Abstract
Rationale
Transverse (t-) tubules regulate cardiac excitation contraction coupling and exhibit inter-chamber and inter-species differences in expression. In cardiac disease t-tubule loss occurs and affects the systolic calcium transient. However, the mechanisms controlling t-tubule maintenance and whether these factors differ between species, cardiac chambers and in a disease setting remain unclear.
Objective
To determine the role of the BAR domain protein amphiphysin II (AmpII) in regulating t-tubule maintenance and the systolic calcium transient.
Methods and Results
T-tubule density was assessed by di-4-ANEPPS, FM4-64 or WGA staining using confocal microscopy. In rat, ferret and sheep hearts t-tubule density and AmpII protein levels were lower in the atrium than the ventricle. Heart failure was induced in sheep using right ventricular tachypacing and ferrets by ascending aortic coarctation. In both heart failure models, AmpII protein and t-tubule density were decreased in the ventricles. In the sheep, atrial t-tubules were also lost in heart failure and AmpII levels decreased. Conversely junctophilin 2 levels did not show inter-chamber differences in the rat and ferret nor did they change in heart failure in the sheep or ferret. Additionally, in rat atrial and sheep heart failure atrial cells where t-tubules were absent, junctophilin 2 had sarcomeric intracellular distribution. Small interfering RNA induced knockdown of AmpII protein reduced t-tubule density, calcium transient amplitude and the synchrony of the systolic calcium transient.
Conclusions
AmpII is intricately involved in t-tubule maintenance. Reducing AmpII protein decreases t-tubule density, reduces the amplitude and increases the heterogeneity of the systolic calcium transient.
Keywords: T-tubules, calcium, heart failure
INTRODUCTION
The synchronous rise of the systolic Ca2+ transient in mammalian ventricular myocytes is due to the presence of an extensive and regular transverse (t) tubular system 1. The presence of t-tubules ensure close apposition of L-type Ca2+ channels and sarcoplasmic reticulum (SR) Ca2+ release channels (ryanodine receptors, RyRs) forming dyads or couplons where excitation contraction coupling commences 2,3. The t-tubules are also surrounded by a continuous network of SR which is believed to assist with amplification of the initial Ca2+ entry during the action potential and contribute to the synchronous rise of systolic Ca2+ 4,5. The t-tubule and SR networks are however labile with disorganization and loss commonly observed in heart failure 5-9. In such circumstances the loss of t-tubules leads to dyssynchronous Ca2+ release patterns, a smaller systolic Ca2+ transient and altered β-adrenergic (β-AR) signalling e.g. 6-10. Conversely, recovery from heart failure is associated with restoration of the t-tubule network along with normalization of β-AR signalling and re-synchronization of the systolic Ca2+ transient 9,11.
More extensive differences in t-tubule organization and density than those occurring in the ventricle during heart failure are known to exist between the atrium and the ventricle. For example, small mammals (mouse, rat, rabbit etc) completely lack or possess only a very rudimentary, predominantly axially arranged, t-tubule network e.g. 12-14. Conversely, some studies have suggested that limited numbers of atrial cells from smaller laboratory species such as the rat have a more ventricular like t-tubule pattern e.g. 15 although these particular cells may be of different lineage and a feature of the pulmonary vein sleeve region 16. The poorly developed t-tubule network in these atrial myocytes leads to the characteristic early peripheral and delayed central Ca2+ transient 12,17. More recently however, a well-developed t-tubule system has been noted in atrial myocytes of larger species including man 18-20. Although remaining less extensive than in the corresponding ventricle 19, the t-tubule system in atrial myocytes of these larger species substantially reduces the spatial heterogeneity of the systolic Ca2+ transient 19. Moreover, as in the ventricle, the atrial t-tubule network is disrupted in heart failure and atrial fibrillation resulting in increased Ca2+ transient heterogeneity and dys-synchronous Ca2+ release 18,19. Several proteins have been implicated in the biogenesis and maintenance of t-tubules including titin cap protein (telothonin, TCAP), junctophilin 2 (JPH2) and the Bin/Amphiphysin/Rvs (BAR) domain protein amphiphysin II (AmpII or BIN1) (reviewed in 21,22). Of these, the BAR domain proteins are ubiquitously expressed, highly conserved in eukaryotes and have pleiotropic roles including sensing membrane curvature, endocytosis and regulation of actin filament function (reviewed in 23,24). Certain splice variants of amphiphysin, AmpII (BIN1) 25-27, appear not to be involved in the formation of clathrin coated vesicles and endocytosis 28,29. They are however, highly expressed in striated muscles, localises to t-tubules and gene deletion leads to fatal perinatal cardiomyopathy 28,30. More recently these BIN1 splice variants have been identified, in the mouse heart, as being involved in both t-tubule formation and causing extensive folding of the inner t-tubule membrane 27. However, the lack of a densely folded inner t-tubule membrane in other species e.g. sheep and rat as used in the present study 5 suggests that the particular BIN1 (AmpII) splice variants responsible may be a feature of the murine myocardium.
Mutations in AmpII also lead to the inherited condition centronuclear myopathy (CMN) 31 which is characterised by a severe cardiomyopathy, myocyte disarray e.g. 32 and arrhythmias 33. Moreover Drosophila AmpII mutants show flight muscle t-tubule disarray that is reversed by AmpII cDNA transfection 29. Finally, AmpII transfection induces tubule formation in CHO and HepG2 cells, cell types that do not ordinarily possess t-tubules 26,34,35 and, using the 13 + 17 splice variant, causes t-tubule rescue in cultured BIN1 (AmpII) heterozygous knockout cardiac myocytes 27.
In cardiac muscle AmpII protein levels are decreased in heart failure, which as noted above, is associated with t-tubule loss and disorganisation 11,25. Additionally, AmpII directs L-type Ca2+ channel expression to the t-tubule 26 and thereby provides a potential link between t-tubule loss and the decrease in L-type Ca2+ current observed in some models of heart failure 25,36. In the recently developed BIN1 knockout mouse model, the heterozygote shows a decreased intensity of t-tubule staining although t-tubules in the ventricle appear to be localised correctly. However, no information was presented on how such perturbations in BIN1 (AmpII) levels impact cellular Ca2+ homeostasis or inter-chamber differences in t-tubule density. This study therefore sought to determine; i) if inter-chamber differences t-tubule density are related to AmpII protein levels, ii) if AmpII protein levels and t-tubule density change in heart failure, iii) whether AmpII gene silencing reduces t-tubule density and, iv) how AmpII gene silencing influences the synchronicity of the systolic Ca2+ transient. We demonstrate that; i) the amount of AmpII protein is lower in the atria, ii) AmpII protein levels and t-tubule density are lower in heart failure, iii) transfection of adult rat ventricular myocytes with small interfering RNA (siRNA) decreases both AmpII protein levels and t-tubule density and, iv) loss of AmpII increases heterogeneity (dyssynchrony) of the systolic Ca2+ transient. We conclude therefore that AmpII is required for the maintenance of t-tubules in cardiac muscle and loss of AmpII is responsible for t-tubule disruption and increases the heterogeneity of the systolic Ca2+ transient. In contrast, using the same experimental approaches, we show that JPH2 is present, with sarcomeric intracellular distribution, in rat atrial and sheep heart failure atrial cells (which lack t-tubules) and also that JPH2 has an important role in determining t-tubule orientation rather than the overall density of t-tubules.
METHODS
A detailed Methods section is available in the Online Supplement.
All procedures involving animals accord to The United Kingdom, Animals (Scientific Procedures) Act of 1986 and have been approved by The University of Manchester Ethical Review Process.
Myocyte isolation, t-tubule quantification and animal models
Single cardiac myocytes were isolated from the left ventricle and left atria of sheep and rats using collagenase and protease digestion methods described in detail previously 19,36-38. Myocytes and PFA fixed tissue sections (ferret) were stained with di-4-ANEPPS or Alexa Fluor 488 conjugated wheat germ agglutinin and imaged confocally on a Leica SP2 microscope to visualise t-tubules which were then quantified after image processing as described previously 19,20. Heart failure was induced using either right ventricular tachypacing in the sheep 19,36,39 or ascending aortic coarctation in the ferret 40,41.
