Abstract
Introduction
Irreversible electroporation (IRE) is considered superior to thermoablations for tumors in the vicinity of larger vessels and the liver hilum. We report on an initial clinical experience of IRE.
Materials and Methods
Indications included focal liver lesions <3 cm, irresectability due to contraindications and expected complications and/or irradicality following radiofrequency ablation (RFA). Ultrasound was chosen for guidance and needle placement.
Results
IRE was intended to perform in 14 patients with 1 procedure aborted due to technical failure. Among the 13 successfully treated were 7 percutaneous, 4 laparoscopic, and 2 open surgical procedures. The average age was 63 ± 10 years. Twelve solitary nodules and one bifocal disease were treated with an average size of 1.5 cm ± 0.5 cm. Median follow-up was 6 months. Three incomplete ablations account for 21% (3/14), 2 of them occurring in 2 metastases larger than 2 cm percutaneously treated with 5 needles instead of 4 used for smaller tumor sizes.
Conclusion
IRE was introduced without difficulties into clinical practice. As a main obstacle emerged in visualization of the needles, computed tomography may offer advantages in the guidance of percutaneous IRE of liver metastases larger than 2 cm. Local failure occurred in 21%.
Keywords: colorectal liver metastases, hepatocellular carcinoma, irreversible electroporation, minimal invasive liver surgery, ultrasound guided ablation
Introduction
The oncological value of local ablative treatment modalities thoroughly depend on achieving local tumor control. Risk factors for local failure are well known at least for radiofrequency ablation, and were profoundly examined by Mulier et al. in 2005 (Table I) [1]. Due to the high variability of local recurrence rates and for technical reasons influencing susceptibility for local recurrence of one or the other local ablative treatment modality, various alternative technologies have been suggested. The recently introduced irreversible electroporation (IRE) as a nonthermal ablation method seems to overcome some obstacles of extant local ablation techniques, namely, thermoablations.
Table I.
Risk factors of local recurrence following radiofrequency ablation according to univariate analysis (abbreviated, courtesy by S. Mulier, reprinted with permission)
| Risk factor for local recurrence | No. of patients included | p |
|---|---|---|
| Tumor size (>3/>5 cm) | 1817 | <0.001 |
| Entity metastasis (vs. HCC) | 4605 | <0.001 |
| Proximity to major vessel | 375 | <0.001 |
| Superficial tumor site | 70 | <0.001 |
| Percutaneous approach | 4424 | <0.001 |
| Safety margin ≤5 mm (vs. 10 mm) | 5224 | <0.001 |
| No pringle maneuvre performed | 4690 | <0.05 |
| Local anesthesia/sedation | 2491 | <0.001 |
| Modality of image guidance | 4341 | n.s. |
| Unexperienced interventionalist | 4495 | <0.001 |
| Early years of intervention | 5224 | n.s. |
| n.s. = not significant | ||
IRE utilizes an electrical high frequency field with at least 1000 to 1500 V/cm strength. At a molecular level, establishing such a field induces the opening of nanometer-sized pores in the cell membrane. According to the field strength, this event may be reversible, and the cells survive. In case of a voltage exceeding 1000 V/cm and more, the pores do not close again. Subsequently, cell content (mostly of the cell plasma) pours out of the cell. Further internal cell structures remain intact; the membrane does not depolarize. Afterwards – within 16 to 18 h – the cells undergo apoptosis. The mechanism of action leads to some unique features of IRE: tissue with a comparably high density of viable cells will receive most injury, whereas fibrotic tissue with a paucity of cells will almost be left unaffected. After the first experimental proof of concept research, preliminary data exist from clinical work and preclinical “non nocere” trials.
This report focuses on the feasibility and technical success of IRE under ultrasound guidance in the treatment of predominantly small solitary tumors with particular attention to the local control rate.
