Abstract
The nonessential metal cadmium (Cd) is toxic only after entering the cell. Proteins possibly relevant to intracellular Cd accumulation include the divalent metal transporter-1 (DMT1) and all 14 zinc-like iron-like protein (ZIP) importers, 10 zinc transporter (ZnT) exporters, and metallothionein chaperones MT1 and MT2. Comparing oral Cd-treated ZIP14 knockout (KO) with wild-type (WT) mice, we predicted Cd uptake and distribution would be diminished in the KO—because ZIP14 is very highly expressed in GI tract and liver; this was indeed observed for Cd content in liver. However, the reverse was found in kidney and lung from 6 or 12 h through 10 days of Cd exposure; at these times, Cd accumulation was unexpectedly greater in KO than WT mice; mRNA levels of the 27 above-mentioned genes were thus examined in proximal small intestine (PSI) versus kidney to see if these paradoxical effects could be explained by substantial alterations in any of the other 26 genes. PSI genes highly expressed in untreated WT animals included seven ZIP and five ZnT transporters, DMT1, MT1, and MT2; kidney genes included 11 ZIP and 7 ZnT transporters, DMT1, MT1, and MT2. Over 10 days of oral Cd, a bimodal response was seen for Cd content in PSI and for various mRNAs; initially, acute effects caused by the toxic metal; subsequently, the up- or down-regulation of important genes presumably to combat the sustained adversity. These data underscore the complex interplay between the gastrointestinal tract and renal proteins that might be relevant to Cd uptake and distribution in animals exposed to oral Cd.
Keywords: oral cadmium, ZIP influxors, ZnT effluxors, divalent metal transporter DMT1, metallothioneins, zinc transport, SLC39 family, SLC30 family, SLC11A2, pharmacokinetics of metal uptake
Cadmium (Cd0, Cd2+) is a ubiquitous nonessential toxic heavy metal.@ Heavy industrial usage of Cd began in the 1940s (Stoeppler, 1991). Today Cd is used principally in combination with nickel, e.g., battery manufacturing, pigments, and plastic stabilizers. Major occupational Cd exposures occur in non-ferrous metal smelters and recycling of electronic waste (Waisberg et al., 2003). Cigarette smoke is by far the largest source of Cd exposure (Zalups and Ahmad, 2003); for nonsmokers, Cd-contaminated food is a major cause of Cd exposure—fish and shellfish, organ meat (especially liver and kidney), and grains and cereal products (ATSDR, 1999; Thévenod and Lee, 2013). In the 2013 Substance Priority List (http://www.atsdr.cdc.gov/spl/), Cd was ranked seventh by the Agency for Toxic Substances and Disease Registry and U.S. Environmental Protection Agency.
A major route of Cd exposure is via the gastrointestinal (GI) tract. Even among smokers, more than half the Cd and other toxicants are swallowed and enter the GI tract, compared with that absorbed by lung (Chen et al., 1989; Järup 2003; Rozman and Klaassen, 2007). Intriguingly, several epidemiological studies (Hsu et al., 2009; Meltzer et al., 2010; Satarug et al., 2004) showed greater Cd accumulation among humans experiencing hypozincemia—which usually accompanies malnutrition, anemia, and/or infection; these data implicate an intricate interplay between Zn2+ and Cd2+ uptake.
Following GI tract absorption in lab animals, Cd binds to various polypeptides such as metallothionein (MT) and reduced glutathione (GSH); dissociation constants of Cd-MT and Cd-GSH complexes are exceptionally low and therefore exhibit extremely high binding affinities: ∼10−23 M (Klaassen and Liu, 1998) and ∼10−10 M (Quig, 1998), respectively. Cd-MT and GS-Cd-SG complexes in blood appear to be the principal means by which Cd is transported from portals-of-entry; ultimately, Cd is deposited mostly in kidney. In double-knockout (KO) mice, after 3 or 10 days of Cd exposre, Mt1/Mt2(−/−) mice had much lower Cd than WT in their tissues (Klaassen et al., 1999), demonstrating in the intact animal an important role of MT1 and/or MT2 in Cd transport.
In addition to being classified by International Agency for Research on Cancer (IARC) as a Category I (i.e., highest level of correlation) human lung carcinogen, Cd also causes severe renal toxicity. Average accumulation of Cd in kidneys of a smoker with ≥100 cigarette-pack-year history, e.g., is close to threshold levels sufficient for overt Cd nephrotoxicity. Estimated half-life of Cd in humans is between 15 and 20 years (Jin et al., 2004).
Cd can damage a cell only after being internalized. Once inside, intracellular Cd2+ causes prominent oxidative stress—via mechanisms not well understood—thus forming disulfide bonds in many sulfhydryl-containing proteins (Go et al., 2014), which in turn likely inactivates critical intracellular functions. Several decades of mammalian cell culture studies (reviewed in [He et al., 2009]) had suggested two fundamental mechanisms of Cd uptake: voltage-gated calcium channels (Lopin et al., 2012); and the divalent metal transporter-1 (DMT1; also called NRAMP2; standardized nomenclature SLC11A2). DMT1 is a proton-coupled importer that prefers Fe2+, but is also involved in Pb2+ and Cd2+ uptake (Bressler et al., 2004).
More recently, however, Cd influx by the ZIP8 and ZIP14 transporters was discovered (reviewed in [He et al., 2009]). The mouse Slc39a8 gene encodes ZIP8, a divalent metal/bicarbonate symporter involved in uptake of Zn2+, Mn2+, and Fe2+ (Dalton et al., 2005; He et al., 2006; Liu et al., 2008). ZIP14, product of the mouse Slc39a14 gene, exhibits strikingly similar functions to that of ZIP8; both transport the electroneutral complex Zn2+/(HCO3−)2, in which Zn2+ can be displaced by Fe2+ or Mn2+, as well as nonessential metal cations including Cd2+ (Liu et al., 2008; Nebert et al., 2012). Most important to this study, ZIP14 expression is highest in GI tract and liver, whereas ZIP8 expression is highest in kidney, lung, and testis (reviewed in [He et al., 2009]).
Other proteins of possible relevance to Cd uptake and distribution include metallothioneins MT1 and MT2 (reviewed in [Klaassen et al., 2009]) and the SLC30 (ZnT) divalent cation effluxors (reviewed in [Huang and Tepaamorndech, 2013]). Each of the 14 ZIP and the 10 ZnT transporters, as well as DMT1, MT1, and MT2, are widely expressed, but often reveal strikingly distinct tissue- or cell-type-specific expression. Currently, it is not known whether any of the ZnT transporters are able to export Cd from the cell.
The mouse genome contains 4 Mt genes encoding metallothioneins (MTs). MT1 and MT2 are the most widely expressed, rapidly induced by numerous metals, drugs and inflammatory stimuli; MT3 exhibits specific neuronal growth-inhibitory activity; MT4 is located in stratified epithelium (Coyle et al., 2002). MTs are low-molecular-weight cysteine-rich proteins, ubiquitous in prokaryotes and eukaryotes. MTs display potent metal-binding, chelation, and redox capabilities. In GI tract and pancreas, hypozincemia causes MT up-regulation. MT1 and MT2 participate in Zn homeostasis and regulation of metabolic pathways, as well as protection against heavy metal toxicity (e.g., Cd) and other oxidant damage (Coyle et al., 2002).
