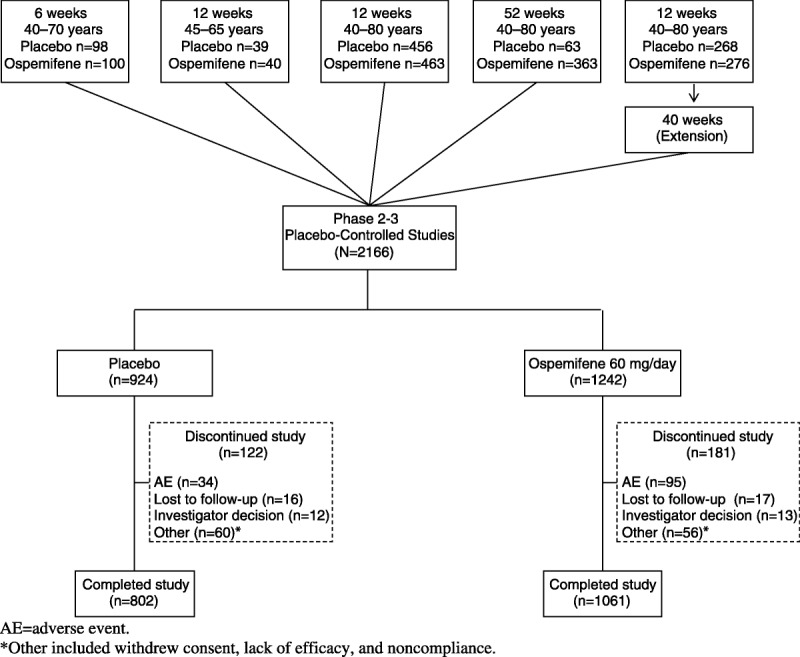
Disposition diagram of phase 2/3 double-blind, placebo-controlled studies included in this analysis: all participants.
The participant’s demographics—age, race, and body mass index (BMI)—at baseline were comparable among women with or without an intact uterus in the ospemifene and placebo treatment groups (Table 1). The mean age at entry for ospemifene versus placebo was 59.4 versus 58.9 years; 93.3% versus 90.6% of participants were white; and the mean (SD) BMI was 25.7 (4.0) versus 26.0 (4.2) kg/m2 among the women enrolled (Table 1). In three of the studies, participants were required to have an intact uterus; in one of these studies, there were approximately six times more actively treated participants than placebo participants because of the prespecified randomized allocation ratio, with a mean (SD) treatment duration of 199 (144) days for ospemifene 60 mg/day versus 124 (110) days for placebo (Table 2). Thus, in the overall analysis population, more women treated with ospemifene had an intact uterus (851 of 1,242 [68.5%]) than women treated with placebo (543 of 924 [58.8%]). There were baseline differences in weight and BMI in participants with an intact uterus versus participants without an intact uterus, regardless of treatment group randomization (two-way analysis of variance, P < 0.0001; Table 1).
TABLE 1.
Demographic characteristics in phase 2/3 double-blind, placebo-controlled studies: total population and participants with an intact uterus
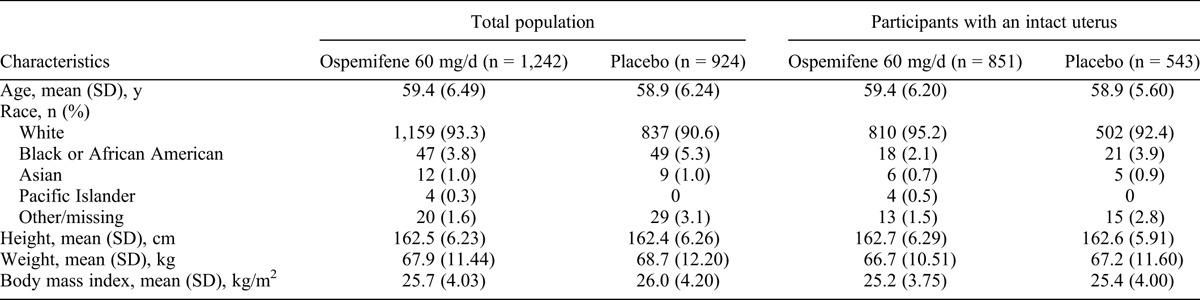
TABLE 2.