Materials and Methods
Indications for IRE were selected according to usual clinical criteria for local ablation in general (irresectability, small solitary tumor centrally sited in combination with a high probability for recurrent disease and/or not more than 3 tumors, absence of coagulation disorders, and normal blood cell count) with IRE chosen rather than radiofrequency ablation (RFA) or microwave coagulation therapy (MCT) in case of tumor nodules in the vicinity of larger vessels (predominantly portal or hepatic veins) and small tumors, as tumor size was initially limited to a largest diameter of 2 cm. Additionally, severe cardiac arrhythmia and inability to undergo general anesthesia were contraindications to IRE. All indications have been thoroughly discussed and approved by an interdisciplinary tumor board.
IRE was performed under ultrasound guidance in a fully equipped operation theatre. Patients underwent general anesthesia with deep sedation and complete muscle relaxation down to a zero train of four. We used the NanoKnife™ system of Angiodynamics (Queensbury, NY, US) as a source for generating alternating current (AC) energy. In all procedures, the AccuSync™ device for synchronization of the pulses using simultaneous electrocardiography (ECG) was employed. In general, the intention was to treat every tumor with a single ablation using four needles regardless of the actual size unless exceeding 2 cm in order to standardize needle placement under ultrasound control. For the most recent two applications, tumor sizes were allowed to increase up to 2.4 cm by adding a fifth needle placed least in the center of the nodule after having set the four first as usually. Replacement of the needles and repeated ablations were performed, if the sonographic appearance or the ablation data provided by the software of the generator suggested improper performance or an ablation area inadequately covering the tumor. The ultrasound platform in use was a Toshiba CoreVision Pro (Toshiba Medical Systems, Tokyo, Japan) with distinct sterile probes for intraoperative use in percutaneous, laparoscopic, and open surgical procedures. No additional image augmentation, navigation, contrast media, or virtual reality was applied.
Follow-up included contrast-enhanced magnetic resonance imaging after 2 and 6 weeks and every 3 months. As a contrast media, gadoxetic acid (Primovist™, Schering, Berlin, Germany) was used in a dosage adapted for the actual body weight. In case of recurrence, the patients were recommended to undergo any further treatment, which was reasonably felt feasible. Complications and follow-up data were prospectively collected.
Contiguous data are displayed as mean ± standard deviation in case of standard normal distribution as assessed by the Kolmogorov–Smirnov test, and median ± range in case of exclusion of standard normal distribution. Comparisons were accordingly performed using t-test, Mann–Whitney U‑test or Fisher’s exact test, respectively, where appropriate. Categorical data are shown as raw figures and are compared using the chi-square test. Due to the short follow-up period, neither survival analysis was obtained nor was logistic Cox regression accomplished for the small number of patients.
The authors of this article certify that they comply with the principles of ethical publishing in Interventional Medicine and Applied Science.
Results
Demographic data
Within 10 months of recruitment, 14 patients were identified meeting the inclusion criteria. One procedure was prematurely aborted due to technical failure of the generator; the patient subsequently underwent a successful microwave coagulation therapy and is currently free of disease. Among the 13 successfully treated IRE patients were 4 female and 9 male. The average age was 63 ± 10 years. Indications were colorectal liver metastases (n = 6), hepatocellular carcinoma (n = 5), and intrahepatic recurrent cholangiocarcinoma (n = 2). Twelve solitary nodules were treated; in one case, bifocal disease underwent percutaneous IRE. Thus, a total of 14 tumors were treated. Seven procedures were performed percutaneously, four laparoscopically, and two open surgically, each of the latter combined with a hepatic resection. Two laparoscopies were also accompanied by additional resections. One percutaneous IRE was combined with simultaneous hernia repair. The tumor size averaged out at 1.5 ± 0.5 cm. The median follow-up period was 6 months (range 3 to 12 months). Table II shows all patients at a glance.
Table II.