Because ZIP14 appears to be the major participant in Cd uptake from GI tract (Girijashanker et al., 2008; He et al., 2009), we postulated that oral Cd-exposed Slc39a14(−/−) KO mice (Hojyo et al., 2011) might exhibit less Cd uptake and distribution in proximal small intestine (PSI), liver, kidney, and lung. We tested this hypothesis during 10 days of oral Cd. We examined concomitantly Cd content, via inductively-coupled plasma mass spectrometry (ICP-MS), and mRNA expression by quantitative real-time polymerase chain reaction (qRT-PCR)—of the DMT1 and 14 ZIP importers, 10 ZnT exporters, and MT1 and MT2. Our results were different from what we had expected.
MATERIALS AND METHODS
Chemicals
CdCl2 anhydrous (American Chemical Society-certified), sucrose (reagent grade), and nitric acid and hydrogen peroxide (trace-metal grade) were purchased from Fisher Scientific (Pittsburgh, PA).
Animals, Treatment, and Isolation of Organs
Four male and four female Slc39a14(+/−) heterozygote breeders (having a “mixed” genetic background, derived from B6 × 129+Ter/SvJcl hybrid M1 ES cells [Hojyo et al., 2011]) were shipped from the Fukada mouse colony (Yokohama, Japan); these mice were continuously bred in the Nebert mouse colony (Cincinnati, OH). Dozens of Slc39a14(+/−) × Slc39a14(+/−) intercross matings resulted in sufficient numbers of age-matched Slc39a14(+/+) wild-type (WT), Slc39a14(+/−) heterozygote, and Slc39a14(−/−) KO littermates which were used in these studies. All mouse experiments were conducted in accordance with the National Institutes of Health standards for the care and use of experimental animals and University Cincinnati Medical Center Institutional Animal Care and Use Committee.
To male and female mice of all 3 genotypes, at 6–8 weeks of age, we continuously administered Cd in drinking water (0.4 mg/ml CdCl2 and 20 mg/ml sucrose) and sacrificed animals (N = 3 per genotype) at zero-time, 6 h, 12 h, and 1, 2, 5 and 10 days of treatment. No gender differences were observed. The Cd-laced drinking water was changed every third day. Mice that were sacrificed at 6 h were initially gavaged at zero-time with Cd-laced water at a dose of 10 µl/g body weight and then maintained with this type of water for the 6-h time-point. The amounts of oral Cd exposure described herein caused no overt histological or biochemical alterations. The Cd dose is consistent with other studies in the literature. We added sucrose to the drinking water in order to mask the metallic taste of Cd; this ensured no decrease in water intake by the mice over the 10-day experiment (Schneider et al., 2014).
Untreated animals of each genotype were used as controls; Cd levels for controls (N = 5 WT, N = 7 Slc39a14(+/−) heterozygotes, and N = 8 KO) represented the zero-time-points. For confirmation, various time-points were repeated several times.
At each of the seven time-points, organs collected from animals included: PSI, liver, kidney, and lung. PSI samples represent the first 8 cm beyond the pyloric sphincter, i.e., duodenum and portion of the proximal jejunum. Aliquots of ∼100 mg of each organ were collected and immediately placed in dry ice. These aliquots were then stored at −80°C until isolation of mRNA. The rest of each organ was weighed and stored at −20°C until preparation for Cd analysis.
Preparation of Tissues for Cd Analysis
At each time-point, individual organs were placed in acid-washed glass digestion vials (10% nitric acid) and pre-digested with 0.50 ml of 50% (v/v) HNO3 overnight in a closed container at 70°C; then 1.0 ml of 50% (v/v) HNO3 and 1.0 ml of doubly deionized water were added, and the samples were subjected to microwave digestion (300 W; 5.00-min ramp time, 10.0-min hold time; 250 psi; 190°C), using the CEM Explorer system equipped with the Discover auto-sampler (Matthews, NC). To oxidize fat, liver samples were treated with 250 µl of hydrogen peroxide 30% (v/v) for a second digestion step, using the same program conditions. Digested samples were then diluted to 10.0 ml with doubly distilled water containing a yttrium internal standard (100 µg/l).
Inductively Coupled Plasma Mass Spectrometry
An Agilent 7500ce (Agilent Technologies; Tokyo, Japan) inductively coupled plasma mass spectrometer, equipped with shielded-torch, and collision/reaction-cell technology was used for the element-specific detection of 111Cd. The collision/reaction cell consisted of an octopole-ion guide, operated in “rf-only” mode, which also served to remove polyatomic interferences. Forward power was 1500 W (with shielded torch); plasma gas-flow rate was 15.6 l/min; auxiliary gas-flow rate was 1.0 l/min; carrier gas-flow rate was 1.00 l/min; the nebulizer was glass-expansion microcentric; spray chamber (Scott double-channel) was kept at ∼2°C; sampling depth was 8 mm; sampling and skimmer cones were composed of nickel; dwell time was 0.10 s. Isotopes monitored for these experiments included 111Cd, 66,68Zn, 57Fe, 63Cu, 55Mn, and yttrium 89Y (internal standard). The octopole-reaction system used helium (flow optimized before each experiment). All experiments were blank-corrected (N = 5); to validate the results, three samples of DOR-3 (Fish Protein, Certified Reference Material for Trace Metals, National Research Council Canada) were digested and analyzed alongside the experimental samples. Tissue Cd content was expressed in µg/g wet weight of tissue.
Preparation of mRNA
With RNAzol® RT (RN 190; Molecular Research Center, Inc.; Cincinnati, OH), mRNA was isolated from the four above-mentioned organs, following protocol recommended by the vendor.
Reverse Transcription and qRT-PCR
The mRNA (1.0 µg) from each sample was used as template for reverse transcription using the iScript TM cDNA Synthesis Kit (170-8890, Bio-Rad Laboratories, Richmond, CA) following protocol recommended by the vendor. We performed qRT-PCR in the Bio-Rad DNA Engine Opticon 2TM (Bio-Rad Laboratories; Richmond, CA), using iQTM SYBR Green Supermix (170-8882, Bio-Rad Laboratories). The “housekeeping gene” tubulin α-1a mRNA (TUBA1A) was employed as the internal control. Primers used to target all 14 ZIP mRNAs, 10 ZnT mRNAs, DMT1 mRNA, MT1 and MT2 mRNAs, and the TUBA1A internal control mRNA are listed in Supplementary Table 1. The relative gene expression (RGE) of different genotypes for each transporter was calculated with respect to the control untreated WT [Slc39a14(+/+)], by applying this formula:
RGE for genotype = 2[(Ct gene − Ct TUBA1A)WT − (Ct gene − Ct TUBA1A)Genotype]. Further details are provided in the figure legends.
Statistical Analysis
Graphs and all calculations were generated using Microsoft Windows Excel (Microsoft Corporation) and SPSS 13.0 for Windows (2004, Apache Software Foundation). Independent-sample t-tests were used to compare groups’ means, and Levene’s test was applied to evaluate equality of variances. P values of <.05 were regarded as statistically significant.
RESULTS
Constitutive Expression of Genes Possibly Relevant to Cd Accumulation in PSI and Kidney
It should be emphasized that not all ZIP transporters, and even fewer ZnT effluxors, have been sufficiently studied, especially as far as divalent cation substrate-specificity. Hence, in any particular organ or cell type in which a specific ZnT might be more efficient at Cd efflux than ZIP importers are proficient at Cd influx—this imbalance might contribute substantially to Cd content in that organ or cell type. At the present time, however, it is not known whether any of the ZnT transporters is efficient at Cd efflux from any cell type.
