Abstract
Objectives:
The decision tree underlying current practice guidelines for post polypectomy surveillance relies on risk stratification based on predictive attributes gleaned from adenomas removed on screening colonoscopy examination. Our primary aim was to estimate the magnitude of association between baseline adenoma attributes and the risk of adenoma recurrence and invasive colorectal adenocarcinoma (CRC). Our secondary aims were to estimate the adenoma detection rate (ADR) of surveillance compared with screening colonoscopies and describe time trends in preventive colonoscopy utilization.
Methods:
We used prospective analyses of retrospectively collected clinical data from electronic health records. A cohort of primary care patients eligible for colorectal cancer screening was assembled encompassing 110,452 subjects, of which 3,300 had adenomas removed on screening examination. Of those patients who had a follow-up surveillance colonoscopy (defined as a patient with a documented adenoma on prior colonoscopy) recorded during the study period, 537 had a recurrent adenoma.
Results:
Of those recurrent adenomas, 354 had a high-risk attributes. High-risk attributes were described at >3 adenomas, at least one adenoma >10 mm in size, high-grade dysplasia, or villous features. The risk of developing invasive CRC among post polypectomy patients was significantly higher if the baseline adenomas displayed any of the following attributes: more numerous than 3 (4.3-fold higher risk, 95% confidence interval (CI) low, high 1.4, 12.9), larger than 10 mm in size (5.2-fold higher risk, 95% CI low, high 1.8, 15.1), high-grade dysplasia (13.2-fold risk, 95% CI low, high 2.8, 62.1), or villous features (7.4-fold higher risk, 95% CI low, high 2.5, 21.5). These attributes combined added a net value of 22.8% to the probability of correctly predicting CRC. There was a threefold increase in surveillance utilization relative to screening from 2005 to 2011. The ADR of surveillance (34.1%) was 1.5-fold higher than that of screening (23.1%).
Conclusions:
These results emphasize the need to mitigate excessive risk by performing timely surveillance colonoscopies in patients with baseline adenomas displaying high-risk attributes as recommended in practice guidelines.
INTRODUCTION
Colonoscopy with polypectomy has been used as the first-line modality for colorectal cancer (CRC) prevention at Geisinger Health System (GHS) and many other provider systems in the developed world for well over a decade.1, 2, 3, 4, 5, 6, 7 Adenomatous polyps can be identified in 20–40% of patients undergoing screening colonoscopy,8 and their occurrence is associated with increased risk of CRC.9, 10, 11, 12, 13, 14 Even when all visible baseline adenomas are completely removed by polypectomy, the risk of future recurrence of metachronous adenomas remains,15 and the patient is in need of periodic surveillance examinations.16 There are four attributes of the baseline adenomatous polyp for which there is evidence of strong effects on the risk of recurrence, namely the number (≥3) and size (≥10 mm) of adenomas, and the presence of high-grade dysplasia or villous morphology.3, 5, 17
Timing of recall surveillance colonoscopy examinations is based on a decision rule that incorporates information on these four high-risk attributes of baseline lesions,18 as set forth in published3, 5, 17 and revised2 practice guidelines for surveillance colonoscopy. Briefly, the American College of Gastroenterology practice guidelines dictates the following surveillance intervals in patients with baseline average risk. The presence of 1–2 small (<10 mm) tubular adenomas requires a 5- to 10-year recall. If there are 3–10 adenomas, adenomas >10 mm, villous adenoma, adenomas high-grade dysplasia, or serrated lesions, recommended surveillance intervals are 3 years. In patients with >10 tubular adenomas or serrated polyposis syndrome, recall exam is suggested in <3 and 1 years, respectively.2 Use of these 4 features is instrumental in determining what time interval between repeat surveillance examinations will provide sufficient mitigation of interval CRC risk without exposing the patient to excessive cost and procedural risk associated with overly intensive surveillance regimens.5, 7, 8, 19, 20, 21, 22, 23 Yet, the authors of the guideline point out a need for more evidence to add precision to the magnitude of effect estimates used in the guideline's underlying decision rule.2
This study aims to contribute such evidence for the next iteration of the guideline's updating cycle. We used prospective analyses of retrospectively collected data to estimate the strength of associations between baseline adenoma attributes and the risk of adenoma recurrence and invasive CRC. We also estimated the adenoma detection rate (ADR) of surveillance and screening colonoscopy, thereby contributing data for the formation of quality benchmarks. Finally, we described a time trend in colonoscopy utilization.
METHODS
Setting
GHS is a not-for-profit integrated health-care organization providing primary, specialty and tertiary health-care services to residents of 31 counties in central and northeastern PA. Facilities relevant to this study included 42 community-based primary care clinics, 6 gastrointestinal endoscopy platforms and an anatomic pathology center. GHS maintains a proactive CRC prevention program in which colonoscopy is offered to all age-eligible subjects during primary care visits, as well as via mail, telephone and public awareness campaigns. This study was deemed minimal risk human subjects research using previously collected data and approved by the Geisinger Institutional Review Board on 16 May 2011 (protocol no. 2011-0198).
