Abstract
Streptococcus pneumoniae neuraminidase has been implicated as a virulence factor in the pathogenesis of pneumococcal otitis media. In this study, native neuraminidase was partially purified from cultures of S. pneumoniae by serial chromatography with DEAE-Sepharose and Sephacryl S-200. Recombinant neuraminidase, a 3,038-bp fragment of the neuraminidase A (nanA) gene, was cloned into the pET-28b vector and then expressed at high levels in Escherichia coli. Chinchillas were immunized subcutaneously with either the gel-purified native or recombinant neuraminidase, and all responded with elevated titers of antineuraminidase antibody in serum. Immunization with neuraminidase resulted in a significant reduction in nasopharyngeal colonization as well as in the incidence of otitis media with effusion. These data demonstrate for the first time that neuraminidase affords protection against S. pneumoniae nasopharyngeal colonization and experimental otitis media.
Streptococcus pneumoniae is the most frequent cause of otitis media (OM) in children, accounting for 30% of the cases of acute OM and 5% of the cases of chronic OM with effusion (OME) (14). All S. pneumoniae isolates to date have been shown to produce neuraminidase (9). S. pneumoniae neuraminidase has been detected in 78% of culture-positive human middle ear effusions from patients with acute OM and in 96% of S. pneumoniae-positive middle ear effusions from patients with chronic OME (7). Neuraminidase is an enzyme that cleaves N-acetylneuraminic acid from mucin, glycolipids, glycoproteins, and oligosaccharides on host cell surfaces (18, 20). Results of studies conducted in our laboratory indicate that during S. pneumoniae-induced OM in the chinchilla, terminal sialic acid residues are removed from the surface of the epithelium lining the lumen of the eustachian tube, presumably by neuraminidase, resulting in the exposure of GlcNAc β 1-4 Gal, which is part of one of the S. pneumoniae eukaryotic receptors (10, 11). Additional studies conducted by our laboratory implicate neuraminidase as a virulence factor for S. pneumoniae (23, 24). We found that the ability of a neuraminidase-deficient mutant to colonize and persist within the nasopharynx and middle ear was significantly impaired relative to the parent strain (21). These data indicate that neuraminidase plays an important role in the ability of S. pneumoniae to colonize and persist in the nasopharynx and induce OM. For this reason, we have chosen to investigate whether neuraminidase is a protective antigen and a potential protein-based vaccine candidate in the prevention of S. pneumoniae nasopharyngeal (NP) colonization and OM.
Purification of native neuraminidase.
Neuraminidase was purified from whole-cell lysates as previously described by Lock et al. (12). S. pneumoniae 6A (EF3114) was used for purification of native neuraminidase as well as intranasal inoculation in the chinchilla model of OM and has been described in detail previously (1, 22). Fractions from a DEAE-Sepharose column containing neuraminidase (identified by use of a neuraminidase assay described below) were pooled, washed, and concentrated at 4°C with buffer D (50 mM Tris base, 0.15 M NaCl, 0.1 mM Na-EDTA, 200 mM phenylmethylsulfonyl fluoride, 0.01% sodium azide) by using an Amicon stirred ultrafiltration cell model 8200 fitted with a polyethersulfone membrane with a 50,000 molecular weight cutoff (Millipore Corporation, Bedford, Mass.). The concentrated product was then applied to a Sephacryl S-200 (XK-26/100 column; Amersham Pharmacia, Piscataway, N.J.) and eluted with buffer D. Those fractions with the highest level of neuraminidase activity were pooled, washed, and concentrated by ultrafiltration. The final column-purified neuraminidase product was stored at −20°C.
Cloning and expression of nanA gene fragments in Escherichia coli.
A 3,083-bp fragment of the nanA gene was PCR amplified from template DNA from S. pneumoniae serotype 2 strain D39, which has been described previously (21). The forward primer was 5′-ACGCTAGCATGAATCGGAGTGTTCAAGAACG-3′, and the reverse primer was 5′-ACCTCGAGTTGTTCTCTCTTTTTCCCTAGC-3′, with the boldface type indicating the additional bases introduced for cloning. The PCR fragment of the expected size was then generated and cloned to the pET28b vector (Novagen, Madison, Wis.) and used to transform competent E. coli Top10 cells (Invitrogen, Carlsbad, Calif.). The presence and integrity of the recombinant nanA-pET28b plasmid were examined in the kanamycin-resistant transformants by restriction endonuclease digestion and by sequencing of the nanA gene.