The Unigene dbEST expressed-sequence tags database [http://www.ncbi.nlm.nih.gov/unigene] lists ubiquitous expression: of all ZIP proteins (except ZIP2, ZIP5, and ZIP12); of ZnT1, ZnT5, ZnT6, ZnT7, and ZnT9 proteins; and of DMT1, MT1, and MT2 proteins. “Ubiquitous expression” is defined here as “detected in substantial amounts from human, mouse and/or rat cDNA libraries of intestine, liver, kidney, lung, and usually 10 or 20 other tissues/cell types”. ZIP2 is located only in embryo, endocrine tissues, and brain. ZIP5 has been found in intestine, liver, and kidney but not lung. ZIP12 is expressed in embryo, kidney, and brain. ZnT2 and ZnT4 are found in intestine, kidney, and lung but not liver. ZnT3 is located only in endocrine tissues and brain. ZnT8 is expressed in liver, kidney, and lung but not intestine. ZnT10 is expressed in intestine and liver but not kidney or lung.
We first chose to measure mRNA levels of all 14 ZIP importers, all 10 ZnT exporters, the DMT1 importer, and the MT1 and MT2 chaperone proteins—in untreated mice. Because such a systematic study had never before been carried out in the same untreated animal species, we first wished to quantify the degree of these pertinent mRNA levels for each of the 27 genes.
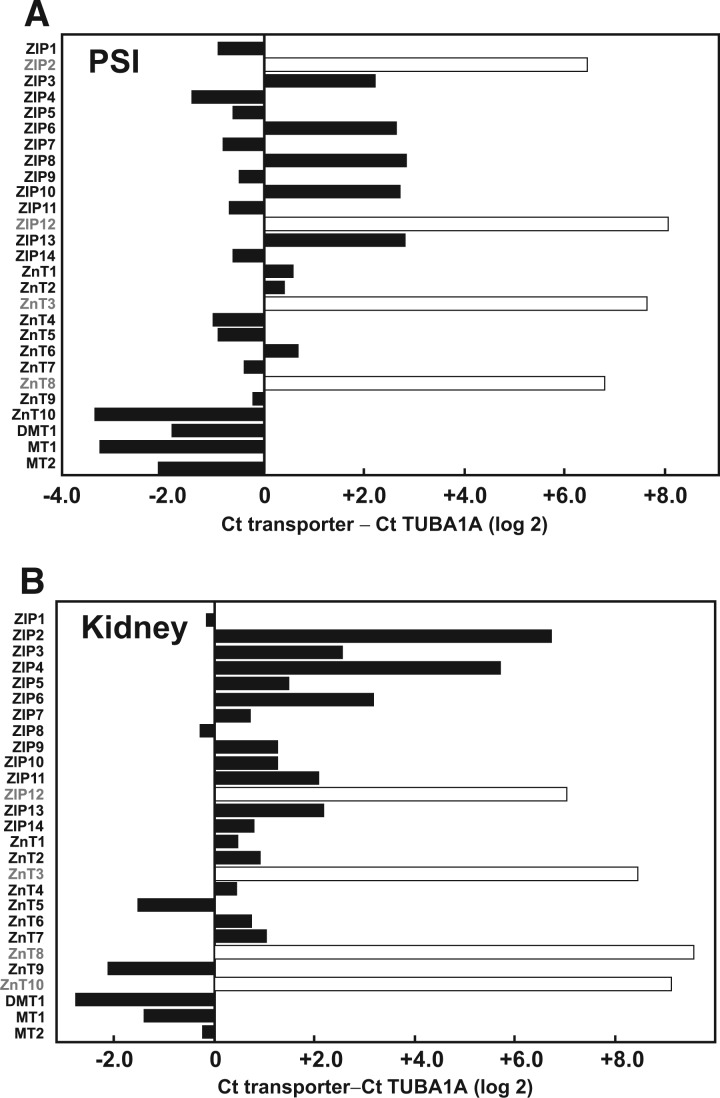
Analyzing by qRT-PCR and using TUBA1A mRNA (encoded by housekeeping gene Tuba1a) as control in the same plate, we sought to determine the extent of expression of these 27 genes in PSI and kidney; large differences in constitutive mRNA levels were seen (Fig. 1A and B; Supplementary Tables 2 and 3). Including importers, exporters and metallothioneins (Table 1), more genes were found to be highly constitutively expressed in kidney (total of 21) than PSI (total of 15). On the other hand, DMT1, MT1 and MT2 were all highly expressed in both PSI and kidney.
FIG. 1.
The constitutive expression of each transport gene was compared with that of a “housekeeping gene” that is strongly expressed in most tissues (tubulin-1α; Tuba1a) by subtracting Ct counts of TUBA1A from Ct counts of the respective gene at a fixed threshold. For each individual WT mouse, all genes and TUBA1A from the tissue of interest were inspected simultaneously on the same qRT-PCR plate. By using differences in Ct counts as our unit of comparison, we show results in log 2 scale, either from genes that are less expressed (+ values) or more expressed (− values) than TUBA1A. Open bars represent genes with high Ct counts and no observable product (band) when the qRT-PCR final reaction was examined on an agarose gel (Supplementary Tables 2 and 3). On the other hand, closed bars denote genes that are expressed at some level (high, low or intermediate). Abbreviations: WT, wild-type; qRT-PCR, quantitative real-time polymerase chain reaction
TABLE 1.
Qualitative Summary of Cd-Relevant Genes that are Highly Constitutively Expressed in (Untreated WT) PSI versus Kidneya
| Transporter | PSI | Kidney |
|---|---|---|
| ZIP1 | X | X |
| ZIP2 | ||
| ZIP3 | X | |
| ZIP4 | X | |
| ZIP5 | X | X |
| ZIP6 | X | |
| ZIP7 | X | X |
| ZIP8 | X | |
| ZIP9 | X | X |
| ZIP10 | X | |
| ZIP11 | X | X |
| ZIP12 | ||
| ZIP13 | X | |
| ZIP14 | X | X |
| ZnT1 | X | |
| ZnT2 | X | |
| ZnT3 | ||
| ZnT4 | X | X |
| ZnT5 | X | X |
| ZnT6 | X | |
| ZnT7 | X | X |
| ZnT8 | ||
| ZnT9 | X | X |
| ZnT10 | X | |
| DMT1 | X | X |
| MT1 | X | X |
| MT2 | X | X |
| TUBA1A | X | X |
aThe expression levels in PSI (Fig. 1A) can be seen below zero (i.e., genes that are more highly expressed [– values] than TUBA1A). In kidney (Fig. 1B), because TUBA1A shows lower Ct counts (21.84 at threshold 0.97) than in PSI (24.57 at threshold 0.038), transporter genes that are highly expressed can be seen below the value of +3.2.