Study design
The denominator population included 110,452 primary care patients of an integrated health-care organization in central and northeastern Pennsylvania who were age-eligible for screening colonoscopy (50+ years of age) between 1 January 2004 and 31 March 2011, of which 34,254 underwent ≥1 colonoscopies. There were 8,619 patients excluded as their indication for the “screening” examination was for signs or symptoms of underlying pathology (iron deficiency anemia, gastrointestinal bleeding, diarrhea, etc.). There were then 25,635 patients included in the study that had a true “screening” examination (Figure 1). Nested within the denominator of patients who had a screening colonoscopy was the post polypectomy cohort, which included all patients with pathology-verified adenomas removed at screening colonoscopy (N=3,300). Denominator cohort inclusion: (i) age ≥50 years old on the cohort entry date; (ii) at least 1 encounter at a GHS primary care office in 1 of 42 community-based practice sites for any chief complaint or well visit; (iii) at least 2 encounters on record with GHS providers between 1 January 2004 and 31 March 2011, which included the above-stated primary care visit and (iv) did not have (as of the cohort entry date) a record of previous colonoscopy or prior diagnosis of CRC or colonic polyps in the proceeding 8 years. Post polypectomy subcohort inclusion: (i) met criteria for inclusion in the denominator cohort; (ii) had a screening colonoscopy; and (iii) had at least 1 pathology-verified adenoma diagnosed at that screening colonoscopy.
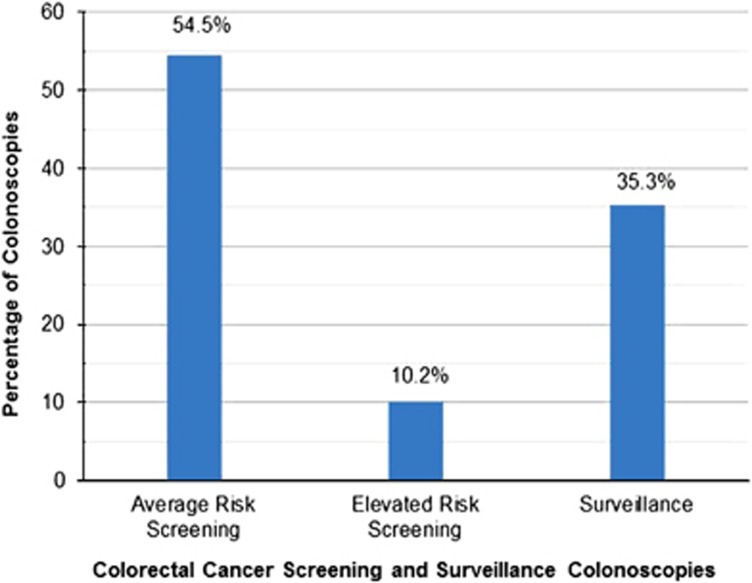
Figure 1.
Colonoscopies according to clinical indication. The clinical indication for each of the 25,635 preventative maintenance colonoscopies was ascertained. The indications were classified into average risk screening, elevated risk screening, and surveillance examinations. Average risk screening was defined as a person who did not have symptoms, or had a family history of colorectal cancer in only one first-degree relative older than 60 years of age. Elevated risk screening was defined as an asymptomatic person who had a family history of one first-degree relative diagnosed with colorectal cancer at age 60 years or younger or who had two first-degree relatives diagnosed with colorectal cancer at any age.
Temporal definitions
The denominator cohort entry date was defined as the first encounter on record between 1 January 2004 and 31 March 2011. The exit date was defined as the earliest of the following: (i) death and (ii) loss to follow-up (i.e., 365 days without an encounter). An encounter includes any activity in the electronic health record (EHR), which includes, but is not limited to medication refills, patient communication in any aspect of the medical record, clinic, laboratory, or ancillary service visit; (iii) first diagnosis of invasive colorectal adenocarcinoma; or (iv) administrative censoring on 31 March 2011. For patients included in the post polypectomy subcohort, follow-up began 1 day after baseline (baseline was defined as the date of the screening colonoscopy) and ended on the exit date.
Data collection
EHR data review
GHS has used EpicCare (Epic Corporation, Verona, WI) since 2001 as its central EHR platform. Two other specialized EHR systems are relevant: (1) ProVation (ProVation Medical, Minneapolis, MN), serving the Endoscopy Centers; and (2) CoPathPlus (Sunquest Information Systems, Tucson, AZ), serving the Anatomic Pathology Laboratory. All predictor and outcome variables for this study were retrieved from the EHR. Text parsing of colonoscopy and pathology notes was performed using a PERL Regular Expression-based text parser implemented in SAS 9.3 for Windows (SAS Institute, Cary, NC), similar to that used by Hinchcliff et al.24
Endoscopy utilization
The total number of colonoscopies was evaluated by the above data collection parameters. A review of available endoscopy time slots was accomplished by evaluating the allotted monthly number of staffed endoscopy time slots and the actual number of cases performed during that time period. This utilization data was reviewed for the time course during which the data was collected.
Study variables
Clinical indication was ascertained from the colonoscopy report and classified into the following mutually exclusive categories: (i) screening (average risk); (ii) screening (elevated risk); (iii) post polypectomy surveillance; (iv) signs and symptoms (i.e., workup for iron deficiency anemia, rectal bleeding, heme positive stool, etc.); or (v) inflammatory bowel disease (IBD; follow-up examination of subjects with pre-existing diagnosis of IBD). Average risk screening was defined as a person who did not have symptoms or had a family history of colorectal cancer in only 1 first-degree relative older than 60 years of age. Elevated risk screening was defined as an asymptomatic person who had a family history of 1 first-degree relative diagnosed with colorectal cancer at age 60 years or younger or who had 2 first-degree relatives diagnosed with colorectal cancer at any age.