Myocyte culture, siRNA mediated gene silencing and [Ca2+]i measurements
Single rat ventricular myocytes were maintained in myocyte growth medium (Promocell, UK) and transfected following manufacturers recommendations (Santa Cruz, USA) with 10 μmol/L siRNA (Sigma Mission siRNA or Santa Cruz scrambled siRNA) targeting AmpII, JPH2 or using a scrambled (control) siRNA. Cells were transfected for 24 hours and then imaged as described above or processed for immunoblotting or immunolabeling. Changes in [Ca2+]i were monitored using Fluo-3 AM loaded cells on a Leica SP2 confocal microscope.
Statistics
All data are presented as mean ± standard error of the mean (SEM) from n observations / N experiments. To account for multiple observations (n) from the same animal (N) linear mixed modeling was performed (SPSS Statistics; IBM, USA). Where multiple observations or technical replicates were not performed Students t-test, paired t-test, or Mann-Whitney Rank Sum tests (where either the data were not normally distributed or had unequal variance) were used. Pearson’s coefficient was performed on paired data points using GraphPad Prism 5.0. Data were considered significant when P<0.05.
RESULTS
Inter-chamber differences in t-tubule density and amphiphysin II expression
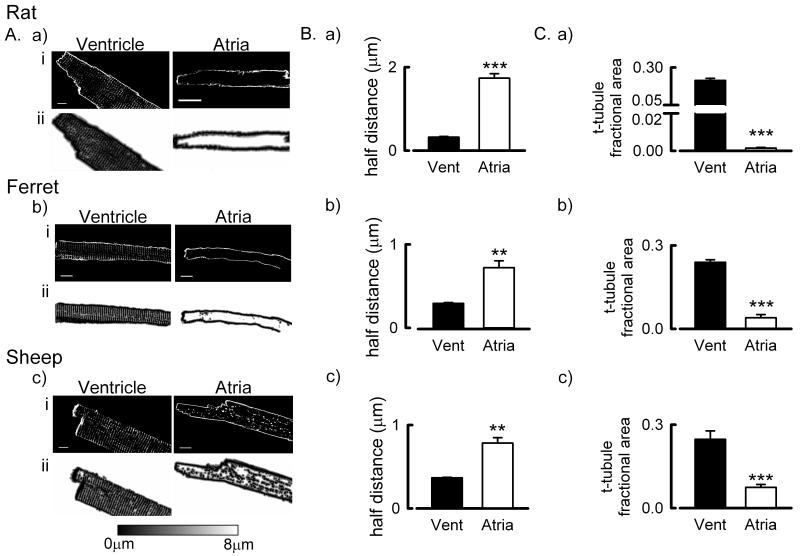
The presence of t-tubules was examined in the ventricles and atria of the rat, ferret and sheep (fig 1A). In agreement with previous work 1,19,20, a well-developed t-tubule network was found in all of the ventricular samples studied. In the rat atrium we found essentially no t-tubules whereas in the ferret atrium a rudimentary t-tubule network was present in some cells (fig 1A.b.). To determine that the cell isolation process had not led to t-tubule loss in rat atrial cells we also performed confocal imaging of intact hearts stained with FM4-64 42; both right and left ventricular myocytes had a well developed t-tubule network whereas t-tubules were again essentially absent in both the left and right atria with the occasional short potential membrane invagination present in very few cells (Online Figure III.) However, in the sheep atrium a well-developed t-tubule network was present (fig 1A.c.). As a major role of the surface membrane and t-tubules is to facilitate Ca2+ flux in and out of the cell we next assessed the impact of the inter-chamber and species differences in t-tubule occurrence on the distance any point is inside the cell to the surface membrane or t-tubule membrane. This was assessed using two approaches; firstly, as described previously 19,20, by calculating distance maps (fig 1A.a.ii – c.ii.) profiling the distance any voxel is within the cell (in either the vertical or horizontal planes) from a membrane (surface sarcolemma or t-tubule) and calculating the distance which 50 % of voxels are from a membrane (referred to as the half-distance, fig 1B.a-c. and Online Figure II.). The second approach provides a measure of t-tubule density independent of the effects of cell width on half-distance measurements. Here we determined the fraction of all voxels within the central 2 μm thick section of the cell (excluding surface sarcolemma) occupied by t-tubules identified using either di-4-ANEPPS or Alexa Fluor 488 conjugated WGA staining (referred to as the t-tubule fractional area, fig 1C.a-c.). It is clear that, in all species, there are more t-tubules in the ventricular samples than the corresponding atrial samples (fig 1B-C. P < 0.01). Moreover, in agreement with previous studies 19,20, the fractional area occupied by t-tubules in the atrium was greatest in the sheep (fig 1C).
Figure 1. Quantification of species and inter-chamber differences in t-tubule density.
A. Representative examples showing membrane staining (upper panels) and distance maps (lower panels) in ventricular and atrial myocytes from rat (a), ferret (b) and sheep (c). Cells have been stained with either di-4-ANEPPS (a & c) or WGA (b). B. Mean data summarizing the distance 50 % of voxels (half distances) are from the nearest cell membrane (t-tubule or surface sarcolemma) in ventricular and atrial cells from (a-c) rat, ferret and sheep. C. Mean data summarizing the fraction of intracellular pixels occupied by t-tubules in ventricular and atrial cells from (a – c) rat, ferret and sheep. **, P < 0.01, ***, P < 0.001. Rat ventricle, 20 cells / 5 hearts; Rat atria, 15 cells / 3 hearts; Ferret ventricle, 8 cells / 4 hearts; Ferret atria, 8 cells / 4 hearts; Sheep ventricle, 20 cells / 5 hearts; Sheep atria, 21 cells / 5 hearts.
We next sought to determine if the inter-chamber differences in t-tubule density were associated with AmpII protein levels. Representative immunoblots from rat, ferret and sheep samples are shown in fig 2A. Despite the absence or limited presence of t-tubules in the atria of rat and ferret, AmpII protein is detectable, although at lower levels compared to the respective ventricle (lower by 61 ± 4.0 %, P < 0.001 and 38.2 ± 8.1 %, P < 0.05 respectively). In the sheep myocardium, as is observed in skeletal muscle 30 and mouse ventricle 27, two major isoforms were detected and densitometric analysis of each band alone or both combined yielded the same qualitative result (not shown); we therefore quantified both bands. In the sheep atrium, where the t-tubule network is relatively well developed, AmpII levels were still lower than in the corresponding ventricle. However, the extent of the decrease (26.7 ± 6.7 %, P < 0.05) is less than that in rat and ferret where t-tubules are absent.
Figure 2. Inter-chamber differences in Amphiphysin II and Junctophilin 2 protein levels in the rat, ferret and sheep.
A. Representative Western blots for AmpII and GAPDH (upper panels) and mean data for ventricular and atrial tissues from rat (a), ferret (b) and sheep (c). (note, GAPDH cannot be used for sheep samples). B. Representative Western blots for JPH2 and GAPDH (upper panels) and summary data (lower panels) for ventricular and atrial tissues from rat (a), ferret (b) and sheep (c). IC, internal control (rat, ferret and sheep ventricular samples respectively); *, P < 0.05; ***, P < 0.001. For Western blots data have been normalized to the respective internal control relative to GAPDH (where used). N = 6, rat ventricle; 5, rat atria; 5, ferret ventricle; 6, ferret atria; 7, sheep ventricle; 7, sheep atria.