Complete record of patients
| Pat. | Age | Access | Diagn. | Sim. proc. | n | Size (mm) | f-u (months) |
|---|---|---|---|---|---|---|---|
| 1 | 49 | Percut. | CRC | None | 1 | 20 | 12 |
| 2 | 55 | Laparoscop. | HCC | Resection | 1 | 13 | 11 |
| 3 | 68 | Laparoscop. | HCC | None | 1 | 14 | 10 |
| 4 | 73 | Open surg. | CRC | Resection | 1 | 6 | 10 |
| 5 | 63 | Laparoscop. | HCC | Resection | 1 | 15 | 10 |
| 6 | 65 | Percut. | CCC | None | 2 | 17/14 | 9 |
| 7 | 66 | Percut. | CRC | None | 1 | 16 | 8 |
| 8 | 58 | Percut. | CCC | Hernia repair | 1 | 15 | 8 |
| 9 | 61 | Percut. | HCC | None | 1 | 14 | 7 |
| 10 | 52 | Open surg. | CRC | Resection | 1 | 12 | 7 |
| 11 | 87 | Percut. | CRC | None | 1 | 22 | 6 |
| 12 | 72 | Percut. | CRC | None | 1 | 24 | 4 |
| 13 | 53 | Laparoscop. | HCC | None | 1 | 10 | 3 |
| Abbr.: pat. – patient identification, age – in years, CRC – colorectal liver metastases, HCC – hepatocellular carcinoma, CCC – recurrent cholangiocarcinoma, sim. proc. – simultaneous procedure | |||||||
Intraoperatively
From a technical point of view, all procedures but one succeeded. No difficulties occurred during the stay in the operation theatre with handling or mutual interdisciplinary collaboration, since, besides an interventional radiologist and a surgeon, both intraoperative electrocardiography and anesthesiologic muscle relaxation and sedation had to be engaged. No treatment associated complication occurred. Average hospital stay was 2, 4, and 9 postoperative days, respectively, according to the chosen access route. Duration of the procedure varied depending on the access route, again, and simultaneous procedures (Table III).
Table III.
Comparison of different ways of access to IRE
| Percutaneous | Laparoscopy | Open surgery | p | |
|---|---|---|---|---|
| Number of patients | 7 | 4 | 2 | – |
| Duration of the procedure a | 62 ± 27 min | 155 ± 75 min | 240 ± 10 min | 0.031e |
| Hospital stay b | 2 days | 4 days | 9 days | 0.015f |
| Ratio HCC:Mets | 3d:4 | 4:0 | 0:2 | 0.592g |
| Local recurrencies | 3 | 0 | 0 | 0.192g |
| Abbr.: min – minutes, HCC –
hepatocellular carcinoma, Mets – metastases a Mean ± standard deviation b Median d Including two cases of recurrent cholangiocarcinoma e t-test f Kruskal–Wallis test g Fisher’s exact test | ||||
Outcome
All patients survived until the end of the follow-up period. Three tumors were incompletely ablated (21.4% [3/14]), all of them after percutaneous IRE: the only treated bifocal case experienced local recurrence in one of both treated tumor sites, and two further local failures were found in the most recently treated patients with colorectal metastases, both exceeding 2 cm in size, which have been treated using five needles instead of four. The former patient suffered from multifocal recurrent cholangiocarcinoma and had already been scheduled to interstitial brachytherapy (CT-HDBRT) before, whereas the other patients underwent interstitial brachytherapy as a salvage treatment. One patient with a colorectal carcinoma metastasis (Figs 1 and 2) received chemotherapy and developed another metastasis treated by microwave coagulation therapy. She later underwent hepatic resection for recurrent metastases in combination with intestinal reanastomosing. One laparoscopically ablated patient later received transarterial chemoembolization (TACE) for diffuse intrahepatic recurrence of hepatocellular carcinoma. The eventual rate of overall recurrence (i.e., all local, regional and systemic tumor recurrence detected), hence, was 38%. The rate of local recurrence due to incomplete ablations resembles 21% on a per-tumor basis. Notably, no case of local recurrence emerged at the end of the comparably short follow-up period following a surgical approach (laparoscopic or open surgical).