Disposition in phase 2/3 double-blind, placebo-controlled studies: participants with an intact uterus
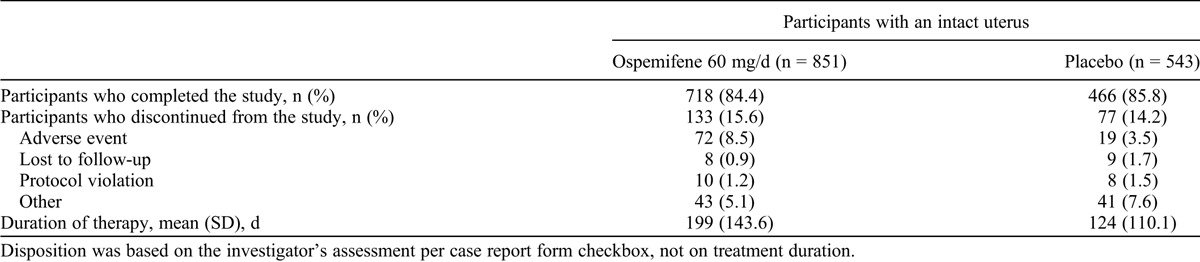
The percentage of participants who were considered study completers was similar for the ospemifene 60 mg/day (1,061 of 1,242 [85.4%]) and placebo (802 of 924 [86.8%]) groups. The most common reason for discontinuation in the ospemifene group was AE; 95 (7.6%) of 1,242 participants in the ospemifene 60 mg/day group discontinued because of AEs. In the ospemifene 60 mg/day group, the AEs that most frequently led to study discontinuation were hot flushes (13 of 1,242 [1.0%]), muscle spasms (7 of 1,242 [0.6%]), headache (6 of 1,242 [0.5%]), and vaginal discharge (6 of 1,242 [0.5%]). In the placebo group, hot flushes (3 of 924 [0.3%]) and diarrhea (3 of 924 [0.3%]) were the most common AEs leading to discontinuation. The most common reason for discontinuation in the placebo group was “other” (60 of 924 [6.5%]). “Other” included reasons such as withdrawal of consent, lack of efficacy, and noncompliance. In the ospemifene group, 718 of 851 participants (84.4%) with an intact uterus were study completers; in the placebo group, 466 of 543 participants (85.8%) with an intact uterus were study completers (Table 2).
Vaginal bleeding
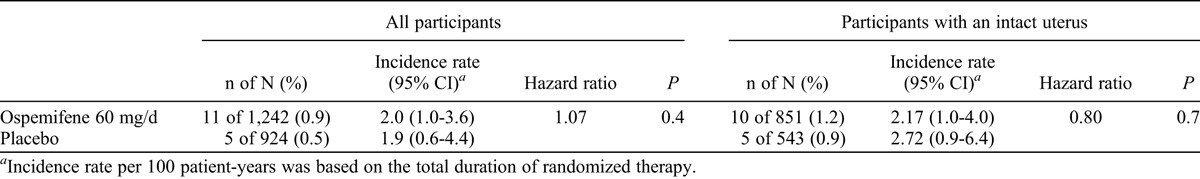
Vaginal bleeding or spotting was reported in 10 of 851 women (1.2%) with an intact uterus in the ospemifene 60 mg/day group (incidence rate, 2.17 per 100 patient-years) and in 5 of 543 women (0.9%) in the placebo group (incidence rate, 2.72 per 100 patient-years; P = 0.7; Table 3). One of 391 women who had had a hysterectomy in the ospemifene 60 mg/day group experienced a TEAE related to vaginal spotting; this event occurred on day 56 of treatment and was not associated with vaginal lesions on visual inspection on day 79. None of the vaginal bleeding or spotting TEAEs led to discontinuation.
TABLE 3.