The membrane bridging protein JPH2 has also been implicated in determining t-tubule orientation and formation 43,44. We therefore examined whether the abundance of JPH2 protein differs between cardiac chambers in line with the changes in t-tubule density and AmpII protein expression noted above. This data is summarised in Fig 2B and shows that, irrespective of the presence or absence of t-tubules, there are no inter-chamber differences in JPH2 in the rat and ferret. For example, in the rat atrium JPH2 levels are 96 % of those in the ventricle (Fig 2Ba. P = 0.87). Conversely, in the sheep atrium where t-tubules are well developed, JPH2 levels are lower than in the ventricle (by 32.1 ± 6.5 %, P < 0.001)
Reduced amphiphysin II expression in heart failure and t-tubule loss
Heart failure is known to lead to loss of t-tubules in the ventricle e.g. 8 and atrium 19 and therefore we next determined if changes in AmpII or JPH2 protein levels occur in parallel to t-tubule loss. Firstly, we examined t-tubule density in ventricular and atrial cells in an ovine tachypacing model of heart failure. Clinical signs of heart failure including breathlessness and lethargy were present after 50.5 ± 4.3 days of right ventricular tachypacing. As reported previously 36,39, left ventricular internal diastolic dimension increased (pre-pacing, 2.41 ± 0.14 cm; heart failure, 3.87 ± 0.09 cm. P < 0.001) and fractional shortening decreased (pre-pacing, 0.68 ± 0.02; heart failure 0.27 ± 0.02, P < 0.001) with the development of heart failure (supplementary data, Table 1). Isolated myocytes were stained with di-4-ANEPPS to visualise the t-tubule network and it is clear that in both ventricular (fig 3A.) and atrial cells (fig 3B.) there is t-tubule loss in heart failure. We characterised the extent of t-tubule loss by determining the half distance value and fractional area occupied by t-tubules (fig 3A.b. & B.b.). The original images and voxel distance maps show that in the ventricle t-tubule loss was evident at the cell end and to varying extents throughout the cell. Conversely, in the atrium there was an almost complete loss of t-tubules in heart failure and correspondingly larger increase in the half-distance and smaller fractional area occupied by t-tubules compared to the ventricle. The half-distance increased from 0.38 ± 0.01 to 0.45 ± 0.01 μm in the ventricle (P < 0.001) and 0.78 ± 0.07 to 1.94 ± 0.12 μm in the atrium (P < 0.01) and the fractional area occupied by t-tubules decreasing from 0.22 ± 0.01 in control to 0.15 ± 0.01 in heart failure in the ventricle (fig 3A.b.iii, P < 0.001) and from 0.075 ± 0.01 in control to 0.014 ± 0.003 in heart failure in the atrium (fig 3B.b.iii, P < 0.05). The reduction / loss of t-tubules in the ventricle and atrium in heart failure was associated with a reduction in AmpII protein levels in both chambers (fig 3C.a & b; ventricle by 24.1 ± 5.7 % and atrium by 34.5 ± 6.9 %, both P < 0.05). However, despite the loss of t-tubules in heart failure there was no change in JPH2 protein levels in either the ventricle or atrium (fig 3D.).
Figure 3. Decreased t-tubule density and Amphiphysin II protein levels but unchanged Junctophillin 2 protein levels in ventricular and atrial cells in ovine tachypacing induced heart failure.
A. Representative membrane staining (a), distance maps (b.i.) and mean data summarizing half distances (b.ii.) and t-tubule fractional occupation (b.iii.) from control sheep (left) and heart failure sheep (right) ventricular myocytes. B. Representative membrane staining (a), distance maps (b.i.) and mean data summarizing half distances (b.ii.) and t-tubule fractional occupation (b.iii.) from control sheep (left) and heart failure sheep (right) atrial myocytes. Data from (cells/hearts); Ventricle, 67/6 control, 56/7 heart failure; Atria, 21/5 control, 18/2 heart failure. C. Representative Western blots (upper panels) and summary data (lower panels) showing changes in AmpII protein levels in ventricular (a) and atrial (b) tissues. D. Representative Western blots (upper panels) and summary data (lower panels) showing JPH2 protein levels in ventricular (a) and atrial (b) tissues. IC, internal control; *, P < 0.05; **, P < 0.01; ***, P < 0.001. For Western blots data are normalized to the respective internal control sample used in each experiment. For Western blotting N = 7 control ventricle, 7 heart failure ventricle, 7 control atria, 6 heart failure atria.
To establish that the changes noted above were not restricted to the tachypacing model used in the sheep we also examined the t-tubule network, AmpII and JPH2 protein levels in a thoracic aortic coarctation / pressure overload model of heart failure in the ferret 40,41. Clinical signs of heart failure took 39 ± 2 days to develop following aortic coarctation and resulted in an increase in left ventricular end diastolic dimensions from 1.99 ± 0.03 cm to 2.25 ± 0.06 cm and decrease in ejection fraction from 0.41 ± 0.04 to 0.11 ± 0.02 (both P < 0.005; supplementary data, Table 2). In the heart failure ventricle, t-tubule loss was evident (fig 4A.) resulting in an increase in the voxel half-distance from 0.294 ± 0.008 μm in sham-operated hearts to 0.353 ± 0.007 μm in heart failure (P < 0.01). The increase in half-distance was accompanied by a decrease in the fractional area occupied by t-tubules in the ferret ventricle in heart failure from 0.236 ± 0.007 in control to 0.186 ± 0.005 in heart failure (fig 4A.b.iii, P < 0.001). As in the sheep tachypacing model of heart failure, AmpII protein levels were decreased in the ferret aortic coarctation model (fig 4B.a, by 61.3 ± 5.6 %, P < 0.05) but no change in JPH2 protein levels were observed (fig 4B.b.). Fig 5A examines the relationship between t-tubule density and AmpII protein levels across each of the species, cardiac chambers and disease models studied thus far. Since different loading controls were used in each experiment we have normalised the AmpII protein levels to those in the appropriate ventricular sample. A significant correlation exists between t-tubule half distance (density) and AmpII protein levels (P < 0.01) indicating that, in common with Hong et al 27, t-tubule density depends on AmpII protein levels. Importantly, whilst the relationship between t-tubule density (half distance) and AmpII protein appears to diverge at low AmpII levels, this significant correlation is maintained if the sheep is examined in isolation or if the rat atria, which lacks t-tubules, are excluded from the analysis (both P < 0.05, data not shown).
Figure 4. Decreased t-tubule density and Amphiphysin II protein levels but unchanged Junctophillin 2 protein levels in ventricular cells following aortic coarctation induced heart failure in the ferret.
A. Representative images showing membrane staining (a), distance maps (b.i.), half-distance summary data (b.ii.) and t-tubule fractional area summary data (b.iii.) from control (left) and heart failure ferret ventricular myocytes (right). Data from (cells/hearts); control, 22/7; heart failure, 15/5. B. Representative Western blots (upper) and summary data for AmpII (a) and JPH2 (b) in control and heart failure ventricular samples. IC, internal control (ferret ventricular sample); *, P < 0.05; **, P < 0.01; *** P < 0.001. For Western blots data are presented normalized to the internal control sample. Data from 5 control and 6 heart failure animals.
Figure 5. T-tubule density correlates with Amphiphysin II protein levels across tissues, differing disease states and following siRNA mediated gene silencing.
A. Dependence of t-tubule half-distances on AmpII protein levels in tissues and species indicated. Data are presented as mean ± SEM and the solid line through the data is a best-fit linear regression (Pearsons correlation coefficient, #, P < 0.01). Half distances and AmpII protein levels are expressed relative to the respective control samples (e.g. ventricular samples when comparing to atrial expression and, control atrial or ventricular samples when comparing to changes in heart failure which are plotted at coordinate 1,1). B. Representative images showing immuno-localisation of AmpII (a) and di-4-ANEPPS membrane staining (b) in freshly isolated (left), scrambled siRNA treated (centre) and AmpII siRNA treated (right) rat ventricular cells. C. Representative Western blot (upper) for AmpII and β-actin and summary data showing reduced AmpII protein abundance following siRNA treatment. ***, P < 0.001. N = 18 experiments. D. Mean data summarizing half-distances (solid bars) and t-tubule fractional area (open bars) in freshly isolated, scrambled siRNA treated and AmpII (target) treated rat ventricular myocytes. (Scrambled vs. Freshly Isolated; $, P < 0.05: Target vs. Scrambled; ***, P < 0.001: Target vs. Freshly Isolated; †, P < 0.001). Data from (cells/experiments); Freshly isolated, 20/5; scrambled, 199/20; target, 196/20. E. Dependence of half distance on AmpII protein levels following siRNA mediated AmpII gene silencing. The solid line through the data is a best-fit linear regression (#, P < 0.01; N = 18).