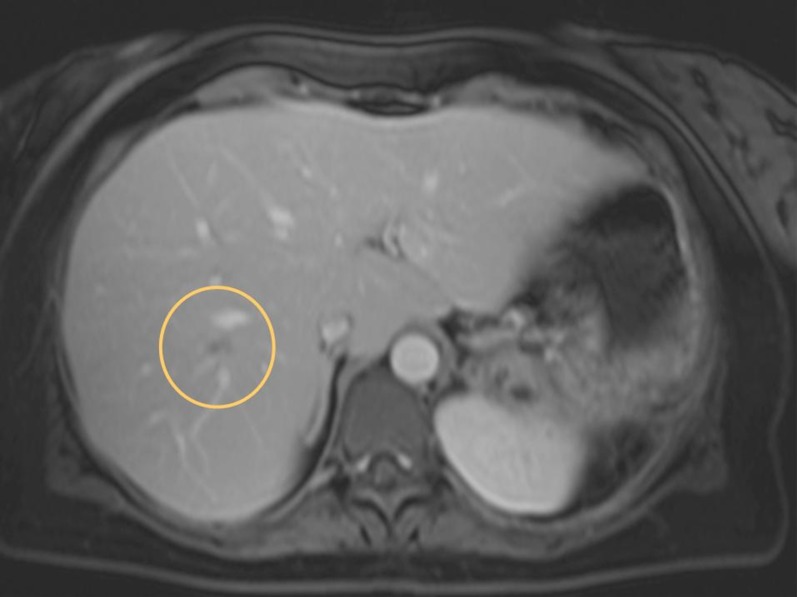
Fig. 1.
Colorectal liver metastasis prior to irreversible electroporation (encircled). Note the centrally sited tumor localisation in the vicinity of large hepatic venous branches
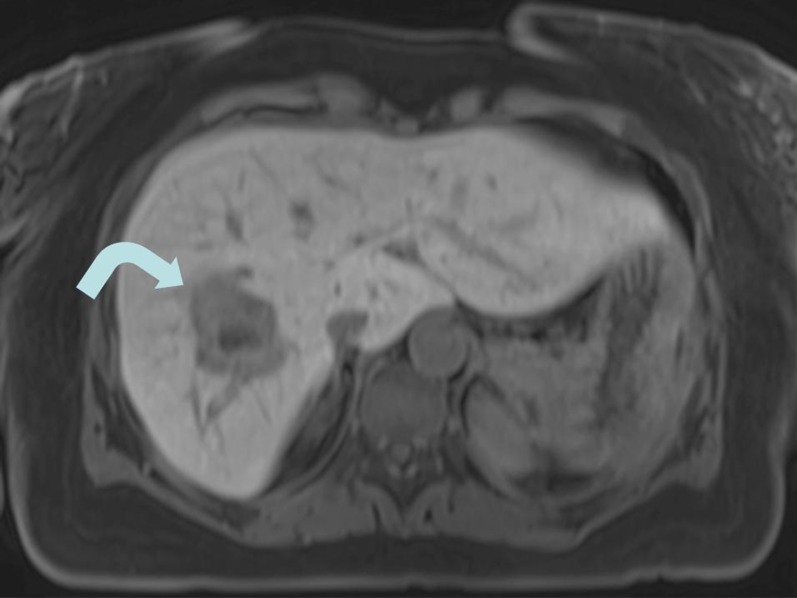
Fig. 2.
Ablation area (arrow) after irreversible electroporation, encompassing the tumor volume thoroughly
Discussion
Apart from case reports, clinical experience with hepatic IRE is limited so far. Altogether, preliminary reports account for 72 patients in two publications [2, 3]. Apart from a couple of case reports [4–6], abstracts displayed at the 2012 meeting of the Society for Interventional Radiology report on another 106 treatments thoroughly reviewed by Charpentier [7]. Melbourne/Australia reports on 11 patients with hepatocellular carcinoma (HCC) [8]. A Dutch group presented further 5 patients in an abstract presented at the Interventional Treatment of Liver Tumor meeting 2013 [9]. The same group published a review this year summarizing the first 129 patients treated worldwide [10]. Our single-center experience adds another 13 cases to the worldwide experience, thus, contributing to an emerging knowledge in how to use the comparably new IRE within the context of the armamentarium of interventional treatment modalities.