PSI and Kidney Constitutive Gene Expression in WT versus KO Mice
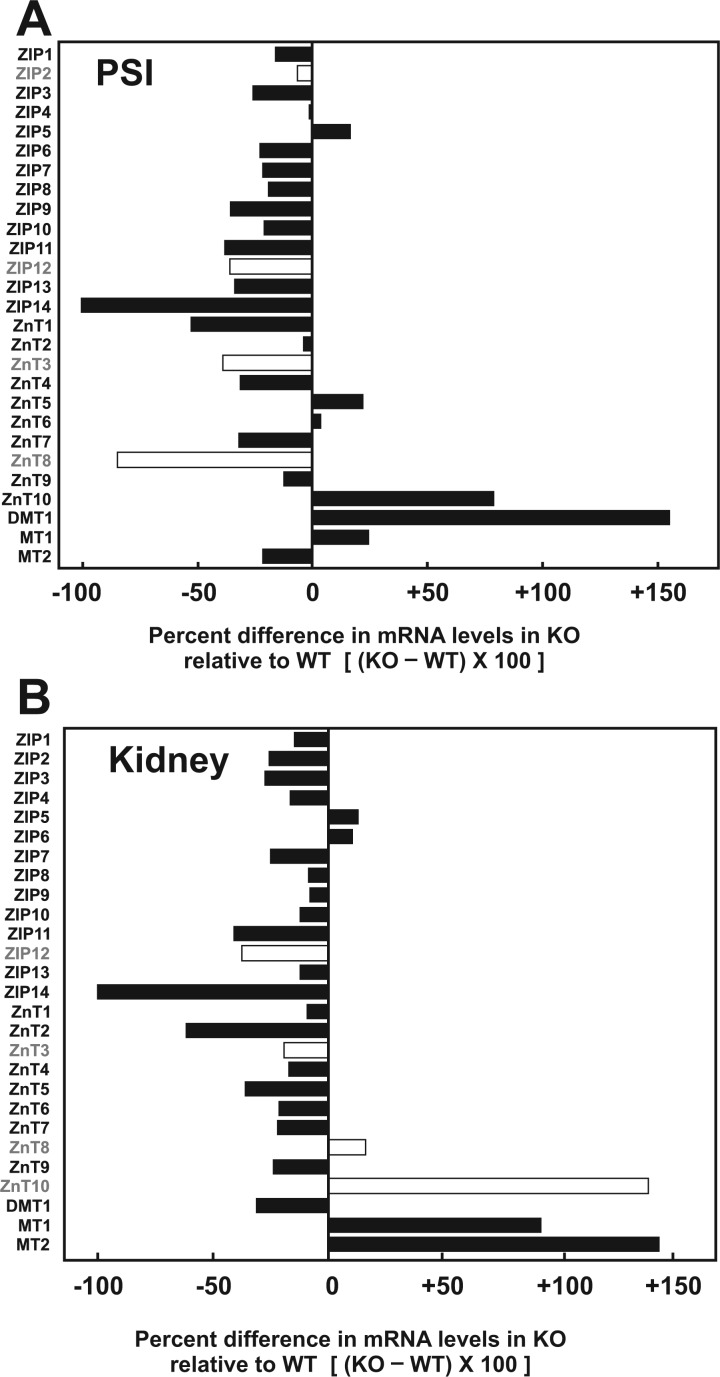
The next step was to compare constitutive mRNA levels of the 27 genes in PSI and kidney of untreated Slc39a14(+/+) WT to that of untreated Slc39a14(−/−) KO mice, to see if any of the other 26 genes are up- or down-regulated when ZIP14 expression is globally ablated. Percent differences in KO gene expression, when compared with WT, are illustrated for PSI in Fig. 2A. As expected for the KO, ZIP14 expression was virtually 100% absent. Most strikingly, DMT1 expression in KO was increased more than 150%, suggesting that DMT1 in PSI might function more prominently (presumably for endogenous substrates such as Fe2+ or Zn2+) when the Slc39a14 gene is absent. An 80% increase in ZnT10 mRNA expression was also found. Curiously, substantial decreases in ZnT1, ZnT4, and ZnT7 were observed in ZIP14 KO mice; substantial decreases in ZnT3 and ZnT8 mRNA levels were also seen (Fig. 2A), but can be disregarded because of their low copy-number abundance in PSI (Fig. 1A and Supplementary Table 2).
FIG. 2.
Relative gene expression (RGE) for the different genotypes (WT and KO) were obtained by linearizing the average normalized Ct counts (with respect to TUBA1A) for each genotype, using the untreated WT as control, and applying this equation: RGE for genotype = 2[(Ct gene − Ct TUBA1A)WT − (Ct gene − Ct TUBA1A)Genotype].RGE for WT is always 20 = 1.0. The percent difference in gene expression of KO relative to WT is (RGEKO − RGEWT) × 100. Open bars indicate genes with negligible constitutive expression in WT (cf. Fig. 1). Abbreviations: WT, wild-type; KO, knockout
In kidney (Fig. 2B), ZIP14 mRNA expression again was virtually 100% absent, as expected. Of note, in the KO, renal ZnT2 was diminished ∼66%, whereas MT1 and MT2 mRNAs were elevated ∼85% and ∼145%, respectively. Also, there was a significant increase of 140% in ZnT10 mRNA expression levels in kidney of the KO (Fig. 2B); this is particularly intriguing because this exporter was not found to be constitutively expressed in WT kidney (Fig. 1B and Supplementary Table 3).
Comparison of Oral Cd Distribution in Four Tissues
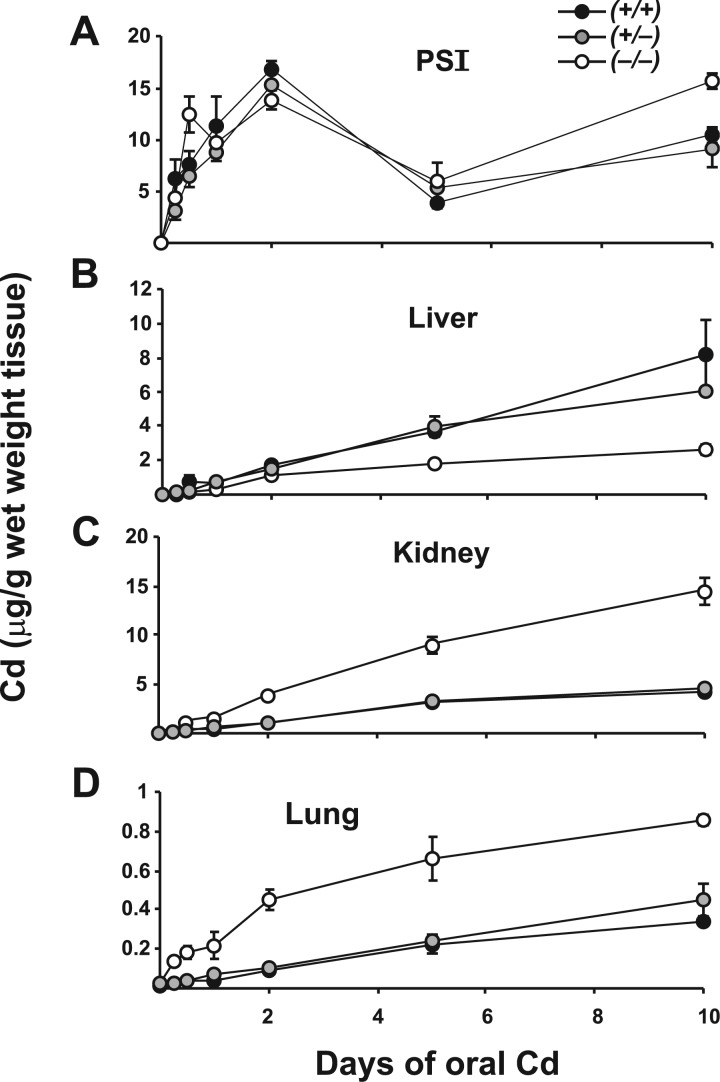
Slc39a14(+/+) WT, Slc39a14(+/−) heterozygote, and Slc39a14(−/−) KO mice were provided drinking water containing Cd for 10 days (Fig. 3). PSI, liver, kidney, and lung from groups of mice (N ≥ 3 per genotype) were collected at 0, 6 h, 12 h, and 1, 2, 5, and 10 days of exposure to oral Cd.