Prevention colonoscopies
were defined as those performed on asymptomatic subjects for clinical indications of “screening” or “surveillance”.
Lesion type was ascertained from the pathology report and classified into the following mutually exclusive categories: (i) invasive adenocarcinoma; (ii) adenoma (any adenoma including tubular, sessile, or flat). High-risk lesions including villous changes and high-grade dysplasia are further subcaterogized as stated below; (iii) hyperplastic polyp; or (iv) other findings (e.g., leiomyomas, ganglioneuroma, condyloma, melanosis coli, neurofibroma, lipoma, histiocytoma, secondary tumors, findings consistent with inflammatory etiology (active or inactive, infectious or IBD), findings consistent with ischemic etiology, etc.
High-risk baseline adenoma attributes
Four dichotomous variables (1 per attribute) were assessed per colonoscopy: (i) multiplicity (positive if found ≥3 adenomas); (ii) size (positive if found ≥1 adenoma of size ≥10 mm); (iii) high-grade dysplasia (positive if found ≥1 adenoma exhibiting high-grade dysplasia); and (iv) villous features (positive if found ≥1 adenoma exhibiting villous morphology).
Surveillance guidelines
The surveillance program was based on the American College of Gastroenterology guidelines from that time period (2000). Once an adenoma was detected in a patient with an average risk baseline, they were placed in the following colonoscopy surveillance program. Patients who had 1–2 small tubular adenomas with low-grade dysplasia required a 5- to 10-year recall. Patients with 3–10 adenomas, an adenoma >10 mm, a villous adenoma, or any polyp with high-grade dysplasia, required a 3-year recall.25 Additional recommendations from the 2008 screening guidelines were added to our clinical practice. Patients with >10 adenomas on an examination require a recall <3 years after initial polypectomy. Sessile adenomas that were removed piecemeal required 2–6 months follow-up to verify complete removal.3
Adenoma detection rate
The ADR is a calculated value based on the number of total colonoscopies performed with the detection of at least 1 adenoma divided by the total number of colonoscopies performed for each indication (i.e., the ADR was calculated independently for screening and surveillance colonoscopies). The ADR is based on individual polyp data extracted from pathology notes for each colonoscopy using the text parsing as stated above.
Smoking status was ascertained from the Epic Rooming Tool (a tool designed by GHS using epic functionality to gather specific information required by joint commission) and dichotomized (ever vs. never).
History of IBD was coded as present if ≥2 occurrences of International Classification of Diseases, Ninth Revision, (ICD-9) codes 555.xx, 556.xx or 558.xx were found in the patient's electronic record.
Family history of cancer (any site) was ascertained from the Epic Family History Questionnaire and coded as present if the patient reported having ≥1 first-degree or ≥2 second-degree relatives with any type of cancer. This variable represents history taken by the primary care provider, and was used in multivariate analysis of the denominator cohort (Table 1).
Table 1. Population characteristics.
|
Colonoscopy uptakea |
Odds ratio (95% CI low, high) | P value | ||
|---|---|---|---|---|
| Yes | No | |||
| Sex, n (row %) | ||||
| Female | 13,179 (21.6) | 47,830 (78.4) | 0.80 (0.77, 0.82) | <0.0001 |
| Male | 12,456 (25.2) | 36,987 (74.8) | 1.00 (reference) | |
| Mean age (s.d.) | 62.7 (8.7) | 66.5 (10.7) | 0.96 (0.96, 0.97) | <0.0001 |
| Mean BMI (kg/m2)b (s.d.) | 33.9 (7.2) | 33.4 (7.6) | 1.00 (0.99, 1.00) | 0.8 |
| FHx of cancerb n, (row %) | ||||
| Yes | 11,797 (29.1) | 28,780 (70.9) | 1.50 (1.49, 1.58) | <0.0001 |
| No | 13,838 (19.8) | 56,037 (80.2) | 1.00 (reference) | |
| IBDc n, (row %) | ||||
| Yes | 2,056 (41.2) | 2,936 (58.8) | 2.50 (2.30, 2.60) | <0.0001 |
| No | 23,579 (22.4) | 81,881 (77.6) | 1.00 (reference) | |
Abbreviations: BMI, body mass index; CI, confidence interval; IBD, inflammatory bowel disease.
Subjects deemed accepting if received ≥1 colonoscopies in query period.
FHx of cancer: ≥1 first-degree or ≥2 second-degree relatives with cancer at any site.
History of diagnosed IBD.
Family history of CRC was ascertained from the colonoscopy note. This variable represents history taken by the endoscopist on the day of the procedure and was used in multivariate analysis of the post polypectomy subcohort (Tables 2, 3, 4). Family history defines “average risk screening” as a person without a family history of colorectal cancer or a family history of colorectal cancer in only 1 first-degree relative >60 years of age. Family history defines “elevated risk screening” as a person who has a family history with at least 1 first-degree relative diagnosed with colorectal cancer at age 60 years or younger or who had 2 first-degree relatives diagnosed with colorectal cancer at any age. Of note, the family history was taken by patient recollection and the charts of the relatives were not entered.