Lee et al. summarized the history and advantages of IRE 2010 [11] and described six particularly outstanding features of IRE: short ablation time, preservation of vital structures within IRE-ablated zone, avoidance of heat/cold-sink effect, IRE-induced complete ablation with well-demarcated margin, IRE-induced apoptotic cell death, and real-time monitoring of IRE ablation. We were not able to compare different imaging modalities during IRE, since electrode placement and targeting the procedure were only performed under ultrasound control. Apart from difficulties in defining the appropriate needle paths, the IRE applicator needle itself proved to be adequately visible in real-time ultrasound. Whereas some of the characteristics suggested by Lee et al. could not be confirmed, as no specimens for histological examination have been sampled, our initial impressions suggest feasibility and efficacy of IRE in tumor localizations, where we would have been reluctant to consider a thermoablation due to the vicinity of a large blood vessel or a vital structure in the liver hilum. However, the comparably short ablation time seems to be compensated by the difficult multiple placement of parallel needles.
Our clinical experience led us to analyze the incomplete ablations in the presented series, which resulted in the identification of three clearly distinct risk factors for local failure of IRE: 1) use of percutaneous access for IRE, 2) treatment of colorectal liver metastasis as an indication for IRE instead of primary liver cancer, and 3) tumor size exceeding a maximum of 2.0 cm even in case of five needles used for IRE.
The tumor size seems to be limited to the four-needle approach; however, even the addition of a fifth needle did not ameliorate the problems of correct needle placement under ultrasound control. The software of the generator suggests a certain number of needles for the treatment of a given target area; however, occasionally, the designated ablation area may only be created, if the needles can be placed correctly and accurately without pardoning a tiny degree of deviation. Not only the distance of the electrodes has to remain exact, but also the parallelism of the insertion path has to be maintained. Obviously, even in the treatment of small tumors, the choice of more than the recommended number of needles seems to be appropriate.
There is no data available comparing IRE of different types of tumor tissue. So far, it remains highly speculative, whether metastases are more difficult to treat with IRE than primary liver tumors like hepatocellular carcinoma. If so, the application of IRE resembled some similarities to thermoablative treatment modalities, since tumors in cirrhotic liver tissue tend to be more amenable to heating due to the insulating effect of the surrounding liver tissue. This may partly be explained by the observation that a certain degree of tissue damage induced by IRE may also be induced by a kind of thermoablation mimicking effects [12].
Percutaneous access accounts for the majority of local failures in this series of patients. This may be explained by sonographic guidance using a nondigital intraoperative ultrasound platform without the opportunity of contrast enhancement, three-dimensional image reconstruction, image fusion, or virtual reality scenarios. Cross-sectional imaging modalities are in widespread use for guiding and targeting local ablations. They offer the advantage of reformatted slices in different – also oblique – levels prior to the application of alternating current in order to confirm the exact needle positions. In the future, the use of more than one imaging modality at once for targeting IRE is most likely, i.e., needle placement under real-time ultrasound control and confirmation of correct needle positions using a cross-sectional imaging modality, e.g., either computed tomography or nuclear magnetic resonance.
In accordance with several “non nocere” trials, we did not find significant morbidity following IRE. Contrasting to occasionally fatal complications following a thermal ablation [13], no major complication classified III or higher according to Clavien et al. [14] occurred. The intraoperative accomplishment of sedation and muscle relaxation was not challenging. Ball et al. pointed out some key elements for anesthesia supporting IRE [15]. Use of ECG triggering ameliorated much embarrassment concerning cardiac arrhythmia potentially induced by the electric pulses. Postoperative pain level was unequivocally low. A noninferiority analysis of postprocedural pain after RFA and IRE revealed no significant difference among the different local ablative techniques [16]. Our overall impression confirms these data: none of our patients suffered from intractable pain following IRE postoperatively.