FIG. 3.
Cd content in four tissues of Slc39a14(+/+), Slc39a14(+/−) and Slc39a14(−/−) mice at various time-points while ingesting Cd in drinking water. Note the ∼20-fold variation in Cd concentrations on the ordinate. Symbols and brackets denote means ± S.E.M. Comparing KO with WT, significant P values included: <.05 for liver (day 10), kidney (12 h and day 1), lung (day 5); <.02 for PSI (day 2), kidney (day 2), lung (day 2); ≤.01 for PSI (day 10), liver (day 1), kidney (days 5 and 10), lung (6 and 12 h); and ≤.001 for liver (day 5), lung (day 10). Analysis included Student’s t-test and Levene’s test for equality-of-variance. Abbreviations: WT, wild-type; KO, knockout
In PSI at most time-points (Fig. 3A), no significant differences in Cd levels were seen between WT and heterozygotes; however, Cd content in KO was statistically significantly lower at 2 days and higher at 10 days, compared with that in the other two genotypes. For all three genotypes, we saw a general rise in Cd levels up to 2 days, a substantial drop at 5 days, which began to recover, perhaps slightly increasing at 10 days of oral Cd. We did not see striking decreases in Cd content in PSI of Slc39a14(−/−) mice, compared with the other two genotypes; in other words, genetic ablation of ZIP14, considered the main Cd influxor in the GI tract (He et al., 2009), had little effect on Cd concentration in this tissue. This pattern for Cd accumulation in the KO GI tract might implicate participation of other Cd transporter(s) in GI tract. However, as mentioned above, “Cd uptake” and “Cd content” do not necessarily need to go hand-in-hand.
In liver (Fig. 3B), a steady rise in Cd content was similar between WT and Slc39a14(+/−) mice, whereas rising Cd concentrations were significantly lower in KO animals, especially at the 5- and 10-day time-points. This pattern differs from that in PSI but is consistent with our hypothesis, i.e., that ZIP14 is the major transporter for Cd uptake in liver and no other hepatic transporter assumes this function in the absence of ZIP14.
Intriguingly, a different pattern prevailed in kidney and lung (Fig. 3C and D), except Cd accumulation in lung was ∼20-fold lower than that in kidney. When compared with WT and Slc39a14(+/−) kidney, KO kidney displayed higher Cd content, from 12 h onward, reaching ∼3.5-fold greater amounts by 10 days. When compared with WT and Slc39a14(+/−) lung, KO exhibited higher Cd levels, from 6 h onward, but reaching only ∼2-fold greater amounts by 10 days. This pattern is opposite to that seen in liver. It is worth noting that the Cd-importer ZIP8 is much more highly expressed than ZIP14 in both kidney and lung (Girijashanker et al., 2008; He et al., 2009; Wang et al., 2007); on the contrary, ZIP14 is much more highly expressed than ZIP8 in GI tract and liver (Girijashanker et al., 2008; He et al., 2009; Wang et al., 2007).
How might ZIP14 ablation in these tissues lead to enhanced, rather than diminished, Cd content? Over-expression of ZIP8 [or other Cd influxor(s)], down-regulation of Cd effluxors, or changes in MT chaperone levels, are 3 possible mechanisms that might explain elevated Cd content—as a function of time in oral Cd-treated KO, compared with that in WT and Slc39a14(+/−) mice. Hence, analysis of these mRNA levels, following oral Cd exposure, was studied for the remainder of this report.
PSI Gene Expression in Oral Cd-Treated WT versus KO Mice
Because the patterns of Cd levels found in PSI and kidney (Fig. 3) were different from what we had expected (i.e., kidney KO showed greater Cd accumulation than WT, whereas PSI was not remarkably different between KO and WT), we proceeded to examine expression of these 27 genes in PSI and kidney following exposure to oral Cd. Except for the thorough functional characterization of Cd uptake by ZIP8 and ZIP14 (He et al., 2009) and DMT1 (Shawki et al., 2012), and interactions of Cd with metallothioneins (Babula et al., 2012; Freisinger and Vasak, 2013), little is known about effects of oral Cd on the other 12 ZIP importers as well as any of the 10 ZnT exporters—in either WT mice or following ablation of the Slc39a14 gene.
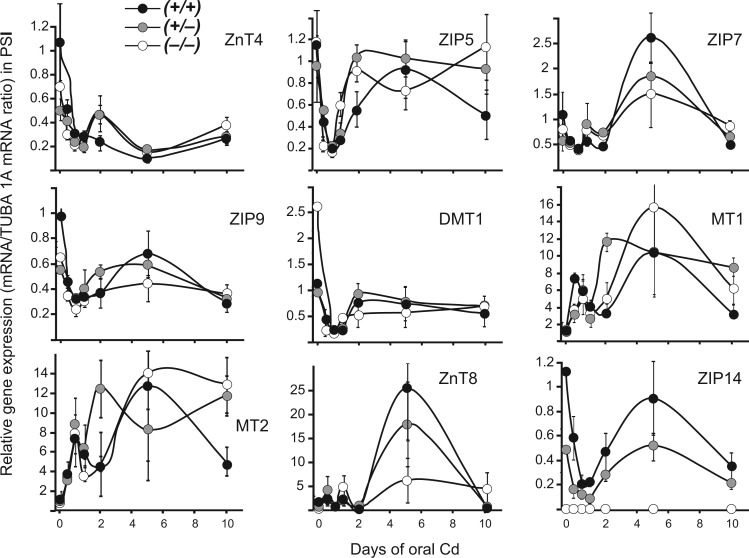
In PSI (Fig. 4 and Supplementary Figure 1), with regard to general expression patterns of most genes, we observed a fall in mRNA levels at shorter time-points (i.e., 6 h to 2 days) of exposure to oral Cd; this decline might reflect Cd-mediated toxicity by way of oxidative stress (Go et al., 2014), which likely occurs when Cd enters GI tract epithelial cells at early stages of oral Cd exposure. A somewhat different pattern was seen with MT1 and MT2 (Fig. 4): 6 h of oral Cd caused immediate increases in mRNA levels, then a fall at 1 or 2 days, followed by a dramatic rise.
FIG. 4.
Selected patterns of rises and falls in Cd-relevant gene expression, as a function of time of oral Cd exposure, in PSI of all three genotypes. Relative gene expression is measured with respect to the untreated WT (Fig. 2 legend for equation). Circles and brackets denote means ± S.E.M. In this figure as well as Fig. 5, Supplementary Figures 1 and 2, P values comparing genotypes at any particular time-point are available upon request. However, the patterns of rises and falls are regarded as more important than cluttering the panel with P values and/or symbols denoting statistically significant differences at any specific time-point. Abbreviations: WT, wild-type
Beyond 2 days of oral Cd, there was a tendency for increased expression for most of the 27 genes (Fig. 4 and Supplementary Figure 1); in many cases there appeared to be a recovery, as mRNA levels returned to those of untreated controls (e.g., ZIP5 and DMT1). However, others (e.g., ZnT4) presented a pattern of sustained suppression, even at longer exposures to oral Cd. For others such as MT1, MT2 (especially WT), and ZnT8 and ZIP2, there were strong increases in mRNA levels at 5 days of Cd, followed by decreases after 10 days of oral Cd. Of particular note is the pattern of the ZnT8 effluxor, which is not expressed constitutively in PSI (Fig. 1A and Table 1); ZnT8 expression in WT and heterozygote was strikingly increased (25- and 15-fold, respectively), but less so in the KO (Fig. 4). If ZnT8 were to function as a Cd exporter, this dramatic increase in ZnT8 mRNA at 5 days of Cd could explain the unexpected fall in Cd levels at this time-point.