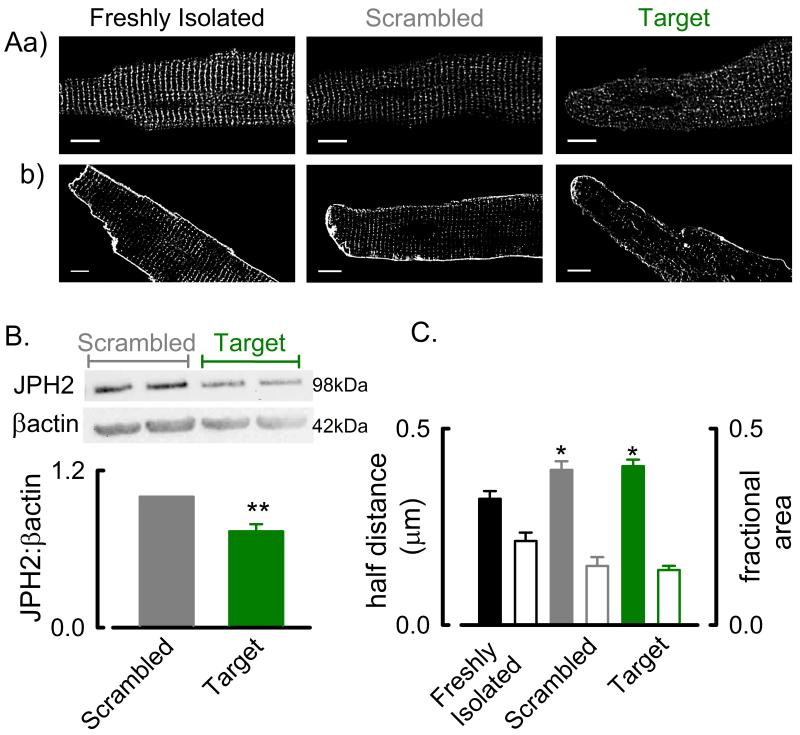
Amphiphysin II gene silencing reduces t-tubule density and increases the heterogeneity of the systolic Ca2+ transient
We next sought, using siRNA mediated gene silencing, to determine the role AmpII plays in t-tubule maintenance and synchronisation of the systolic Ca2+ transient. The immuno-localisation in fig 5B.a. shows that AmpII has a sarcomeric (~ 2μm spacing) distribution in freshly isolated and scrambled siRNA transfected rat ventricular cells. However, in AmpII targeted siRNA (target) transfected cells the distribution of AmpII is markedly altered becoming noticeably punctate and heterogeneous. The altered AmpII immuno-localisation following siRNA gene silencing is associated with disorganised t-tubule staining (fig 5B.b.). Consistent with previous studies showing some degree of t-tubule loss in cultured ventricular cells e.g. 45,46 there was a slight increase in half-distance and decrease in fractional area occupied by t-tubules in scrambled siRNA treated cells compared to freshly isolated cells (fig 5D; half-distances: freshly isolated, 0.32 ± 0.02 μm; scrambled siRNA treated, 0.38 ± 0.01 μm, P < 0.05; fractional areas: freshly isolated, 0.21 ± 0.01; scrambled siRNA, 0.17 ± 0.01, P < 0.05). However, siRNA gene silencing had a more pronounced effect on half-distance and fractional occupation with the siRNA mediated 12 ± 3.2 % decrease in AmpII protein abundance (fig 5C, P < 0.001) resulting in an increase in the voxel half-distance from 0.37 ± 0.01 μm to 0.57 ± 0.01 μm (fig 5D, P < 0.001) and a decrease in the fractional area occupied by t-tubules from 0.17 ± 0.01; target, 0.08 ± 0.01 (fig 5D, P < 0.001). Importantly, siRNA mediated gene silencing did not alter JPH2 protein abundance (Online Figure IV.A).
Given the potential role of AmpII in trafficking the L-type Ca2+ channel (LTCC) to the t-tubule 26, we also examined the cellular distribution of the LTCC, AmpII and t-tubules in freshly isolated, scrambled siRNA and AmpII targeted siRNA transfected cells (Online Figure V). In freshly isolated cells (fig S5A) AmpII and the LTCC have regular striated intracellular distribution. The LTCC is also present to some extent on the surface sarcolemma; nevertheless, there is strong colocalisation of the LTCC and AmpII. In scrambled siRNA treated cells t-tubules were visualised by WGA staining and are clearly well-maintained as is the cellular distribution of, and colocalisation with, the LTCC (fig S5B). Following AmpII siRNA mediated gene silencing there is loss of t-tubules and LTCC predominantly from the centre of the cell whilst at the cell edges colocalisation of the t-tubule and the LTCC is maintained (Online figure VC).
Since transient transfection techniques have limited efficiency and can be variably successful we examined the post-transfection relationship between AmpII protein levels and the voxel half-distance as a measure of t-tubule density. This data is summarised in fig 5E and shows an inverse correlation between voxel half-distance and AmpII protein levels (P < 0.01). Thus the density of t-tubules depends on the level of AmpII within cardiac myocytes.
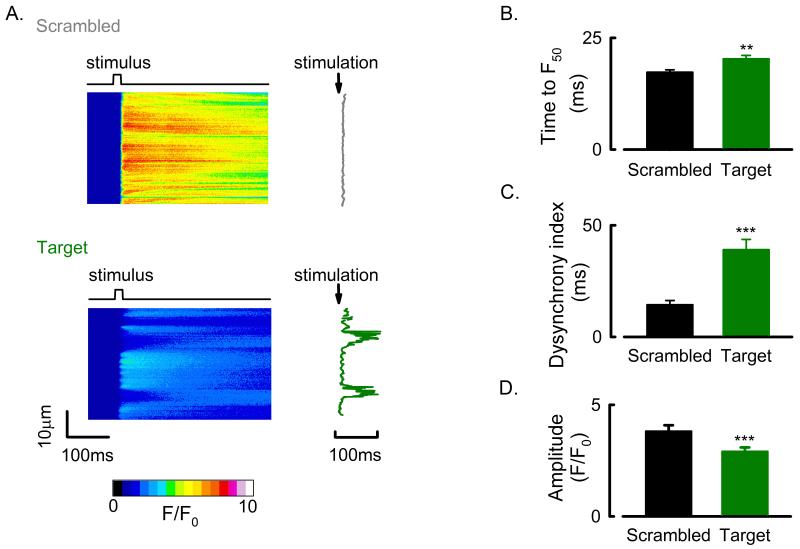
Finally, we sought to determine the effect that AmpII gene silencing mediated t-tubule loss had on the systolic Ca2+ transient. Cells were field stimulated and changes in [Ca2+]i measured using xt scanning confocal microscopy (fig 6A.). In scrambled siRNA transfected cells the systolic Ca2+ transient rose synchronously along the length of the cell whereas in the target siRNA treated cell there are areas where the rise of [Ca2+]i was delayed. On average the time for the systolic Ca2+ transient to reach 50 % of its peak at each point along the linescan image (F50) increased from 17.3 ± 0.5 ms in scrambled siRNA treated cells to 20.2 ± 0.8 ms in target siRNA treated cells (fig 6B. P < 0.01). In order to assess the degree of spatial heterogeneity of the systolic rise of [Ca2+]i the dyssynchrony index was calculated from the standard deviation of the F50 times along each linescan image 7. The dyssynchrony of the systolic Ca2+ transient increased by 163 ± 33 % (fig 6C; scrambled, 13.9 ± 1.3 ms; target, 36.9 ± 3.2 ms. P < 0.001). In addition to the delayed and dyssynchronous rise of [Ca2+]i the amplitude of the systolic Ca2+ transient was also reduced in the target siRNA treated cells, in this instance by 20.6 ± 4.1 % (fig 6D, P < 0.001).
Figure 6. Amphiphysin II gene silencing mediated changes to the systolic Ca2+ transient.
A. Confocal xt line-scans (left) and line-by-line systolic Ca2+ rise times (right) from a representative scrambled (upper) and AmpII siRNA targeted (lower) rat ventricular cell. B. Mean data showing delayed rise time (F50) of the systolic Ca2+ transient in AmpII gene silenced rat ventricular cells. C. Mean data showing increased dyssynchrony index (standard deviation of rise times) in AmpII silenced rat ventricular cells. D. Mean data showing reduced Ca2+ transient amplitude following AmpII gene silencing in rat ventricular cells. **, P < 0.01; ***, P < 0.001. Data from (cells/experiments); scrambled, 80/12; target, 94/12.