Incidence rates of vaginal bleeding for ospemifene 60 mg/day versus placebo
Endometrial thickness
The mean (SD) increase in endometrial thickness based on TVUS was 0.51 (1.5) versus 0.06 (1.2) mm at 12 weeks, 0.56 (1.6) versus 0.05 (1.3) mm at 6 months, and 0.81 (1.5) versus 0.07 (1.2) mm at 12 months for participants in the ospemifene and placebo groups, respectively. The differences between participants receiving ospemifene and participants receiving placebo were statistically significant (P ≤ 0.001, Welch’s test) at all three evaluations.
We assessed for a possible association between endometrial thickness and vaginal bleeding, which demonstrated no statistically significant correlation (ospemifene 60 mg/day: Spearman ρ = 0.0625, P = 0.2527; placebo: Spearman ρ = 0.3152, P = 0.0847; Table 4). One woman with vaginal bleeding and endometrial thickness greater than 10 mm had a diagnosis of endometrial polyp with simple hyperplasia without atypia.
TABLE 4.
Endometrial thickness (by TVUS) at the final visit and vaginal bleeding among participants with an intact uterus
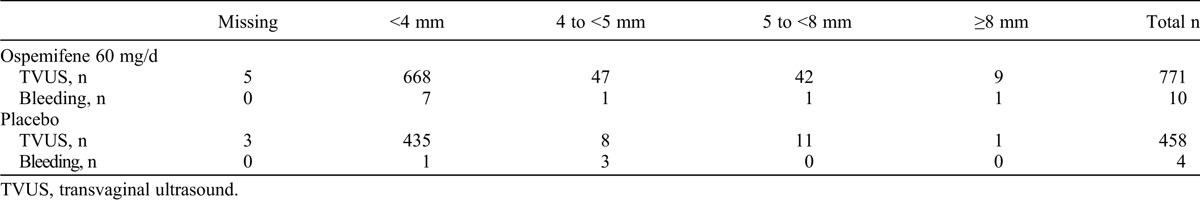
An endometrial thickening of 5 mm or more during a participant’s last visit demonstrated no statistically significant increase in incidence rate (hazard ratio, 1.58; P = 0.2; Table 5). The mean (95% CI) incidence rate per 100 patient-years for an endometrial thickening of 5 mm or more, based on the total duration of randomized therapy, was 11.3 (8.4-14.8) for ospemifene 60/day mg versus 7.1 (3.7-12.5) for placebo.
TABLE 5.
Endometrial thickness in participants with an intact uterus
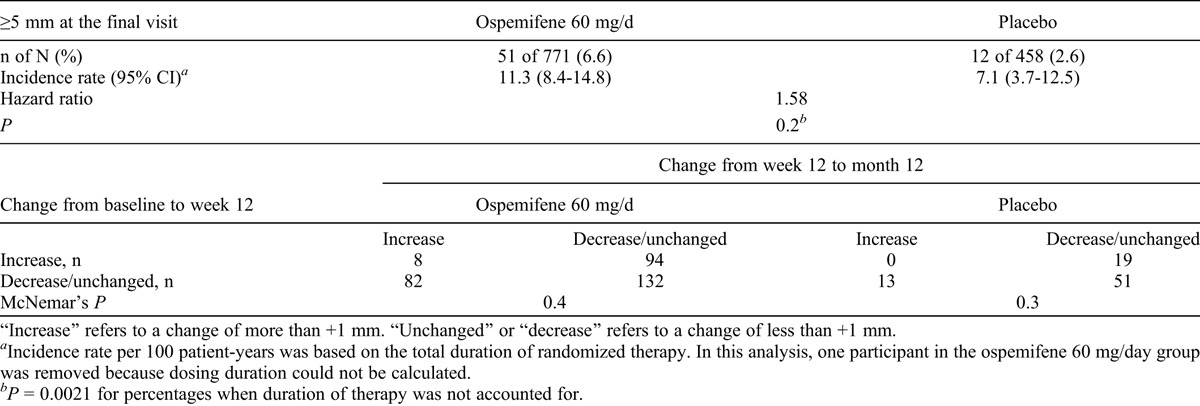
The consistency of small changes in endometrial thickness (≤1 mm) was evaluated. Changes in each participant’s endometrial thickness from baseline to week 12 and from week 12 to month 12 were examined among participants receiving ospemifene and placebo to determine whether there was any significant difference in the two correlated proportions. Endometrial thickness was evaluated with TVUS in two studies in which women had endometrial thickness evaluation at baseline, at week 12, and at month 12. Using McNemar’s test for correlated proportions, we compared endometrial ultrasounds with increases or decreases at week 12 with endometrial ultrasounds with increases/decreases at month 12. Table 5 summarizes the findings for participants in the ospemifene and placebo groups. In both the ospemifene and placebo groups, there was no statistically significant difference in the proportion of participants whose endometrial thickness increased between the two periods (from baseline to week 12 and from week 12 to month 12); there was also no significant difference between the proportion of participants whose endometrial thickness changed direction (increased in the first period, then