Table 2. Adenoma detection rate and adenocarcinoma detection yield in screening (baseline) and surveillance (follow-up) colonoscopies.
| Indication |
Number of colonoscopies (rate and percentage of total in category) |
|||
|---|---|---|---|---|
| Any adenoma | Advanced adenoma | ICRC | Total in indication category | |
| Screening (average risk) | 3,048 (23.3) | 2,001 (15.3) | 100 (0.8) | 13,087 |
| Screening (elevated risk) | 542 (22.2) | 356 (14.6) | 17 (0.7) | 2,436 |
| Surveillance | 2,890 (34.1) | 1,890 (22.3) | 110 (1.3) | 8,472 |
Advanced adenoma: adenoma with ≥1 risk attributes; any adenoma: any adenoma (including tubular, sessile, or flat) with or without risk attributes; ICRC: incident invasive colorectal adenocarcinoma.
Average risk screening was defined as a person who did not have symptoms or had a family history of colorectal cancer in only one first-degree relative older than 60 years of age. Elevated risk screening was defined as an asymptomatic person who had a family history of one first-degree relative diagnosed with colorectal cancer at age 60 years or younger or who had two first-degree relatives diagnosed with colorectal cancer at any age.
Table 3. Effects of baseline adenoma attributes on the odds of adenoma recurrence, development of an advanced adenoma, or incident invasive colorectal adenocarcinomaa.
| Predictor | Grouped as |
Most advanced lesion in follow-up
Odds ratio (95% CI low, high;
P
value) |
||
|---|---|---|---|---|
| Any adenoma N=537/3,300 | Advanced adenoma N=354/3,300 | ICRC N=14/3,300 | ||
| Number of adenomas | ≥3 vs. 1 or 2 | 1.8 (1.5, 2.2; <0.0001) | 2.4 (1.9, 3.0; <0.0001) | 4.3 (1.4, 12.9; 0.01) |
| Size | ≥10 mm vs. 1–9 | 3.1 (2.5, 3.8; <0.0001) | 3.6 (2.8, 4.5; <0.0001) | 5.2 (1.8, 15.1; 0.03) |
| High-grade dysplasia | Present vs. absent | 3.9 (2.1, 7.4; <0.0001) | 4.3 (2.2, 8.4; <0.0001) | 13.2 (2.8, 62.1; 0.001) |
| Villous morphology | Present vs. absent | 2.7 (2.2, 3.4; <0.0001) | 3.7 (2.9, 4.7; <0.0001) | 7.4 (2.5, 21.5; 0.01) |
Abbreviations: ICRC, incident invasive colorectal adenocarcinoma.
Advanced adenoma: adenoma with ≥1 risk attributes. Risk attributes were defined at namely the number (≥3) and size (≥10 mm) of adenomas, and the presence of high-grade dysplasia or villous morphology; any adenoma: any adenoma (including tubular, sessile, or flat) with or without risk attributes; ICRC, incident invasive colorectal adenocarcinoma.
Analysis adjusted for age, sex, smoking status, and family history of CRC.
Table 4. Predictive performance of baseline adenoma attributes for predicting adenoma recurrence and invasive colorectal adenocarcinoma.
| Any recurrent adenoma, N=537/3,300 |
ROC-AUC (P
value) |
||
|---|---|---|---|
| Advanced adenoma, N=354/3,300 | ICRCN=14/3,300 | ||
| A. Covariates onlya | 59.4% (<0.0001) | 58.6% (<0.0001) | 65.5% (0.03) |
| B. Covariates and attributesa | 69.0% (<0.0001) | 72.2% (<0.0001) | 88.3% (<0.0001) |
| Net gainb | 9.6% (<0.0001) | 13.6% (<0.0001) | 22.8% (<0.0001) |
Abbreviations: ICRC, invasive colorectal cancer; ROC-AUC, receiver operating characteristic-area under the curve.
Any recurrent adenoma: any adenoma (including tubular, sessile, or flat) with or without risk attributes.
Attributes: Advanced adenoma: adenoma with ≥1 risk attributes. Risk attributes were defined at namely the number (≥3) and size (≥10 mm) of adenomas, and the presence of high-grade dysplasia or villous morphology.
Covariates include age, sex, smoking status, and family history of colorectal cancer.
Contrasted with the null model (i.e., the non-discrimination line).
Model B contrasted with Model A.
Body mass index (BMI) was calculated from the most recent height and weight measurements taken on or before the cohort entry date.
Colonoscopy uptake (Table 1) for each subject was a dichotomous variable classified as “yes” if there was record of ≥1 colonoscopies between 1 January 2004 to 31 March 2011 and “no” if not.
Analytic procedures
All data operations and tests of hypothesis were performed using SAS 9.3 for Windows (SAS Institute). Logistic regression was used to estimate odds ratios and 95% confidence intervals (CIs).
Association of colonoscopy uptake
with age, sex, family history (any cancer), IBD status, and BMI was tested using multivariate logistic regression in the 110,452 subjects of the denominator cohort (Table 1).