Junctophilin 2 gene silencing does not affect t-tubule density but does alter t-tubule orientation
The previous experiments point to a key role for AmpII in regulating t-tubule maintenance in cardiac muscle. However, a number of additional proteins have also been implicated in controlling t-tubule maintenance / formation. We investigated a potential role for the membrane bridging protein JPH2 which is thought to be responsible for tethering the sarcoplasmic reticulum to the t-tubule and maintaining t-tubule orientation 42,47. Firstly, the cellular localisation of JPH2 was examined (fig 7A.a.). In both freshly dissociated and scrambled siRNA transfected cells JPH2 has a primarily sarcomeric distribution with some surface membrane staining. In JPH2 siRNA transfected cells there is a 26± 5.4 % decrease in JPH2 protein levels (fig 7B, P < 0.01) with no changes in AmpII protein abundance detected (Online Figure IV.B). Notably however, in JPH2 siRNA targeted cells the cellular distribution of JPH2 appears more diffuse throughout the cell. The altered JPH2 cellular distribution is reflected in a less organised t-tubule network in JPH2 siRNA transfected cells (fig 7A.b.). However, despite the disorganisation of the t-tubule network the voxel half distance and fractional area occupied by t-tubules are unaltered by JPH2 gene silencing (fig 7C).
Figure 7. Junctophilin 2 gene silencing does not reduce t-tubule density in rat ventricular cells.
A. Representative images showing immuno-localisation of JPH2 (a) and membrane staining with di-4-ANEPPS (b) in freshly isolated (left), scrambled siRNA treated (centre) and JPH2 siRNA treated (right) rat ventricular cells. B. Example Western blots of JPH2 and β-actin in scrambled and JPH2 target treated rat ventricular cells (upper) and mean data showing reduced JPH2 protein abundance in JPH2 gene silenced cells (lower). **, P < 0.01. N = 4 experiments. C. Mean data showing no change in half-distance (solid bars) or fractional t-tubule area (open bars) measurements following JPH2 gene silencing in adult rat ventricular myocytes. *, P < 0.05 vs freshly isolated myocytes. Data from (cells/experiments); freshly isolated, 20/5; scrambled, 48/5; target, 51/5.
Whilst the decrease in JPH2 protein abundance (fig 7B) does not alter the distance any point within the cell is from either the surface or t-tubule membrane, the orientation of t-tubules does change. We quantified changes in t-tubule orientation by skeletonising the di-4-ANEPPS stained cells and calculating the probability distribution of the angular orientation of t-tubules 48 (fig 8A). In scrambled siRNA transfected cells t-tubules are predominantly oriented perpendicular to the long axis of the cell whereas in JPH2 siRNA transfected cells t-tubules have both perpendicular and horizontal orientation such that the ratio of transverse (90 ± 15 ° to long axis) to longitudinal (0 ± 15 ° to long axis) t-tubules decreased by 31 ± 7.8 % in JPH2 siRNA transfected cells (fig 8B. P < 0.01). T-tubule orientation was also examined in ventricular cells from the ovine tachypacing and ferret pressure overload models of heart failure (Online Figure VI). In the sheep, but not the ferret, longitudinal t-tubules were more evident in heart failure.
Figure 8. Junctophilin 2 gene silencing leads to t-tubule re-orientation in adult rat ventricular cells but no change in t-tubule density.
A. Representative images showing di-4-ANEPPS membrane staining (a), skeletonised images (b) and t-tubule orientation (c) from a scrambled siRNA treated (left) and JPH2 siRNA (right) targeted rat ventricular myocytes. B. Mean data showing a reduction in the ratio of transverse (perpendicular) to longitudinal (axial) t-tubules in JPH2 gene silenced rat ventricular cells. **, P < 0.01. Data from (cells/experiments); scrambled 48/5; target, 51/5.
We also examined the cellular distribution of JPH2 and the RyR in atrial and ventricular tissues. Where t-tubules are essentially absent (heart failure sheep atria and rat atria) there is regular Online Figure VII). A similar colocalising JPH2 and RyR sarcomeric distribution is observed in rat ventricular tissue (Online Figure VII.B.b.) where t-tubules are known to be present. Conversely, AmpII and WGA (cell membrane) staining are present around the cell surface rat atrial myocytes but colocalise with sarcomeric distribution throughout the cytoplasm of rat ventricular myocytes (Online Figure VII.C).
DISCUSSION
Five main findings are presented in this paper: i) across species there are inter-chamber differences in t-tubule density that are paralleled by differences in AmpII but not JPH2 protein levels; ii) t-tubule density and AmpII protein levels, but not JPH2 protein levels, are decreased in two distinct models of heart failure; iii) AmpII gene silencing in adult rat ventricular cells decreased t-tubule density, AmpII protein levels, Ca2+ transient amplitude and increased the dyssynchrony of the systolic Ca2+ transient, iv) JPH2 gene silencing did not reduce the overall density of t-tubules in rat ventricular cells but did alter t-tubule orientation and, v) JPH2 has sarcomeric intracellular distribution colocalising with RyRs in cells lacking t-tubules. Taken together these findings indicate that AmpII has a major role in t-tubule maintenance in cardiac muscle. Conversely however JPH2 appears to be more important for controlling t-tubule orientation and localisation of non-junctional RyRs rather than controlling t-tubule density in cardiac muscle.
Differences in t-tubule density, Amphiphysin II and Junctophilin 2 protein levels between atrial and ventricular cardiac myocytes
In accordance with many e.g. 1,49, but not all studies e.g. 15,42, we found that rat atrial mycoytes lack a discernible t-tubule network. We also found that the ferret atrium like that of the cat 50 and rabbit 13 had only a very sparse t-tubule network present in some cells. However, as described previously 18-20, the sheep atrium has a well-developed t-tubule network. Functionally, where the t-tubule network is absent in atrial myocytes the systolic Ca2+ transient rises initially at the cell periphery and then propagates as a wave of Ca2+-induced Ca2+ release to the cell centre e.g. 1,49. However, the relatively well-developed t-tubule network in the sheep atrium ensures that the systolic Ca2+ transient rises synchronously at the cell periphery and the cell centre 18,19. Despite the inter-species differences in t-tubule density in the atria we noted that all ventricular cells from the rat, ferret and sheep possessed a regular t-tubule network throughout the entire volume of the cell and in all species the density of the ventricular t-tubule network was greater than that in the atrium.
Given the inter-chamber differences in t-tubule density one of the first aims of this study was to elucidate which proteins may be involved in t-tubule formation. To this end several candidates exist and we have studied two of these in detail, AmpII and JPH2. Expression of the BAR domain protein bridging integrator-1 (BIN1), or AmpII, in non-muscle CHO cells is sufficient to induce tubule formation 34. AmpII has also been shown to be responsible for trafficking of the L-type Ca2+ channel to the cell membrane in cardiac myocytes 26. JPH2 on the other hand tethers the SR/RyR to the sarcolemma/Z-line maintaining the geometry of the dyad 4,47,51. Deletion of either AmpII and JPH2 results in perinatal lethality with evidence of cardiac failure and structural disorganization 28,44,47. It is also noteworthy that the recently described murine cardiac conditional BIN1 (AmpII) knockout heterozygote heart retains a regular, albeit less intensely stained, t-tubule network 27. However in the same study, short hairpin RNA gene silencing of AmpII caused t-tubule loss as was observed herein and moreover, adenoviral mediated expression of the 13+17 splice variant of BIN1 increased t-tubule intensity in cultured heterozygote cells. Additionally, in the BIN1 knockout heart the main secondary effect of BIN1, after maintaining t-tubule density, appears to be the generation of a tightly folded inner t-tubule membrane. Interestingly, in agreement with several other studies e.g. 52,53 we do not note inner t-tubule membrane folding in the rat or sheep ventricular myocardium by serial block face scanning electron microscopy (sbfSEM) 5 and the ~ 250 nm resolving capability of confocal microscopy is insufficient to visualize such structures or subtle changes in t-tubule lumen diameter. As such the inner t-tubule membranes noted by Hong et al 27 may be features specific to the mouse or reflect the role of species differences in BIN1 (AmpII) isoform expression.