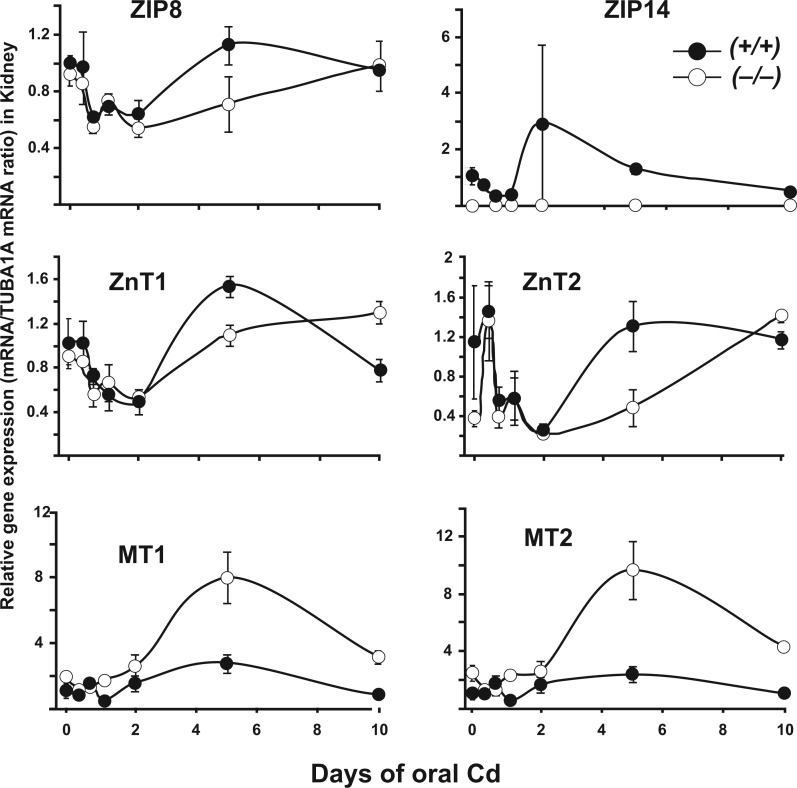
Kidney Gene Expression in Oral Cd-Treated WT versus KO Mice
In kidney (Fig. 5 and Supplementary Figure 2), we chose to study gene expression patterns in only the WT versus the KO, because renal Cd content in Slc39a14(+/−) was found to be generally very similar to that of the WT. Interestingly, the pattern of decreased expression at short Cd exposures, followed by recovery or slight increases at later time-points (i.e., which was usually observed in PSI), was similar in kidney with few exceptions. Notably, larger increases in MT1 and MT2 mRNA levels were found, especially at the 5-day time-point, but, overall, KO mice exhibited dramatically elevated levels of mRNA, when compared with WT. MT1 and MT2 over-expression might be due to greater accumulation of Cd in KO kidney, although the reason for such MT induction by elevated Cd levels is not understood (Babula et al., 2012). It is noteworthy that ZIP8, a principal importer of Cd in kidney (He et al., 2009; Wang et al., 2007), did not show substantial changes in mRNA levels when Slc39a14 was globally ablated (Fig. 5).
FIG. 5.
Selected patterns of rises and falls in Cd-relevant gene expression, as a function of time of oral Cd exposure, in kidney of WT and KO mice. Relative gene expression is measured with respect to the untreated WT (Fig. 2 legend for equation). Circles and brackets denote means ± S.E.M. Abbreviations: WT, wild-type; KO, knockout
Other Divalent Cations During These Experiments
Finally, in addition to Cd, we examined iron and zinc in PSI, liver, kidney, and lung (data not shown). Untreated WT versus KO, Cd-treated WT versus KO, and Cd-treated versus untreated WT versus KO groups were compared. The only significant differences were observed in lung and included: increased Fe in Cd-treated versus untreated WT (P < 0.002), increased Fe in Cd-treated versus untreated KO (P < 0.05), and a trend in decreased Zn in Cd-treated versus untreated KO (P = 0.061).
DISCUSSION
Comparing oral Cd-treated ZIP14 KO mice with WT, we had postulated that Cd uptake and distribution might be decreased in the KO—due to the Cd-importer ZIP14 being very highly expressed in GI tract and liver. This is indeed what was observed for Cd content in liver, but was not found in kidney or lung from 6 or 12 h throughout 10 days of Cd exposure (Fig. 3); at these time-points, Cd accumulation was significantly greater in KO than in mice carrying 1 or 2 copies of the Slc39a14 gene. During oral Cd exposure, most Cd will go first through the portal vein to liver. Because ZIP14 is a major Cd uptake transporter in hepatocytes, KO mice would likely accumulate less Cd in liver—resulting in more Cd remaining in the blood, which could lead to higher accumulation of Cd in other tissues, namely kidney and lung.
Thus, mRNA levels of all 14 ZIP and all 10 ZnT transporters, the DMT1 importer, and MT1 and MT2 chaperone proteins were thoroughly examined in PSI versus kidney to see if these paradoxical effects of increased, rather than decreased, Cd content in the KO could be explained by compensatory up-regulation of any of the other 26 genes. Constitutive mRNA levels were first assessed in PSI and kidney of untreated WT and compared with that in KO mice. Changes in mRNA levels in oral Cd-treated WT versus KO mice were then evaluated.
PSI genes highly expressed in untreated WT animals included ZIP1, ZIP4, ZIP5, ZIP7, ZIP9, ZIP11, ZIP14, ZnT4, ZnT5, ZnT7, ZnT9, ZnT10, DMT1, MT1, and MT2. Kidney genes highly expressed in untreated WT animals included ZIP1, ZIP3, ZIP5, ZIP6, ZIP7, ZIP8, ZIP9, ZIP10, ZIP11, ZIP13, ZIP14, ZnT1, ZnT2, ZnT4, ZnT5, ZnT6, ZnT7, ZnT9, DMT1, MT1, and MT2 (Fig. 1 and Table 1). These constitutive-level results are generally in good agreement with those listed in the Unigene dbEST described earlier. However, there were two exceptions to the data in dbEST: we did not find ZIP12 or ZnT8 in kidney (Fig. 1B and Table 1).
In PSI, constitutive DMT1 expression levels were substantially up-regulated, and ZnT1 down-regulated, in KO compared with that in WT mice (Fig. 2A). In kidney, constitutive MT1, MT2, and ZnT10 expression was substantially up-regulated, and ZnT2 down-regulated, in KO compared with that in WT mice (Fig. 2B).
Bimodal Response Following Cd Exposure
Over the 10-day period of oral Cd, a bimodal curve was commonly seen for Cd content in PSI (Fig. 3), and for the levels of various mRNAs encoded by the importer, exporter, and MT1 and MT2 chaperone protein genes (Figs. 4 and 5; Supplementary Figures 1 and 2). The first phase of increased Cd content in PSI peaked at 2 days; the first phase of increased MT1 and MT2 mRNA levels occurred at 6–12 h, whereas the first phase of most of the ZIP and ZnT mRNA levels was one of down-regulation during the first 6 h to 1 or 2 days.