The international clinical experience with hepatic IRE is limited so far. Ali et al. report on 29 ablations performed in the liver [17]. Only two explant specimen were available for histological examinations revealing complete local tumor control. Notably, only one local recurrence was radiographically observed following laparoscopic IRE, which markedly resembles our own experience with excellent success obtained by adopting a surgical (laparoscopic, open-surgical) access route. A multicenter European phase II trial is presented by Lencioni et al. [18]: 26 HCC patients have been treated with a local failure rate of 21% resembling a similar rate of incomplete ablations found in our series presented herewith, whereas our follow-up period was significantly longer with 6 months in comparison to 1 month in the presented multicenter trial [18]. The complication rate was low in both reports. Cheung et al. provide additional data on 11 HCC patients [8] resembling a local failure rate with 28% similar to the figure presented herein. He also confirms tumor size as a paramount risk factor for local tumor control. The most recently published report on hepatic IRE in eleven patients emphasizes the risk of local tumor recurrence accompanied with IRE with 27% local failures based on the number of treated tumors [19]. The focus of the study was however on the safety of percutaneous IRE in the treatment of peribiliary tumors, and the median tumor size was with 3 cm comparably large. The colleagues from Erlangen, Germany found likewise either incomplete ablation or local recurrence in 22% on a per-tumor and 29% on a per-patient basis (n = 14). They considered their results satisfying with regard to the so-called “critical lesion locations” [20].
Further investigations are necessary in order to define the role of IRE within the context of local and regional treatment options in the liver. The clinical observations published so far do not suggest superiority of IRE to any of the alternatively available thermoablative treatment options. However, our study reveals some insights in a growing expertise with IRE and a subsequently rising learning curve. If the technical difficulties of IRE and uncertainties of the effects on healthy and tumor tissue are overcome, IRE may complement our armamentarium of local and regional treatment options favorably.
Funding Statement
Funding sources: No financial support was received for this study.
Footnotes
Author's Contributions: RME wrote the paper and performed the procedures; SSC read and corrected the paper; MG read, critically corrected, and approved the paper; BG performed the procedures and reviewed the paper.
Conflict of interest: The authors declare no conflict of interest.
Contributor Information
Robert M. Eisele,
Sascha S. Chopra,
Matthias Glanemann,
Bernhard Gebauer,
References
- 1.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005 Aug;242(2):158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC., 2nd Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013 Apr;107(5):544–549. doi: 10.1002/jso.23280. [DOI] [PubMed] [Google Scholar]
- 3.Kingham TP, Karkar AM, D'Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB, Jarnagin WR, Fong Y. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg. 2012 Sep;215(3):379–387. doi: 10.1016/j.jamcollsurg.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Kasivisvanathan V, Thapar A, Oskrochi Y, Picard J, Leen EL. Irreversible electroporation for focal ablation at the porta hepatis. Cardiovasc Intervent Radiol. 2012 Dec;35(6):1531–1534. doi: 10.1007/s00270-012-0363-7. [DOI] [PubMed] [Google Scholar]
- 5.Mannelli L, Padia SA, Yeung RS, Green DE. Irreversible electroporation of a liver metastasis. Liver Int. 2013 Jan;33(1):104. doi: 10.1111/liv.12000. [DOI] [PubMed] [Google Scholar]