Association of baseline adenoma attributes with recurrent adenoma and invasive colorectal adenocarcinoma was tested using multivariate logistic regression in the 3,300 subjects of the post polypectomy subcohort, adjusting for sex, smoking status, family history (of CRC), and age (age at diagnosis of recurrent adenoma or CRC, or age at the end of follow-up for patients who did not have these outcomes; Table 3). Receiver operating characteristic-area under the curve was used to evaluate predictive performance and compare competing models as previously described.26 The classifier in this analysis was a logistic regression model that included the 4 adenoma attributes (namely, the number (≥3) and size (≥10 mm) of adenomas, and the presence of high-grade dysplasia or villous morphology) along with the covariates age, sex, smoking status, and family history. To estimate the net predictive performance of the adenoma attributes this model was contrasted with the model including covariates alone and the incremental Receiver operating characteristic-area under the curve for the contrast was reported (Table 4).
Evaluation of the text parser's performance
Two hundred randomly selected pathology notes (for “lesion type”) or colonoscopy notes (for “indication”) were manually adjudicated by a pathologist (for “lesion type”) or gastroenterologist (for “indication”) who were blinded to the machine calls. Cohen's κ for inter-rater agreement between the machine calls and the expert calls was 0.94 (for “lesion Type”) and 0.99 (for “indication”), suggesting good agreement. The overall machine call rate was 98.1% (for “lesion type”) and 99.5% (for “indication”).
RESULTS
A cohort encompassing 110,452 primary care patients 50+ years old was assembled. There were 34,254 (31.0%) subjects with 1 or more screening/surveillance colonoscopies. In all, 8,619 subjects were excluded from the analysis as the indication for their screening endoscopies also included signs or symptoms of gastrointestinal pathology (iron deficiency anemia, diarrhea, overt gastrointestinal blood loss, etc.). Therefore, 25,635 (23.2%) subjects were included with 1 or more screening/surveillance colonoscopies and without concern for underlying gastrointestinal pathology (Table 1). Women were 20% less likely than men to accept colonoscopy. Per year of age, older subjects were 3.7% less likely than younger subjects to accept colonoscopy. Subjects with family history of cancer were 50% more likely to accept colonoscopy than subjects who did not report such history. Those with a medical history of diagnosed IBD were 2.5-fold more likely to accept colonoscopy than those without diagnosed IBD.
Clinical indications of colonoscopies performed
The majority of the colonoscopies performed on cohort subjects between their entry and exit dates were performed on asymptomatic subjects for the purpose of CRC prevention (i.e., the clinical indications of screening and surveillance). Of those, 64.7% were indicated for screening (54.5% and 10.2% average and elevated risk indications, respectively) and 35.3% for surveillance (Figure 1).
Time trends in prevention colonoscopy utilization
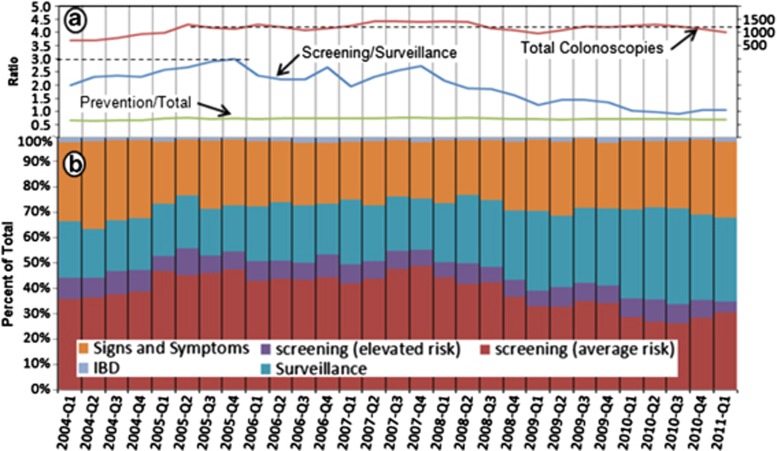
In 2004 the Gastroenterology Service Line at GHS opened access to colonoscopy, enabling primary care providers to order colonoscopies directly. This action resulted in a 1.8-fold increase in the total number of colonoscopies, from 707 in the first quarter of 2004 to 1,303 in the second quarter of 2005 (Figure 2a, total colonoscopies line). Following this period of rapid growth, the overall number of colonoscopies for this cohort stabilized with minor fluctuation around a mean of 1,152 procedures per quarter.
Figure 2.
Trends over time in colonoscopy utilization. (a) Key metrics for monitoring colonoscopy utilization by fiscal year quarter. Total colonoscopies represents the number of procedures performed per quarter for all indications and denoted by the red line (number of exams is on the right axis of the graph). The ratio of screening/surveillance examinations performed by quarter is noted by the blue line. The ratio of screening and surveillance colonoscopies performed to the total colonoscopies performed (including for symptoms) is noted by the green line. (b) Percentage of total colonoscopies per fiscal year quarter for indications as noted in the key (signs and symptoms, average risk screening, elevated risk screening, inflammatory bowel disease, and surveillance). Average risk screening was defined as a person who did not have symptoms, or had a family history of colorectal cancer in only 1 first-degree relative older than 60 years of age. Elevated risk screening was defined as an asymptomatic person who had a family history of 1 first-degree relative diagnosed with colorectal cancer at age 60 years or younger or who had 2 first-degree relatives diagnosed with colorectal cancer at any age. Screening examination was defined as a person who had a baseline colonoscopy with pathology dictating a recall colonoscopy.