decreased in the second period) and the proportion of participants whose endometrial thickness changed in the reverse direction (first decreased, then increased). There was no statistically significant difference in the correlated proportions for the ospemifene group (94 of 176 [53.4%] vs 82 of 176 [46.6%]) or for the placebo group (19 of 32 [59.4%] vs 13 of 32 [40.6%]). Thus, the chance of having a small increase or a decrease in endometrial thickness on week 12 or week 52 was randomly distributed.
Uterine polyps
Among women with an intact uterus, 5 of 851 participants (0.6%) in the ospemifene 60 mg/day group and 1 of 543 participants (0.2%) in the placebo group experienced a uterine polyp–related TEAE. Post hoc assessment revealed that the histologic diagnosis of polyp in the absence of a visible polyp on TVUS could be associated with the shape of the suction curette, which created the impression that the biopsy sample was a polyp even in the absence of pathology. Among the six women with a report of polyp, one woman (in the ospemifene 60 mg/d group) experienced vaginal bleeding and had an endometrial thickness greater than 10 mm on TVUS.
Endometrial histology
There were no cases of endometrial cancer observed with exposure of up to 52 weeks in the ospemifene clinical trials. One woman had an endometrial biopsy consistent with simple hyperplasia without atypia and a polyp that resolved (Table 6). This woman presented with vaginal bleeding, which led to the diagnostic biopsy 3 months after therapy with ospemifene. This one case of simple hyperplasia (of 342 biopsies) at 12 months in a participant taking ospemifene 60 mg/day met the FDA criterion of 1% or less incidence.
TABLE 6.
Summary of endometrial biopsy findings: ospemifene 60 mg/day versus placebo
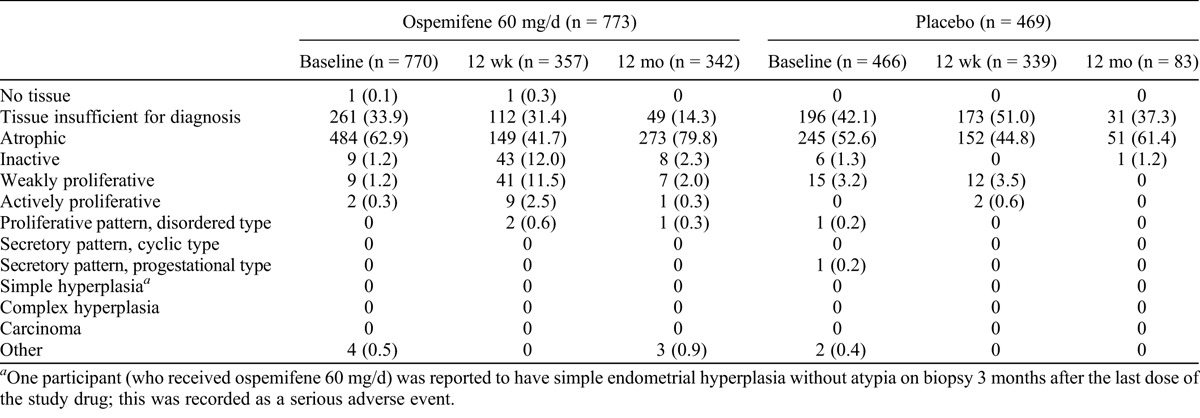
At baseline, most endometrial samples had a histologic interpretation of no tissue, tissue insufficient for diagnosis, or atrophic or inactive endometrium (ospemifene, 98.1%; placebo, 95.9%). In the ospemifene 60 mg/day treatment group, 1.2% of women had weakly proliferative endometrial histology and 0.3% of women had actively proliferative endometrial histology. In the placebo group, 3.2% of endometrial tissue was reported as weakly proliferative, 0.2% of endometrial tissue was reported to be of proliferative pattern (disordered type), and 0.2% of endometrial tissue was reported to be of secretory pattern (progestational type; Table 6). Of the nine ospemifene participants with reports of weakly proliferative endometrium at baseline, two women had weakly proliferative endometrium present at 12 weeks, three participants did not have a follow-up biopsy, and the other four participants had follow-up findings of an atrophic or inactive endometrium. None of these participants had vaginal bleeding or spotting.