We find that AmpII protein levels are lower in the atrium compared to the ventricle which therefore parallels the observed differences in t-tubule density between the atrium and ventricle. Conversely, in the rat and ferret atria where the t-tubule network is either absent or very sparse we do not observe a corresponding decrease in JPH2 protein levels compared to that seen in the ventricle. Paradoxically however in the sheep atrium, where t-tubules are prevalent, JPH2 protein levels are less than that in the corresponding ventricle.
An unresolved question arising from this work is what is the basis of the constancy of JPH2 expression in the rat and ferret atria and ventricle despite the marked differences in t-tubule density? Whilst we do not have a definitive answer, in the rat atrium where t-tubules are virtually absent, RyRs and L-type Ca2+ channels form dyads at the cell surface 54. Additionally, at least in some studies, the L-type Ca2+ current density in the rat atrium and ventricle is the same 37,55 suggesting that the surface density of L-type Ca2+ channels and thus dyads may be greater in the atrium than the ventricle. Furthermore, RyRs are distributed throughout the cell as part of the non-junctional or corbular SR 17,56. Thus JPH2 may still ensure dyad alignment and non-junctional SR alignment in these cells. In support of this we find that RyR and JPH2 have a regular sarcomeric intracellular distribution and colocalise in sheep heart failure atrial myocytes and rat atrial myocytes where t-tubules are absent.
A subsidiary question is why, despite the marked difference in t-tubule density between the atria and ventricles in the rat and ferret, is there only slightly less AmpII protein in the atrium compared to the ventricle? We propose that the explanation for this observation is possibly due to the plurality of roles of AmpII. In a recent study by Hong et al 27 and in Drospophila indirect flight muscle 29 loss of AmpII is associated with t-tubule loss. Conversely AmpII also appears to be required for trafficking of the L-type Ca2+ channel to the cell membrane 26 and, at least in the mouse heart, multiple non-tubule forming AmpII isoforms are expressed 27. Therefore, the existence of L-type Ca2+ channels in atrial cells lacking t-tubules taken together with the other roles known to be performed by various AmpII isforms, would suggest a maintained requirement for AmpII expression even in tissues where t-tubules are lacking. Determing the potential roles for changes in AmpII isoform expression in different tissues and disease settings and the impact that this has on t-tubule formation, maintenance and function are worthy of future elucidation.
Remodeling of t-tubules in heart failure
Further evidence for the importance of AmpII in t-tubule maintenance is provided by the observation that in the two different models of heart failure used in the present study there is a reduction in t-tubule density paralleled by a decrease in AmpII but not JPH2 levels. A number of previous studies have reported t-tubule loss and disorganization in cardiac disease states e.g. 7-9,18,19,48. Whereas some, in line with the present study, have reported reductions in AmpII 11,25,26 others have also reported reductions in JPH2 in heart failure 11,42,48. It is noteworthy however that, in response to SERCA gene delivery 11 or mechanical unloading 9 as treatments for heart failure, t-tubule density 9,11 and AmpII (BIN1) protein levels 11 increased toward control levels but those for JPH2 remained reduced. Similarly, in a pulmonary hypertension model of right ventricular failure sildenafil treatment commenced when ventricular dysfunction was evident lead to an increases t-tubule density but not of JPH2 protein levels 57. Conversely, β-blocker therapy commenced after myocardial infarction was associated with an increase in t-tubule density and JPH2 protein relative to failing tissues 58. However these discrepant findings most likely reflect differences in study design and commencement of β-blocker therapy before ventricular dysfunction was evident and hence attenuation of the progression of heart failure rather than recovery from heart failure; a situation very distinct from those noted above for the effects of mechanical unloading and SERCA gene therapy 9,11.
Gene silencing approaches highlight an important role for Amphiphysin II in t-tubule maintenance in cardiac muscle
The inter-chamber and heart failure differences in t-tubule density and AmpII expression described above, whilst indicating an important role for AmpII in t-tubule maintenance in cardiac muscle, remain only associative observations. We therefore sought to define more precisely if reductions in AmpII expression directly influence t-tubule density in cardiac muscle and adopted a siRNA transient transfection approach in adult rat ventricular myocytes. Here we observed a direct relationship between AmpII protein and t-tubule density even following a relatively short period of ~ 24 hrs gene silencing. A similar linear dependence of t-tubule ‘intensity’ on AmpII protein in cardiac myocytes has also recently been reported using lentiviral mediated shRNA gene silencing over an extended 4 day culture period 27; however, whether the extended culture period also influences these latter observations is unclear given the propensity of adult cardiac cells to de-differentiate relatively rapidly when maintained in culture conditions e.g. 46. The reported-half life of AmpII is approximately 2 hours 59 and therefore amenable to transient transfection techniques and short-term culture of adult ventricular myocytes was therefore used in the present study. Given the low transfection efficiency of adult cadiac myocytes when using non-viral approaches 60, the reduction in AmpII protein levels following siRNA treatment (~ 12 %) is likely an underestimate of the reduction in AmpII in individual cells that have been successfully transfected; although whilst cells were randomly studied, identifying which cells had been successfully transfected using fluorescently labeled siRNA was not successful in the present study.
Whilst AmpII gene silencing leads to a loss of t-tubules and therefore an increase in the distance any point within the cell is from a t-tubule or surface membrane we found that both the half-distance and the fraction of the cell volume occupied by t-tubules were unaltered by siRNA-mediated JPH2 gene silencing. However, JPH2 gene silencing did result in an alteration to the spatial arrangement of the t-tubules from a predominantly transverse (perpendicular to the long-axis of the cell) orientation to one where axially arranged t-tubules were frequently observed. A similar t-tubule re-orientation as a consequence of JPH2 gene silencing has been noted previously 42. More recently it has also been suggested that, during post-natal development, JPH2 may have an important role in determining the formation of transversely oriented t-tubules rather than axially arranged t-tubules as the transversely oriented t-tubules were found to persist following JPH2 gene silencing 61. Similarly, in those models of heart failure where JPH2 levels are decreased, an increase in the proportion of axially arranged t-tubule elements has been noted 48,62. Although in the present study JPH2 was not reduced in either the sheep tachypacing or ferret pressure overload models of heart failure, there was a change in t-tubule orientation in the sheep tachypacing model. This implies that in addition to JPH2, AmpII, potentially even specific AmpII isoforms or other factors may also be responsible for maintaining t-tubule orientation. However, it appears that our findings are at least consistent with AmpII being required for t-tubule maintenance and that JPH2 has a role in ensuring the correct spatial alignment of t-tubules and / or RyRs in the heart.
Consequences of t-tubule loss on the systolic Ca2+ transient
In the present study we show that AmpII gene-silencing mediated depletion of t-tubules leads to a reduced Ca2+ transient amplitude and dyssynchrony of the systolic Ca2+ transient. Previous studies have also shown that t-tubule disorientation following JPH2 gene silencing in various cell types also increases the heterogeneity of the systolic Ca2+ transient 44,61,63. Our findings are also in line with earlier studies showing that the systolic Ca2+ transient amplitude is reduced and becomes dyssynchronous in heart failure 7,9,11. In heart failure there is also a reduction in the t-tubule density; although the loss of t-tubules has not previously been shown to be causative of the dysysnchronous Ca2+ transient. However, acute formamide-induced detubulation of cardiac myocytes and extended culture of adult cardiac myocytes also lead to a reduction in L-type Ca2+ current and reduced synchronicity of the systolic Ca2+ transient suggesting a causal link between t-tubule disruption and Ca2+ transient heterogeneity 1,46,64,65.