Surveillance colonoscopies have increased threefold relative to newcomer screenings. While in the first quarter of 2005 there were 3 screening colonoscopies per every 1 surveillance colonoscopy, this ratio decreased to 1:1 in 2010 (Figure 2a, screening/surveillance line). The proportion of prevention to total was remarkably stable at 72% over time (Figure 2a, prevention/total line).
ADR according to clinical indication
The ADR was 1.5-fold higher in surveillance colonoscopies (34% Table 2) compared with screening colonoscopies (23%). The adenocarcinoma detection yield was 1.6-fold higher in surveillance colonoscopies (1.3%) compared with screening colonoscopies (0.8%).
Association of baseline adenoma attributes with recurrent adenoma and invasive colorectal adenocarcinoma
We performed multivariate analysis to estimate effect magnitudes of baseline adenoma attributes on the risk of adenoma recurrence and CRC, adjusting for age, sex, smoking status and family history of adenoma or CRC. All 4 attributes were significantly associated (Table 3) with the risk of adenoma recurrence and invasive colorectal adenocarcinoma. Effect sizes were larger toward the more severe outcomes. Among the 4 attributes, high-grade dysplasia had the strongest effects overall. Notably, the effect of “size” vanished when “villous morphology” was included in the same model, suggesting that these two predictors are not independent of each other.
We evaluated the net predictive performance of the 4 baseline adenoma attributes using receiver operating characteristic-area under the curve analysis (Table 4). The 4 attributes significantly increased the probability of correctly predicting CRC (i.e., the predictive performance) by a net gain of 22.8%. Gains in performance were also noted for predicting advanced adenoma (13.6%) and any recurrent adenoma (9.6%).
DISCUSSION
Colorectal cancer screening and prevention has been a focus in the United States. The screening guidelines initially included fecal occult blood testing, proctoscope, flexible sigmoidoscopy, and colonoscopy. In keeping with an increased awareness of colorectal cancer prevention, the Healthy Person 2010 initiative targeted a screening exam rate of 50% of adults >50 years in the United States. This includes all the modalities listed above except proctoscopy. Owing to the lower socioeconomic status of our patients, we believe that fecal occult blood testing is used more frequently in our patient population. For example, during this time period, many of our patients (>8,000; ∼7%) had fecal occult blood testing (data not shown) as a method of colorectal cancer screening. Although this accounts for a screening rate of 30.5%, this cohort excluded patients who presented to a specialist for a screening exam and also excluded patients who had any gastrointestinal symptoms. When you include patients 50 years or older in our study who had colonoscopic examinations for this indication, the effective colorectal cancer screening rate in our patient population is 38.3%.
Yet, Xu et al.27 demonstrated that the actual rate of colonoscopic screening examinations ranged from 37.8 to 75.7% of the surveyed population of the United States and territories around 2010, the time around which our study concluded. This disparity is reiterated in a 2006 study by Higgins et al.,28 where disabled Medicare beneficiaries in a family practice setting had a screening rate of 34% as compared with a screening rate of 68% in higher income Medicare beneficiaries receiving care from an internal medicine physician. As our patient population consists of primary older Medicare patients with a lower socioeconomic status, this study helps us to appreciate “real-world” experience in colonoscopy screening programs.
We described a time trend in colonoscopy utilization that reveals a shift toward more numerous surveillance re-examinations and fewer newcomer screenings. We observed a threefold expansion in the rate of surveillance colonoscopies relative to newcomer screening colonoscopies between 2005 and 2010. This trend, which has been noted by others,5, 21, 29, 30, 31 may be the result of maturation of the prevention program over time, whereby the program becomes gradually more occupied with post polypectomy surveillance procedures arising in the wake of large screening campaigns such as the one launched in our clinics in 2004. We also considered capacity constraints as a possible explanation, whereby surveillance procedures might outcompete newcomer screenings in the appointments queue. However, a review of capacity data in our health system revealed no evidence of such capacity shortages. On the contrary, during this time period, a monthly review of endoscopy slots for colonoscopy were filled at a steady state of 77%, and free slots were available throughout the period (data not shown).
The ADR is a commonly used quality metric for colonoscopy.32, 33, 34 In most studies the risk of interval CRC is related inversely to the ADR.34 In keeping with these previous findings and with established guidelines where it is suggested that a provider maintain an ADR of >20% for high-quality screening colonoscopies,35 our ADR in screening colonoscopies was 23%. There are fewer data in the literature on ADR standards for high-quality surveillance exams. One study has suggested an ADR of 37%, although the sample size was smaller.36 The surveillance colonoscopies ADR estimation of 34% in this study gives providers a provisory benchmark of differences in ADR between screening and surveillance colonoscopies.
This study provides further evidence to support the use of number, size, grade of dysplasia, and presence of villous features as predictors of future adenoma recurrence and CRC risk. These attributes are relatively simple to ascertain and provide substantial predictive power and should be included routinely in the triage of post polypectomy patients to determine the appropriate surveillance interval, as recommended in practice guidelines.