Endometrial biopsies were performed on follow-up in women who participated in the clinical trials for 12 months and received ospemifene (n = 342) or placebo (n = 83). The histologic pattern was similar to that at baseline, with 96.5% and 100.0% of participants receiving ospemifene and placebo, respectively, having reports of tissue insufficient for diagnosis or atrophic or inactive endometrium. Of these participants receiving ospemifene, 2.0% had weakly proliferative endometrial histology, 0.3% had actively proliferative endometrial histology, and 0.3% had proliferative pattern (disorder type) tissue; 0.9% had other (various types of polyps). About 3.5% of ospemifene participants had histologic findings other than inactive, atrophic, or insufficient tissue at 12 months, similar to the baseline endometrial biopsy results of placebo participants (4.1%). There were no endometrial carcinomas, complex hyperplasias, or simple hyperplasias with atypia in either the ospemifene group or the placebo group with up to 1 year of study completion.
Pelvic organ prolapse
There were rare cases of pelvic organ prolapse in both the ospemifene and placebo groups. Two women had bladder prolapse (one ospemifene participant and one placebo participant), and one ospemifene participant had a report of cystocele. Each of the two ospemifene participants had delivered two infants vaginally; the placebo participant had given birth vaginally once. Both women who received ospemifene were aged 63 years; the woman who received placebo was aged 53 years.
DISCUSSION
Ospemifene is a tissue-selective estrogen receptor agonist/antagonist that exerts a beneficial effect on vaginal epithelial tissue. Ospemifene also displayed an acceptable endometrial safety profile in studies of VVA treatment in postmenopausal women evaluated up to 52 weeks.
The phase 2/3 studies of ospemifene were powered to provide substantial safety data, allowing a prospectively defined assessment of potential treatment effects on endometrial hyperplasia. In these studies, only one woman who received ospemifene treatment was diagnosed with endometrial hyperplasia (simple hyperplasia without atypia). She also experienced vaginal bleeding and was found at study exit to have a thickened endometrium, which on follow-up biopsy 3 months later was confirmed to be endometrial simple hyperplasia. Based on histologic findings, a similar incidence of proliferative endometrial changes was present at baseline and 12 months in the ospemifene and placebo groups. Although the safety studies of ospemifene were up to 12 months in duration, many women may receive considerably longer treatment in practice. Further studies are needed to evaluate the longer-term risks of endometrial hyperplasia and malignancy in such women.
FDA guidance for VVA trials recommends that clinical trials demonstrate an endometrial hyperplasia rate of 1% or less, with an upper bound less than 4% of the one-sided 95% CI for that rate. With a single case of simple hyperplasia without atypia (0.3%) reported at 12 months, our results are well within the FDA criterion for endometrial safety.