In PSI striking changes in certain mRNA levels, usually peaking at 5 days, were also seen, e.g., ZIP7, MT1, ZnT8, ZIP14 (Fig. 2), ZIP2, ZIP3, ZIP4, ZIP11, ZIP13, ZnT1, ZnT2, ZnT3, ZnT10 (Supplementary Figure 1). In liver, kidney, and lung we saw continued rates of increased Cd content in all 3 genotypes rather than any bimodal distribution (Fig. 3). Substantial changes in certain renal mRNA levels, again usually peaking at 5 days, were also seen, e.g., ZnT1, MT1, MT2 (Fig. 5), and ZIP4, ZIP7, ZIP9, ZIP11, ZIP13, ZnT3, DMT1 (Supplementary Figure 2). These data appear to be consistent with GI tract being the direct portal-of-entry for oral Cd; then, subsequently, liver, kidney, and lung represent downstream targets indirectly receiving Cd via the blood.
Hence, the first phase (6 h to 1 or 2 days of oral Cd) might represent “Cd shock”, i.e., intracellular oxidative stress (Go et al., 2014) when the animal receives its first bolus of oral Cd. The second phase might be the time for cells to begin adapting to subacute oral Cd-mediated adversity by up- or down-regulating relevant transporter and chaperone genes to combat this sustained insult.
Altered Gene Expression in KO Mouse Lines
In many transgenic models, modifications in gene-expression profiles have been reported in mice having one or another gene genetically removed (Bonzo et al., 2012; Chaudhry et al., 2013; Nebert et al., 2013; Smith et al., 2003; Tsutsui et al., 2010; Wang et al., 2013). There are two fundamental reasons for these changes to occur.
First, a similar gene (redundant or overlapping in function) is “called upon” to take the place of the missing gene, i.e., a compensatory response. Examples include up-regulation of Gsx1 and Gsx2 homeobox genes in the Dlx1/2(−/−) homeobox gene double-KO mouse (Wang et al., 2013), up-regulation of CYP1B1 in GI tract of Cyp1a1(−/−) KO mice and up-regulation of CYP3A59 in preputial gland duct of Cyp1a1/1b1(−/−) double-KO mice (Nebert et al., 2013), and up-regulation of Cyp2b genes in Cyp2e1(−/−) KO mice [F. J. Gonzalez, personal communication].
Second, the ablated gene results in downstream effects of altered gene expression. Examples of such effects include modification of lipid homeostasis genes in Cyp1a2(−/−) mice (Smith et al., 2003), cardiovascular developmental homeostasis in the Nos1/2/3(−/−) triple-KO (Tsutsui et al., 2010), hepatic developmental homeostasis in the Hnf4a(−/−) liver-specific conditional knockout (Bonzo et al., 2012), and changes in hepatic P450 enzyme profile when AKR1D1 is over-expressed versus under-expressed (Chaudhry et al., 2013). Our unexpected finding of increased, rather than decreased, Cd accumulation in Slc39a14(−/−) KO kidney and lung, compared with that in WT, is therefore another example of an unanticipated result detected in a KO mouse study.
Conclusions and Future Directions
A major component of this study was to establish constitutive, as well as oral Cd-induced, changes in expression of any of the ZIP, ZnT and DMT1 transporter genes or MT1 or MT2 chaperone genes when the Slc39a14 gene is absent from the genome. Our study thus sheds light on our current understanding of endogenous divalent cation transport in untreated mice, as well as Cd uptake and distribution kinetics following oral Cd. Moreover, further experiments are suggested. For example, does the absence of ZIP14 in PSI, resulting in up-regulation of constitutive DMT1 expression (Fig. 2A), reflect a compensatory requirement for DMT1 such as importing Zn2+, Fe2+ or Mn2+ from the GI tract? In PSI would substantial down-regulation of constitutive ZnT1 expression in Slc39a14(−/−) mice (Fig. 2A) reflect an obligatory decrease in efflux of endogenous substrates such as Zn2+, Fe2+ or Mn2+? In kidney (Fig. 2B) what is the significance of substantial up-regulation of MT1 and MT2 and down-regulation of ZnT2 constitutive mRNA levels when ZIP14 is absent? We suspect that these alterations in constitutive gene expression might reflect compensatory changes to substitute for loss of ZIP14 function in the PSI or kidney.
There are limited studies on comparisons of Zn2+ and Cd2+ uptake, as well as Cd effects on the 27 genes studied herein. It has been reported, for example, that Cd increased MT1, MT2, and ZnT1 expression in human HepG2 hepatoma cell cultures (Urani et al., 2010) and Cd enhanced MT2 in rat uterus and placenta and Cd elevated ZIP14, ZnT2 and DMT1 expression in rat placenta (Nakamura et al., 2012). To date, there are no studies looking at possible Cd efflux from any cell type by any of the 10 ZnT effluxors. Mammalian ZnT proteins have been found to efflux Zn2+, but not Cd2+; by examining a bacterial ZnT ortholog that does not discriminate between the two cations, (Hoch et al., 2012) suggested that a histidine at the ZnT active site might be critical for refined metal transport selectivity in mammals.
When oral Cd is given to WT versus ZIP14 KO mice, we observed during the first 6 h to 2 days of exposure an acute “Cd shock” response to the toxic metal (mRNA up- and down-regulation of specific transporter and chaperone genes). This was followed by a subacute response of these genes, between 2 and 10 days) when oral Cd was continuously administered (Figs. 4 and 5; Supplementary Figures 1 and 2). Further experiments (e.g., DNA-methylation activities of Cd-relevant genes, miRNA participation, and RNA-Seq analysis) will be necessary to understand in more detail the reasons for our observed biphasic response to oral Cd in WT versus Slc39a14(−/−) KO mice.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/
FUNDING
National Institutes of Health (Grants R01 ES010416 to D.W.N., T32 ES016646 to M.G.-P., and P30 ES06096 to D.W.N.).
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues, especially Lei He and Alvaro Puga, for valuable discussions and careful reading of this manuscript. We appreciate very much the expert graphics help by Marian L. Miller, Professor Emerita. None of the coauthors has any conflicts of interest to disclose.