In summary we show that the BAR domain protein AmpII (BIN1) is intricately involved in the maintenance of cardiac t-tubules and thus ensuring the synchronicity of the systolic Ca2+ transient. Our data also supports a role for JPH2 in maintaining the normal orientation of t-tubules. It is therefore possible that AmpII and JPH2, along with a number of other proteins implicated in t-tubule formation e.g. TCAP and PI3K, may form a signalling nexus along the z-line to regulate t-tubule formation, maintenance and orientation. However, from the present study it is clear that t-tubule maintenance depends on AmpII levels and that changes in t-tubule density correlate strongly with AmpII in both the healthy and diseased heart. Thus AmpII may be an attractive target for restoring t-tubules and thus systolic Ca2+ and contractility in heart failure.
Supplementary Material
Novelty and Significance.
What is Known?
Transverse (t) tubules are surface membrane invaginations and are found in all mammalian ventricular myocytes.
Key ion channels linking the action potential to the systolic rise of calcium are located on t-tubules.
In different cardiac diseases t-tubules density is decreased and this leads to heterogeneity of the systolic rise of calcium and reduced contractility.
What New Information Does This Article Contribute?
We demonstrate that the bridging integrator protein, amphiphysin II (AmpII), is vital for maintaining t-tubules in ventricular myocytes.
Differences in t-tubule density between normal and heart failure myocytes and between atrial and ventricular chambers are correlated with AmpII but not junctophillin 2 (JPH2) protein expression.
Gene silencing of AmpII in adult ventricular myocytes leads to t-tubule loss, heterogeneity and reduced amplitude of the systolic calcium transient and restricts expression of the L-type calcium channel to the cell surface
Transverse (t) tubules have a pivotal role in regulating cardiac excitation contraction coupling. Differences in t-tubule distribution and / or density in cardiac disease or between atrial and ventricular mycoytes have a substantial effect on the synchronicity of the systolic rise of calcium. Despite the importance of t-tubules, the factors that are responsible for the formation and / or maintenance of t-tubules remain largely unknown. We show that differences in t-tubule density between atrial and ventricular myocytes and with progression to heart failure correlate with expression of the bridging integrator (BAR domain) protein amphiphysin II (AmpII). We also, using small interfering RNA approaches, demonstrate that loss of AmpII causes t-tubule depletion in adult ventricular myocytes. This loss of t-tubules is associated with a decrease in calcium transient amplitude and reduced synchronicity of the rise of calcium. Conversely, expression of the membrane spanning protein junctophillin-2 does not correlate with inter-chamber differences in t-tubule density nor does it alter in failing ventricular myocytes where t-tubule density is reduced. Our work indicates that AmpII has a vital role in maintaining t-tubules in adult cardiac myocytes and suggests that it could be used to restore t-tubules and the systolic calcium transient in heart failure.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from The British Heart Foundation (FS/12/57/29717, RG/11/2/28701, CH/2000/04, FS/10/52/28678, FS/14/4/30532, PG/12/89/29970), The European Union Framework 6 programme (‘Normacor’), Wellcome Trust Institutional Strategic Support Fund (097280) to The University of Manchester and The Manchester Biomedical Research Centre (George Lancashire Award).
Nonstandard Abbreviations and Acronyms
- AmpII
amphiphysin II (BIN1)
- BAR
Bin/Amphiphysin/Rvs
- β-AR
beta adrenergic receptor
- BIN1
bridging integrator 1 (AmpII)
- HF
heart failure
- JPH2
junctophilin 2
- LTCC
L-type Ca2+ channel
- RyR
ryanodine receptor
- siRNA
small interfering RNA
- SR
sarcoplasmic reticulum
- TCAP
titin cap protein (telothonin)
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Brette F, Komukai K, Orchard CH. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am J Physiol (Heart Circ Physiol) 2002;283:H1720–H1728. doi: 10.1152/ajpheart.00347.2002. [DOI] [PubMed] [Google Scholar]
- 2.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J. 2000;79:2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong J, Baddeley D, Bushong EA, Yu Z, Ellisman MH, Hoshijima M, Soeller C. Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of t-tubules near junctions. Biophys J. 2013;104:L22–L24. doi: 10.1016/j.bpj.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinali C, Bennett H, Davenport JB, Trafford AW, Kitmitto A. 3-D Reconstruction of the Cardiac Sarcoplasmic Reticulum Reveals a Continuous Network Linking T-Tubules: This Organization is Perturbed in Heart Failure. Circ Res. 2013;113:1219–1230. doi: 10.1161/CIRCRESAHA.113.301348. [DOI] [PubMed] [Google Scholar]
- 6.Cannell MB, Crossman DJ, Soeller C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. J Muscle Res Cell Motil. 2006;27:297–306. doi: 10.1007/s10974-006-9089-y. [DOI] [PubMed] [Google Scholar]
- 7.Louch WE, Mørk HK, Sexton J, Strømme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim M, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, Gorelik J, Yacoub MH, Terracciano CM. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca2+-induced Ca2+release in a rodent model of heart failure. Eur J Heart Fail. 2012;14:571–580. doi: 10.1093/eurjhf/hfs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J. β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- 11.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS, Korchev YE, Macleod KT, Harding SE, Gorelik J. Plasticity of Surface Structures and β2-Adrenergic Receptor Localization in Failing Ventricular Cardiomyocytes During Recovery from Heart Failure. Circ Heart Fail. 2012;5:357–365. doi: 10.1161/CIRCHEARTFAILURE.111.964692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996;494(Pt 3):641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiser M, Lederer WJ, Schotten U. Alterations of atrial Ca2+ handling as cause and consequence of atrial fibrillation. Cardiovasc Res. 2011;89:722–733. doi: 10.1093/cvr/cvq389. [DOI] [PubMed] [Google Scholar]
- 14.Woo SH, Cleemann L, Morad M. Ca2+ current-gated focal and local Ca2+ release in rat atrial myocytes: evidence from rapid 2-D confocal imaging. J Physiol. 2002;543:439–453. doi: 10.1113/jphysiol.2002.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisk M, Koivumäki JT, Norseng PA, Maleckar MM, Sejersted OM, Louch WE. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol (Heart Circ Physiol) 2014;307:H609–H620. doi: 10.1152/ajpheart.00295.2014. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto Y, Takano M, Ohba T, Ono K. Arrhythmogenic coupling between the Na+ -Ca2+ exchanger and inositol 1,4,5-triphosphate receptor in rat pulmonary vein cardiomyocytes. J Mol Cell Cardiol. 2012;52:988–997. doi: 10.1016/j.yjmcc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie L, Bootman MD, Berridge MJ, Lipp P. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J Physiol. 2001;530:417–429. doi: 10.1111/j.1469-7793.2001.0417k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D’hooge J, Heidbüchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876–885. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- 19.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 20.Richards MA, Clarke JD, Saravanan P, Voigt N, Dobrev D, Eisner DA, Trafford AW, Dibb KM. Transverse (t-) tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol (Heart Circ Physiol) 2011;301:H1996–H2005. doi: 10.1152/ajpheart.00284.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM. The structure and function of cardiac t-tubules in health and disease. Proc Biol Sci. 2011;278:2714–2723. doi: 10.1098/rspb.2011.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trafford AW, Clarke JD, Eisner DA, Dibb KM. Calcium signalling microdomains and the t-tubular system in atrial myocytes: potential roles in cardiac disease and arrhythmias. Cardiovasc Res. 2013;98:192–203. doi: 10.1093/cvr/cvt018. [DOI] [PubMed] [Google Scholar]