The prevalence of endometrial polyps is dependent on age, menopause status, and hormone therapy use.22 A similar proportion of women with suspected endometrial polyps, based on histology, was found in the ospemifene and placebo groups in the phase 2/3 studies. All polyps were found in the single 52-week study (ospemifene, 5 of 364 [1.4%]; placebo, 1 of 62 [1.6%]). Thus, no increase in the incidence of endometrial polyps was observed in the 1-year clinical trial.
Slight mean increases in endometrial thickness, as assessed on TVUS, were reported in both treatment groups. However, the mean increase was less than 1 mm, with large SDs warranting caution in drawing conclusions. The imprecision of endometrial ultrasounds in assessing very small changes seems consistent with the findings that a woman could have a report of an increase in endometrial thickness at 12 weeks with a subsequent decrease reported at study completion without a change in her therapy. Although, numerically, more participants treated with ospemifene reported endometrial thickness measurements of 5 mm or more, women treated with ospemifene had longer treatment durations than women receiving placebo. There were no statistically significant differences in endometrial thickness between groups (P = 0.2) when the duration of study participation was considered.
The effects on the endometrium were consistent across the phase 2/3 studies. The observed small increases in endometrial thickness after ospemifene treatment were without concomitant cellular proliferation. Such changes in endometrial thickness have also been reported with raloxifene and tamoxifen.23 SERMs may cause glandular cystic atrophy, which can appear to cause endometrial thickening on ultrasound but without evidence of cellular proliferation.23,24 Data from the use of Premarin vaginal cream found an increase in endometrial thickness of 5 mm or more in approximately 10% of women,14 and the incidence of similar increases in endometrial thickness with ospemifene treatment was well within this finding.
In all studies, a statistically significant effect on physiologic vaginal parameters (increased proportion of superficial cells, decreased proportion of parabasal cells, reduced vaginal pH, and improved visual evaluation) was found with ospemifene 60 mg/day compared with placebo.16,17,20 These positive findings are sustained through 52 weeks of treatment. Subjective improvements in VVA symptoms were reported, with consistent improvement in moderate to severe dyspareunia. SERMs such as tamoxifen and raloxifene have not demonstrated similar favorable estrogen agonist effects on vaginal tissue.
A limitation of the current analysis is the combination of disparate studies. Although all studies were undertaken in a generally similar fashion, they differed in some aspects of design, such as requirement for women to have an intact uterus at enrollment, randomization ratio, and study duration. It can be difficult to compare outcomes between short-term and long-term studies; however, we addressed this limitation by using patient-year analyses.
CONCLUSIONS
Although ospemifene exerts beneficial effects on the vagina, endometrial safety is maintained, suggesting that the effects of ospemifene on estrogen receptors vary among the different components of the genital tract. This is in contrast to oral estrogen alone, which has a full agonist effect on both vaginal and endometrial tissues.10 No exogenous progestin use was permitted during the ospemifene clinical trials; thus, the favorable endometrial profile of ospemifene is further distinguished from that of oral steroidal estrogens. The endometrial data in these ospemifene studies, including histology and ultrasound results, seem to be approximately consistent with data for the SERM raloxifene.25 In conclusion, ospemifene exerts positive effects on vaginal tissue in clinical trials,16,17,19,20 yet displays an acceptable endometrial safety profile with up to 1 year of daily oral treatment.
Acknowledgments
We acknowledge Kathleen Burns and Complete Publication Solutions, LLC, for editorial assistance.
Footnotes
The authors had full control over the content, material, and writing and editing of the manuscript, and take full responsibility for its content.
Funding/support: Editorial assistance for this article was funded by Shionogi Inc.
Financial disclosure/conflicts of interest: G.D.C. is a consultant to Shionogi Inc. D.F.A. is a consultant to Shionogi Inc, AbbVie, Agile Therapeutics, Bayer Healthcare, CHEMO, EndoCeutics, Merck, and Warner Chilcott, and is a member of the speakers bureau for Shionogi, Pfizer, Bayer, Besins, Merck, and Noven. He has received institutional grants from AbbVie, Bayer, EndoCeutics, Pfizer, TherapeuticsMD, and Warner Chilcott. He has been compensated for developing educational presentations for Besins. He owns stocks or stock options for Agile. S.R.G. is a consultant to and/or member of the advisory boards for Amgen, Bayer, Cook Ob/Gyn, Philips Ultrasound, and Shionogi Inc, and is a member of the speakers bureau for Shionogi, Noven, and Bayer.