REFERENCES
- ATSDR. (1999). Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- Babula P., Masarik M., Adam V., Eckschlager T., Stiborova M., Trnkova L., Skutkova H., Provaznik I., Hubalek J., Kizek R. (2012). Mammalian metallothioneins: Properties and functions. Metallomics 4, 739–750. [DOI] [PubMed] [Google Scholar]
- Bonzo J. A., Ferry C. H., Matsubara T., Kim J. H., Gonzalez F. J. (2012). Suppression of hepatocyte proliferation by hepatocyte nuclear factor-4α in adult mice. J. Biol. Chem. 287, 7345–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler J. P., Olivi L., Cheong J. H., Kim Y., Bannona D. (2004). Divalent metal transporter-1 in lead and cadmium transport. Ann. NY Acad. Sci. 1012, 142–152. [DOI] [PubMed] [Google Scholar]
- Chaudhry A. S., Thirumaran R. K., Yasuda K., Yang X., Fan Y., Strom S. C., Schuetz E. G. (2013). Genetic variation in aldo-keto reductase-1D1 (AKR1D1) affects the expression and activity of multiple cytochrome P450s. Drug Metab. Dispos. 41, 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. T., Weber R. E., Yeh H. C., Lundgren D. L., Snipes M. B., Mauderly J. L. (1989). Deposition of cigarette smoke particles in the rat. Fundam. Appl. Toxicol. 13, 429–438. [DOI] [PubMed] [Google Scholar]
- Coyle P., Philcox J. C., Carey L. C., Rofe A. M. (2002). Metallothionein: The multipurpose protein. Cell Mol. Life Sci. 59, 627–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton T. P., He L., Wang B., Miller M. L., Jin L., Stringer K. F., Chang X., Baxter C. S., Nebert D. W. (2005). Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc. Natl Acad. Sci. USA 102, 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freisinger E., Vasak M. (2013). Cadmium in metallothioneins. Met. Ions Life Sci. 11, 339–371. [DOI] [PubMed] [Google Scholar]
- Girijashanker K., He L., Soleimani M., Reed J. M., Li H., Liu Z., Wang B., Dalton T. P., Nebert D. W. (2008). Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 73, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y. M., Roede J. R., Orr M., Liang Y., Jones D. P. (2014). Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of Cd toxicity. Toxicol. Sci. 139, 59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Girijashanker K., Dalton T. P., Reed J., Li H., Soleimani M., Nebert D. W. (2006). ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 70, 171–180. [DOI] [PubMed] [Google Scholar]
- He L., Wang B., Hay E. B., Nebert D. W. (2009). Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol. Appl. Pharmacol. 238, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch E., Lin W., Chai J., Hershfinkel M., Fu D., Sekler I. (2012). Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc. Natl Acad. Sci. USA 109, 7202–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo S., Fukada T., Shimoda S., Ohashi W., Bin B. H., Koseki H., Hirano T. (2011). The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS One 6, e18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. W., Lin J. L., Lin-Tan D. T., Yen T. H., Huang W. H., Ho T. C., Huang Y. L., Yeh L. M., Huang L. M. (2009). Association of environmental cadmium exposure with inflammation and malnutrition in maintenance haemodialysis patients. Nephrol. Dial. Transplant 24, 1282–1288. [DOI] [PubMed] [Google Scholar]
- Huang L., Tepaamorndech S. (2013). The SLC30 family of zinc transporters: A review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 34, 548–560. [DOI] [PubMed] [Google Scholar]
- Järup L. (2003). Hazards of heavy metal contamination. Brit. Med. Bull. 68, 167–182. [DOI] [PubMed] [Google Scholar]
- Jin T., Wu X., Tang Y., Nordberg M., Bernard A., Ye T., Kong Q., Lundstrom N. G., Nordberg G. F. (2004). Environmental epidemiological study and estimation of benchmark dose for renal dysfunction in a cadmium-polluted area in China. Biometals 17, 525–530. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Liu J. (1998). Induction of metallothionein as an adaptive mechanism affecting the magnitude and progression of toxicological injury. Environ Health Perspect 106(Suppl. 1), 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D., Liu J., Choudhuri S. (1999). Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 39, 267–294. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Liu J., Diwan B. A. (2009). Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 238, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Li H., Soleimani M., Girijashanker K., Reed J. M., He L., Dalton T. P., Nebert D. W. (2008). Cd2+ versus Zn2+ uptake by the ZIP8 HCO3–dependent symporter: Kinetics, electrogenicity and trafficking. Biochem. Biophys. Res. Commun. 365, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopin K. V., Thevenod F., Page J. C., Jones S. W. (2012). Cd2+ block and permeation of Cav3.1 (α1G) T-type calcium channels: candidate mechanism for Cd2+ influx. Mol. Pharmacol. 82, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Meltzer H. M., Brantsaeter A. L., Borch-Iohnsen B., Ellingsen D. G., Alexander J., Thomassen Y., Stigum H., Ydersbond T. A. (2010). Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT-2 study. Environ. Res. 110, 497–504. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Ohba K., Ohta H. (2012). Participation of metal transporters in cadmium transport from mother rat to fetus. J. Toxicol. Sci. 37, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Galvez-Peralta M., Hay E. B., Li H., Johansson E., Yin C., Wang B., He L., Soleimani M. (2012). ZIP14 and ZIP8 zinc/bicarbonate symporters in Xenopus oocytes: Characterization of metal uptake and inhibition. Metallomics 4, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Shi Z., Galvez-Peralta M., Uno S., Dragin N. (2013). Oral benzo[a]pyrene: Understanding pharmacokinetics, detoxication, and consequences–Cyp1 knockout mouse lines as a paradigm. Mol. Pharmacol. 84, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quig D. (1998). Cysteine metabolism and metal toxicity. Altern. Med. Rev. 3, 262–270. [PubMed] [Google Scholar]
- Rozman K. K., Klaassen C. D. (2007). Absorption, distribution and excretion of toxicants. In Casarett and Doulĺs Toxicology: The Basic Science of Poisons (Klaassen C. D., Ed.), pp. 107–132 McGraw-Hill, New York City. [Google Scholar]
- Satarug S., Ujjin P., Vanavanitkun Y., Baker J. R., Moore M. R. (2004). Influence of body iron store status and cigarette smoking on cadmium body burden of healthy Thai women and men. Toxicol. Lett. 148, 177–185. [DOI] [PubMed] [Google Scholar]
- Schneider S. N., Liu Z., Wang B., Miller M. L., Afton S. E., Soleimani M., Nebert D. W. (2014). Oral cadmium in mice carrying 5 versus 2 copies of the Slc39a8 gene: Comparison of uptake, distribution, metal content, and toxicity. Int. J. Toxicol. 33, 14–20. [DOI] [PubMed] [Google Scholar]
- Shawki A., Knight P. B., Maliken B. D., Niespodzany E. J., Mackenzie B. (2012). H+-coupled divalent metal-ion transporter-1: Functional properties, physiological roles and therapeutics. Curr. Top. Membr. 70, 169–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Davies R., Dalton T. P., Miller M. L., Judah D., Riley J., Gant T., Nebert D. W. (2003). Intrinsic hepatic phenotype associated with the Cyp1a2 gene, as shown by cDNA expression microarray analysis of the knockout mouse. EHP Toxicogenomics 111, 45–51. [PubMed] [Google Scholar]
- Stoeppler M. (1991). Metals and their compounds in the environment. In Cadmium (Merian E., Ed.), 9th edn., pp. 803–851 VCH, Weinheim, New York; Basel; & Cambridge. [Google Scholar]
- Thévenod F., Lee W. K. (2013). Toxicology of cadmium and its damage to mammalian organs. Met. Ions. Life Sci. 11, 415–490. [DOI] [PubMed] [Google Scholar]
- Tsutsui M., Shimokawa H., Otsuji Y., Yanagihara N. (2010). Pathophysiological relevance of NO signaling in the cardiovascular system: Novel insight from mice lacking all NO synthases. Pharmacol. Ther. 128, 499–508. [DOI] [PubMed] [Google Scholar]
- Urani C., Melchioretto P., Gribaldo L. (2010). Regulation of metallothioneins and ZnT1 transporter expression in human hepatoma cells HepG2 exposed to zinc or cadmium. Toxicol. in vitro 24, 370–374. [DOI] [PubMed] [Google Scholar]
- Waisberg M., Joseph P., Hale B., Beyersmann D. (2003). Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192, 95–117. [DOI] [PubMed] [Google Scholar]
- Wang B., Long J. E., Flandin P., Pla R., Waclaw R. R., Campbell K., Rubenstein J. L. (2013). Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J. Comp. Neurol. 521, 1561–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Schneider S. N., Dragin N., Girijashanker K., Dalton T. P., He L., Miller M. L., Stringer K. F., Soleimani M., Richardson D. D., et al. (2007). Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am. J. Physiol. Cell Physiol. 292, C1523–C1535. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Ahmad S. (2003). Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 186, 163–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.