- 6.Niessen C, Jung EM, Stroszczynski C, Wiggermann P. [Ablation of a liver metastasis with irreversible electroporation (IRE) in liver segment II adjoining the area nuda] Rofo. 2012 Oct;184(10):937–938. doi: 10.1055/s-0032-1312961. [DOI] [PubMed] [Google Scholar]
- 7.Charpentier KP. Irreversible electroporation for the ablation of liver tumors: are we there yet? Arch Surg. 2012 Nov;147(11):1053–1061. doi: 10.1001/2013.jamasurg.100. [DOI] [PubMed] [Google Scholar]
- 8.Cheung W, Kavnoudias H, Roberts S, Szkandera B, Kemp W, Thomson KR. Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat. 2013 Jun;12(3):233–241. doi: 10.7785/tcrt.2012.500317. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen K, Scheffer HJ, vanTilborg AAJM, Bouwman A, Vieveen JM, Niessen JWM, Krijnen PAJ, Meijer S, Meijerink MR, van den Tol MP. Irreversible electroporation for the local treatment of tumors: a new kid on the block. Results of an “ablate and resect” pilot-study of resectable colorectal liver metastases. Dig Liver Dis. 2013;45(Suppl 3):S257. doi: 10.1016/S1590-8658(13)60689-1. [DOI] [Google Scholar]
- 10.Scheffer HJ, Nielsen K, de Jong MC, van Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, vanKulik C, vandenTol PM, Meijerink MR. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014 doi: 10.1016/j.jvir.2014.01.028. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver. 2010 Sep;4(Suppl 1):S99–S104. doi: 10.5009/gnl.2010.4.S1.S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faroja M, Ahmed M, Appelbaum L, Ben-David E, Moussa M, Sosna J, Nissenbaum I, Goldberg SN. Irreversible electroporation ablation: is all the damage nonthermal? Radiology. 2013 Feb;266(2):462–470. doi: 10.1148/radiol.12120609. [DOI] [PubMed] [Google Scholar]
- 13.Hatzidakis A, Zervakis N, Krokidis M. Fatal arterial hemorrhage after microwave ablation of multiple liver metastases: The lessons learned. Interv Med Appl Sci. 2013 Sep;5(3):140–143. doi: 10.1556/IMAS.5.2013.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992 May;111(5):518–526. [PubMed] [Google Scholar]
- 15.Ball C, Thomson KR, Kavnoudias H. Irreversible electroporation: a new challenge in "out of operating theater" anesthesia. Anesth Analg. 2010 May 1;110(5):1305–1309. doi: 10.1213/ANE.0b013e3181d27b30. [DOI] [PubMed] [Google Scholar]
- 16.Narayanan G, Froud T, Lo K, Barbery KJ, Perez-Rojas E, Yrizarry J. Pain analysis in patients with hepatocellular carcinoma: irreversible electroporation versus radiofrequency ablation-initial observations. Cardiovasc Intervent Radiol. 2013 Feb;36(1):176–182. doi: 10.1007/s00270-012-0426-9. [DOI] [PubMed] [Google Scholar]
- 17.Ali NS, Cohn S, Raofi V. Single center experience with irreversible electroporation for liver and pancreas tumor ablation. HPB. 2012;14(Suppl 1):2. doi: 10.1111/j.1477-2574.2012.00437.x. [DOI] [Google Scholar]
- 18.Lencioni R, Izzo F, Crocetti L, Vilgrain V, Abdel-Rehim M, Bianchi L, Ricke J, Peck M, Bruix J. A prospective, multicentre phase II clinical trial using irreversible electroporation for the treatment of early stage HCC. J Vasc Interv Radiol. 2012;23:1114. doi: 10.1016/j.jvir.2012.05.018. [DOI] [Google Scholar]
- 19.Silk MT, Wimmer T, Lee KS, Srimathveeravalli G, Brown KT, Kingham PT, Fong Y, Durack JC, Sofocleous CT, Solomon SB. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014 Jan;25(1):112–118. doi: 10.1016/j.jvir.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Eller A, Schmid A, Schmidt J, May M, Brand M, Saake M, Uder M, Lell M. Local control of perivascular malignant liver lesions using percutaneous irreversible electroporation: initial experiences. Cardiovasc Intervent Radiol. 2014 doi: 10.1007/s00270-014-0898-x. epub ahead of print. [DOI] [PubMed] [Google Scholar]