In agreement with prior literature summarized in meta analyses,2, 5, 17, 37 the odds ratios for the 4 predictive baseline adenoma attributes estimated in this study are consistent with very substantial effects. These estimates stress the importance of surveillance colonoscopy for mitigating excess risk of colorectal cancer in post polypectomy patients who had high-risk findings on screening examination.2, 38 There have been differing opinions voiced in the literature with regard to what constitutes an appropriate time interval for surveillance re-examination, with the recent combining of efforts of the United States Multi-Society Task Force (American College of Gastroenterology, American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy) and the American Cancer Society with surveillance recommendations stated previously.2 The United States Preventive Services Task Force does not have a surveillance guideline recommendation. The European Society of Gastrointestinal Endoscopy has similar guidelines except individuals with a low-risk lesion (1–2 small tubular adenomas with low-grade dysplasia) have a recall examination in 10 years. Small serrated lesions also fall into the low-risk classification, with a 10-year recall.39 Under the United Kingdom guidelines, individuals who are high risk (≥5 small adenoma, or ≥3 adenomas, with at least 1 being >10 mm) have a single clearing examination. These guidelines also make no special exceptions for individuals with high-grade dysplasia or villous architecture on pathology, with either no surveillance or a 5-year interval follow-up.40 Shorter surveillance intervals can lead to overutilization,30 whereas longer intervals may expose some patients to unacceptable levels of risk.41 The evidence-based practice guideline for post polypectomy surveillance has attempted to reconcile these conflicting constraints by adjusting the length of the surveillance interval based on attributes of the baseline lesions.17, 42, 43 Our study reinforces this rationale and adds confirmatory evidence to its underlying knowledge base. Our results highlight the importance of surveillance colonoscopies for mitigation of CRC risk in patients post polypectomy and at the same time support the risk stratification strategy recommended by current practice guidelines.
Despite its strengths (a relatively large sample, nested in a primary care population), our study suffers from several limitations. (1) The electronic records we reviewed spanned a period of 7.25 years (with an 8-year look-back period). This combined review and look-back period captured repeat screenings carried out in many average-risk subjects (10-year interval), but not all patients with a later entry into the cohort. (2) In addition, if an individual did not have an encounter in the EHR over a period of 365 days, they were dropped from the study. Although this occurred in a small number of patients, this may have excluded a healthy patient who did not have frequent visits to our health system despite being complaint with the colonoscopy recall program. This limitation is also mitigated by the 8-year look-back period. (3) Neither providers nor patients were blinded in this study to the presence of adenoma risk attributes at baseline and this information is likely to have influenced both the provider's management recommendations and the patient's adherence. This difficult-to-tease-out form of confounding by indication, which can lead to differential ascertainment of post polypectomy adenoma outcomes in subjects with high-risk baseline adenomas may have resulted in inflated estimates.
In conclusion, this study provides further evidence to support the use of number, size, grade of dysplasia, and presence of villous features as predictors of future adenoma recurrence and CRC risk. This study is also unique in that it follows a non-urban cohort in large numbers and provides valuable knowledge for a rather unstudied population not only for the need for expanded screening but also importance of adherence to appropriate surveillance intervals. As a focus of a national strategy for the early detection and prevention of colorectal cancer, defining colonoscopy as a program rather than an individual procedure with systems and community engagement is necessary to overcome ongoing barriers to screening affecting rural populations including lower income and educational levels, need for transportation, scheduling impediments (blue collar or agricultural work responsibilities), and insurance or out-of-pocket cost concerns, particularly with growing non-English-speaking populations in many parts of the country including Pennsylvania. High-quality programs focused on decreasing polyp miss rates and interval cancers by focusing on performance metrics, providing experienced physician, and gastrointestinal endoscopy teams, with timely communication of results and optimal patient satisfaction would also help achieve incremental gains in reducing the incidence and mortality of colorectal cancer regardless of patient demographic or location.
Study Highlights

Guarantor of the article: Porat M. Erlich, PhD.
Specific author contributions: All authors participated in the interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content. K.J.F., J.L., M.K., and P.M.E. also participated in study concept and design and the acquisition and analysis of data. N.S. also provided technical support. P.M.E. also provided statistical analysis and study supervision. P.M.E. accepts full responsibility for the conduct of the study. He has had access to the data and has control of the decision to publish. All authors have approved the final draft submitted.
Financial support: This study was funded in part by the HMO Cancer Research Network, a consortium of research centers embedded in integrated health-care systems and funded by the National Cancer Institute (U19 CA079689 and U24 CA171524). Additional funding was provided from internal sources within the Geisinger Health System. Work was independent and the views and interpretations of data as expressed in this manuscript are those of the authors, and do not necessarily represent those of funding sources.
Potential competing interests: None.