REFERENCES
- 1.The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20: 888- 902. [DOI] [PubMed] [Google Scholar]
- 2. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med 2003; 348: 618- 629. [DOI] [PubMed] [Google Scholar]
- 3. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281: 2189- 2197. [DOI] [PubMed] [Google Scholar]
- 4. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90: 1371- 1388. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein SR, Neven P, Cummings S, et al. Postmenopausal Evaluation and Risk Reduction With Lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause 2011; 18: 17- 22. [DOI] [PubMed] [Google Scholar]
- 6. Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology 2000; 141: 809- 820. [DOI] [PubMed] [Google Scholar]
- 7. Voipio SK, Komi J, Kangas L, Halonen K, DeGregorio MW, Erkkola RU. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas 2002; 43: 207- 214. [DOI] [PubMed] [Google Scholar]
- 8. Unkila M, Kari S, Yatkin E, Lammintausta R. Vaginal effects of ospemifene in the ovariectomized rat preclinical model of menopause. J Steroid Biochem Mol Biol 2013; 138: 107- 115. [DOI] [PubMed] [Google Scholar]
- 9. Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med 1975; 293: 1167- 1170. [DOI] [PubMed] [Google Scholar]
- 10.Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 1996; 275: 370- 375. [DOI] [PubMed] [Google Scholar]
- 11. Pickar JH, Yeh I, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil Steril 2001; 76: 25- 31. [DOI] [PubMed] [Google Scholar]
- 12. Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 2009; 16: 30- 36. [DOI] [PubMed] [Google Scholar]
- 13. Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol 2010; 116: 876- 883. [DOI] [PubMed] [Google Scholar]
- 14. Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009; 16: 719- 727. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Estrogen and Estrogen/Progestin Drug Products to Treat Vasomotor Symptoms and Vulvar and Vaginal Atrophy Symptoms—Recommendations for Clinical Evaluation. Rockville, MD: Center for Drug Evaluation and Research; 2003. [Google Scholar]
- 16. Bachmann GA, Komi JO; The Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17: 480- 486. [DOI] [PubMed] [Google Scholar]
- 17. Portman DJ, Bachmann GA, Simon JA; The Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20: 623- 630. [DOI] [PubMed] [Google Scholar]
- 18. Simon JA, Lin VH, Radovich C, Bachmann GA; The Ospemifene Study Group. One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause 2013; 20: 418- 427. [DOI] [PubMed] [Google Scholar]
- 19. Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas 2014; 78: 91- 98. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein SR, Bachmann GA, Koninckx PR, et al. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17: 173- 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mutter GL, Ferenczy A. Anatomy and histology of the uterine corpus. In: Kurman RJ, ed. Blaustein’s Pathology of the Female Genital Tract. 5th ed New York, NY: Springer-Verlag; 2002: 380- 420. [Google Scholar]
- 22. Dreisler E, Stampe Sorensen S, Ibsen PH, Lose G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20-74 years. Ultrasound Obstet Gynecol 2009; 33: 102- 108. [DOI] [PubMed] [Google Scholar]
- 23. Pinkerton JV, Goldstein SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause 2010; 17: 642- 653. [DOI] [PubMed] [Google Scholar]
- 24. McGonigle KF, Shaw SL, Vasilev SA, Odom-Maryon T, Roy S, Simpson JF. Abnormalities detected on transvaginal ultrasonography in tamoxifen-treated postmenopausal breast cancer patients may represent endometrial cystic atrophy. Am J Obstet Gynecol 1998; 178: 1145- 1150. [DOI] [PubMed] [Google Scholar]
- 25. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol 2000; 95: 95- 103. [DOI] [PubMed] [Google Scholar]