- 23.Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Masuda M, Mochizuki N. Structural characteristics of BAR domain superfamily to sculpt the membrane. Semin Cell Dev Biol. 2010;21:391–398. doi: 10.1016/j.semcdb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H, Hoopes CW, Ocorr K, Chi NC, Shaw RM. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9:812–820. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong T, Yang H, Zhang S-S, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marbán E, Jan LY, Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller AJ, Baker JF, DuHadaway JB, Ge K, Farmer G, Donover PS, Meade R, Reid C, Grzanna R, Roach AH, Shah N, Soler AP, Prendergast GC. Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol Cell Biol. 2003;23:4295–4306. doi: 10.1128/MCB.23.12.4295-4306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razzaq A, Robinson IM, McMahon HT, Skepper JN, Su Y, Zelhof AC, Jackson AP, Gay NJ, O’Kane CJ. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler MH, David C, Ochoa GC, Freyberg Z, Daniell L, Grabs D, Cremona O, De Camilli P. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol. 1997;137:1355–1367. doi: 10.1083/jcb.137.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicot AS, Toussaint A, Tosch V, Kretz C, Wallgren-Pettersson C, Iwarsson E, Kingston H, Garnier JM, Biancalana V, Oldfors A, Mandel JL, Laporte J. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet. 2007;39:1134–1139. doi: 10.1038/ng2086. [DOI] [PubMed] [Google Scholar]
- 32.Verhiest W, Brucher JM, Goddeeris P, Lauweryns J, De Geest H. Familial centronuclear myopathy associated with ‘cardiomyopathy’. Br Heart J. 1976;38:504–509. doi: 10.1136/hrt.38.5.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Böhm J, Yiş U, Ortaç R, Cakmakçi H, Kurul SH, Dirik E, Laporte J. Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation. Orphanet J Rare Dis. 2010;5:35. doi: 10.1186/1750-1172-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Wang D, Liu X, Liu L, Song Z, Zhu T, Adams G, Gao X, Tian R, Huang Y, Chen R, Wang F, Liu D, Yu X, Chen Y, Chen Z, Teng M, Ding X, Yao X. Phosphorylation of the Bin, Amphiphysin, and RSV161/167 (BAR) domain of ACAP4 regulates membrane tubulation. Proc Natl Acad Sci U S A. 2013;110:11023–11028. doi: 10.1073/pnas.1217727110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briston SJ, Caldwell JL, Horn MA, Clarke JD, Richards MA, Greensmith DJ, Graham HK, Hall MC, Eisner DA, Dibb KM, Trafford AW. Impaired β-adrenergic responsiveness accentuates dysfunctional excitation contraction coupling in an ovine model of tachypacing induced heart failure. J Physiol. 2011;589:1367–1382. doi: 10.1113/jphysiol.2010.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–473. doi: 10.1016/j.yjmcc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, Trafford AW. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol. 2004;37:1171–1181. doi: 10.1016/j.yjmcc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Horn MA, Graham HK, Richards MA, Clarke JD, Greensmith DG, Briston SJ, Hall MC, Dibb KM, Trafford AW. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Graham HK, Trafford AW. Spatial disruption and enhanced degradation of collagen with the transition from compensated ventricular hypertrophy to symptomatic congestive heart failure. Am J Physiol (Heart Circ Physiol) 2007;292:H1364–H1372. doi: 10.1152/ajpheart.00355.2006. [DOI] [PubMed] [Google Scholar]
- 41.Díaz ME, Graham HK, Trafford AW. Enhanced sarcolemmal Ca2+ efflux reduces sarcoplasmic reticulum Ca2+ content and systolic Ca2+ in cardiac hypertrophy. Cardiovasc Res. 2004;62:538–547. doi: 10.1016/j.cardiores.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 42.Wei S, Guo A, Chen B, Kutschke WJ, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-Tubule Remodeling During Transition From Hypertrophy to Heart Failure. Circ Res. 2010;107:521–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, Wu HD, Li RC, Zhang HB, Wang M, Tao J, Feng XH, Guo YB, Li SF, Lai ST, Zhou P, Li LL, Yang HQ, Luo GZ, Bai Y, Xi JJ, Gao W, Han QD, Zhang YY, Wang XJ, Meng X, Wang SQ. Mir-24 Regulates Junctophilin-2 Expression in Cardiomyocytes. Circ Res. 2012;111:837–841. doi: 10.1161/CIRCRESAHA.112.277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ, Wehrens XH. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100:44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlović D, McLatchie LM, Shattock MJ. The rate of loss of T-tubules in cultured adult ventricular myocytes is species dependent. Exp Physiol. 2010;95:518–527. doi: 10.1113/expphysiol.2009.052126. [DOI] [PubMed] [Google Scholar]
- 46.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 48.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, Streich JH, Korff B, Tuan HT, Hagen B, Luther S, Hasenfuss G, Parlitz U, Jafri MS, Hell SW, Lederer WJ, Lehnart SE. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of t-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo SH, Cleemann L, Morad M. Diversity of atrial local Ca2+ signalling: evidence from 2-D confocal imaging in Ca2+-buffered rat atrial myocytes. J Physiol. 2005;567:905–921. doi: 10.1113/jphysiol.2005.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei X, Li T, Hagen B, Zhang P, Sanchez PG, Williams K, Li S, Bianchi G, Son HS, Wu C, DeFilippi C, Xu K, Lederer WJ, Wu ZJ, Griffith BP. Short-term mechanical unloading with left ventricular assist devices after acute myocardial infarction conserves calcium cycling and improves heart function. JACC Cardiovasc Interv. 2013;6:406–415. doi: 10.1016/j.jcin.2012.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res. 2009;104:787–795. doi: 10.1161/CIRCRESAHA.108.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulson MN, Scriven DR, Fletcher P, Moore ED. Couplons in rat atria form distinct subgroups defined by their molecular partners. J Cell Sci. 2011;124:1167–1174. doi: 10.1242/jcs.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatano S, Yamashita T, Sekiguchi A, Iwasaki Y, Nakazawa K, Sagara K, Iinuma H, Aizawa T, Fu LT. Molecular and electrophysiological differences in the L-type Ca2+ channel of the atrium and ventricle of rat hearts. Circ J. 2006;70:610–614. doi: 10.1253/circj.70.610. [DOI] [PubMed] [Google Scholar]
- 56.Asghari P, Schulson M, Scriven DR, Martens G, Moore ED. Axial tubules of rat ventricular myocytes form multiple junctions with the sarcoplasmic reticulum. Biophys J. 2009;96:4651–4660. doi: 10.1016/j.bpj.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie YP, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, Wang LC, Weiss RM, Grumbach IM, Anderson ME, Song LS. Sildenafil prevents and reverses transverse-tubule remodeling and Ca2+ handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59:355–362. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, Zimmerman K, Weiss RM, Miller FJ, Anderson ME, Song LS. β-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J. 2012;26:2531–2537. doi: 10.1096/fj.11-199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wechsler-Reya R, Elliott K, Herlyn M, Prendergast GC. The putative tumor suppressor BIN1 is a short-lived nuclear phosphoprotein, the localization of which is altered in malignant cells. Cancer Res. 1997;57:3258–3263. [PubMed] [Google Scholar]
- 60.Kohout TA, O’Brian JJ, Gaa ST, Lederer WJ, Rogers TB. Novel adenovirus component system that transfects cultured cardiac cells with high efficiency. Circ Res. 1996;78:971–977. doi: 10.1161/01.res.78.6.971. [DOI] [PubMed] [Google Scholar]
- 61.Chen B, Guo A, Zhang C, Chen R, Zhu Y, Hong J, Kutschke W, Zimmerman K, Weiss RM, Zingman L, Anderson ME, Wehrens XH, Song LS. Critical roles of Junctophilin-2 in T-tubule and excitation-contraction coupling maturation during postnatal development. Cardiovasc Res. 2013;100:54–62. doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao W, Shi J, Dorn GW, Wei L, Rubart M. Spatial variability in T-tubule and electrical remodeling of left ventricular epicardium in mouse hearts with transgenic Gαq overexpression-induced pathological hypertrophy. J Mol Cell Cardiol. 2012;53:409–419. doi: 10.1016/j.yjmcc.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Landstrom AP, Kellen CA, Dixit SS, van Oort RJ, Garbino A, Weisleder N, Ma J, Wehrens XH, Ackerman MJ. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail. 2011;4:214–223. doi: 10.1161/CIRCHEARTFAILURE.110.958694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horiuchi-Hirose M, Kashihara T, Nakada T, Kurebayashi N, Shimojo H, Shibazaki T, Sheng X, Yano S, Hirose M, Hongo M, Sakurai T, Moriizumi T, Ueda H, Yamada M. Decrease in the density of t-tubular L-type Ca2+ channel currents in failing ventricular myocytes. Am J Physiol (Heart Circ Physiol) 2011;300:H978–H988. doi: 10.1152/ajpheart.00508.2010. [DOI] [PubMed] [Google Scholar]
- 65.Brette F, Sallé L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ Res. 2004;95:e1–e7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.