References
- Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case–control study. Ann Intern Med. 2013;158:312–320. doi: 10.7326/0003-4819-158-5-201303050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- Jacob BJ, Moineddin R, Sutradhar R, et al. Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointest Endosc. 2012;76:355–64.e1. doi: 10.1016/j.gie.2012.03.247. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, St, John J, Bond J, et al. Screening of average-risk individuals for colorectal cancer. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:505–513. [PMC free article] [PubMed] [Google Scholar]
- Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Arends MJ. Pathways of colorectal carcinogenesis. Appl Immunohistochem Mol Morphol. 2013;21:97–102. doi: 10.1097/PAI.0b013e31827ea79e. [DOI] [PubMed] [Google Scholar]
- Goncalves AR, Ferreira C, Marques A, et al. Assessment of quality in screening colonoscopy for colorectal cancer. Clin Exp Gastroenterol. 2011;4:277–281. doi: 10.2147/CEG.S25596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Viney SK, Chapman BA, et al. A prospective study of endoscopist-blinded colonoscopy withdrawal times and polyp detection rates in a tertiary hospital. N Z Med J. 2012;125:52–59. [PubMed] [Google Scholar]
- Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel Am J Gastroenterol 20121071315–1329.quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajobi O, Yiu CY, Sen-Gupta SB, et al. Metachronous colorectal cancers. Br J Surg. 1998;85:897–901. doi: 10.1046/j.1365-2168.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- van Stolk RU, Beck GJ, Baron JA, et al. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology. 1998;115:13–18. doi: 10.1016/s0016-5085(98)70359-2. [DOI] [PubMed] [Google Scholar]
- Rossini FP, Arrigoni A, Pennanzio M. Treatment and follow-up of large bowel adenoma. Tumori. 1995;81:38–44. [PubMed] [Google Scholar]
- Morelli MS, Glowinski EA, Juluri R, et al. Yield of the second surveillance colonoscopy based on the results of the index and first surveillance colonoscopies. Endoscopy. 2013;45:821–826. doi: 10.1055/s-0033-1344582. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Sampliner R, Marshall JR, et al. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120:1077–1083. doi: 10.1053/gast.2001.23247. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151–1162. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Lawson MJ, Tobi M. Cecal stampede: the headlong rush for screening colonoscopy: a position paper. Dig Dis Sci. 2008;53:871–874. doi: 10.1007/s10620-007-9961-7. [DOI] [PubMed] [Google Scholar]
- Vijan S, Inadomi J, Hayward RA, et al. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507–515. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: data from the National Cancer Institute Survey of Colorectal Cancer Screening Practices. Am J Med. 2003;115:129–133. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- Rex DK, Eid E. Considerations regarding the present and future roles of colonoscopy in colorectal cancer prevention. Clin Gastroenterol Hepatol. 2008;6:506–514. doi: 10.1016/j.cgh.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Hinchcliff M, Just E, Podlusky S, et al. Text data extraction for a prospective, research-focused data mart: implementation and validation BMC Med Inform Decis Mak 201212106106-6947-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:3053–3063. doi: 10.1111/j.1572-0241.2000.03434.x. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Xu F, Town M, Balluz LS, et al. Surveillance for certain health behaviors among states and selected local areas—United States, 2010. MMWR Surveill Summ. 2013;62:1–247. [PubMed] [Google Scholar]
- Higgins AY, Doubeni AR, Phillips KL, et al. Self-reported colorectal cancer screening of Medicare beneficiaries in family medicine vs. internal medicine practices in the United States: a cross-sectional study. BMC Gastroenterol. 2012;12:23–230X-12-23. doi: 10.1186/1471-230X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- Ko CW, Dominitz JA, Green P, et al. Utilization and predictors of early repeat colonoscopy in Medicare beneficiaries. Am J Gastroenterol. 2010;105:2670–2679. doi: 10.1038/ajg.2010.344. [DOI] [PubMed] [Google Scholar]
- Saini SD, Nayak RS, Kuhn L, et al. Why don't gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol. 2009;43:554–558. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- Wang YR, Cangemi JR, Loftus EV, Jr, et al. Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am J Gastroenterol. 2013;108:444–449. doi: 10.1038/ajg.2012.429. [DOI] [PubMed] [Google Scholar]
- Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949–956. doi: 10.1136/gutjnl-2012-303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- Jover R, Herraiz M, Alarcon O, et al. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444–451. doi: 10.1055/s-0032-1306690. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Butterly LF, Goodrich M, et al. Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the new hampshire colonoscopy registry. Clin Gastroenterol Hepatol. 2013;11:1308–1312. doi: 10.1016/j.cgh.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunic M, Perkovic N, Rosic-Despalatovic B, et al. Colonoscopic polypectomies and recommendations on the colonoscopy follow-up intervals depending on endoscopic and histopathological findings. Acta Inform Med. 2013;21:166–169. doi: 10.5455/aim.2013.21.166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- Martinez ME, Thompson P, Messer K, et al. One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines. Ann Intern Med. 2012;157:856–864. doi: 10.7326/0003-4819-157-12-201212180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JK, Good CS, Gilbert JM. 22-Year longitudinal study of repetitive colonoscopy in patients with a family history of colorectal cancer. Ann R Coll Surg Engl. 2013;95:586–590. doi: 10.1308/003588413X13781990150419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahi CJ, Anderson JC, Rex DK. Screening and surveillance for colorectal cancer: state of the art. Gastrointest Endosc. 2013;77:335–350. doi: 10.1016/j.gie.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Leddin D, Enns R, Hilsden R, et al. Colorectal cancer surveillance after index colonoscopy: guidance from the Canadian Association of Gastroenterology. Can J Gastroenterol. 2013;27:224–228. doi: 10.1155/2013/232769. [DOI] [PMC free article] [PubMed] [Google Scholar]