For expression, the recombinant plasmid containing the nanA fragment was prepared using an EndoFree plasmid maxi kit (QIAGEN, Valencia, Calif.) and transformed into E. coli BL21(DE3)pLysS cells (Novagen). Expression of nanA was induced with 1 mM isopropylthio-β-d-galactoside (IPTG) for 3 h. Partial purification of the histidine (six-His)-tagged neuraminidase from the cell pellets was accomplished by use of Ni-nitrilotriacetic acid (Ni-NTA) agarose according to the manufacturer's protocol (QIAGEN).
The control for these experiments consisted of the pET-28b expression vector transformed directly into E. coli BL21(DE3)pLysS without the nanA insert. The resultant transformant, E. coli pLysS-Control, was subjected to the same expression, induction, and purification procedures as described above for E. coli pLysS-nanA.
Assay for neuraminidase activity.
Neuraminidase activity was measured in protein preparations using a fluorimetric assay adapted from that of Lock et al. (12, 26).
For detection of neuraminidase in protein samples following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), an overlay assay described by Byers et al. was employed (4). The neuraminidase was visualized by use of UV light (Multi Image light cabinet; Alpha Innotec Corporation, San Leandro, Calif.).
Immunization of chinchillas with gel-purified native or recombinant neuraminidase.
The partially purified native and recombinant neuraminidase preparations were subjected to SDS-PAGE using Prep+2 Criterion precast gels (Bio-Rad Laboratories, Hercules, Calif.), and the protein band containing neuraminidase enzymatic activity was identified using the overlay procedure described above. The thin fluorescent band containing neuraminidase was excised, snap-frozen in liquid nitrogen, and ground to a fine powder. The homogenized gel solution was then diluted with an equal volume of Freund's complete adjuvant. Chinchillas (Chinchilla lanigera; 200 to 300 g) were randomly assigned to two cohorts, and each was immunized by the subcutaneous injection of 100 μg of the native or recombinant neuraminidase preparations. Two additional booster immunizations in Freund's incomplete adjuvant with the same doses of neuraminidase were given at 1-month intervals. The control cohort for the native neuraminidase consisted of chinchillas sham immunized with gel slice in Freund's adjuvant. The control cohort for the recombinant neuraminidase consisted of concentrated eluate from the transformant pLysS-Control. Blood for sera were obtained from all animals prior to the primary immunization and 2 weeks following the final boost. This experiment was repeated once.
Western blot assay for neuraminidase.
Column-purified neuraminidase samples (3 μg per lane) were subjected to SDS-PAGE and then transferred electrophoretically to nitrocellulose membranes. The membranes were blocked overnight at 4°C with 1% bovine serum albumin in phosphate-buffered saline (pH 7.2), incubated with serial dilutions of chinchilla serum (pre- or postimmunization with the gel-purified native or recombinant neuraminidase) in phosphate-buffered saline with 1% bovine serum albumin and 0.05% Tween 20 (Sigma, St. Louis, Mo.), and then incubated with a 1:2,000 dilution of horseradish peroxidase-protein A (Zymed Laboratories Inc., San Francisco, Calif.). The membranes were developed with TMB Single solution (Zymed Laboratories).
Western blotting was also performed using the Penta-His horseradish peroxidase conjugate antibody (QIAGEN) to detect and identify the histidine-tagged recombinant neuraminidase in accordance with the manufacturer's protocol (QIAexpress Detection and Assay Handbook).
Intranasal inoculation with influenza A virus.
Coinoculation of S. pneumoniae with influenza A virus is necessary to establish OM in the chinchilla via the intranasal route. Influenza virus A/Alaska/6/77 (H3N2) has previously been used in combination with S. pneumoniae by our laboratory and others to induce experimental OM in the chinchilla model (8, 25), and it has been described in detail previously (6, 16). Fourteen days following the final immunization with neuraminidase, the chinchillas were anesthetized and inoculated intranasally with approximately 6 × 106 PFU of influenza A virus per our standard protocol (22).
Intranasal challenge with S. pneumoniae and assessment of NP colonization and the development of OME for immunized chinchillas.
For inoculation of S. pneumoniae 6A, log-phase cultures were prepared from chocolate agar plate subcultures as previously described (25). Six days following intranasal inoculation with influenza A virus, all animals (both the experimental and sham-immunized control groups) were challenged intranasally with 5 × 107 CFU of S. pneumoniae 6A. Three days following intranasal challenge with S. pneumoniae, the chinchillas were evaluated by tympanocentesis and NP lavage as previously described (21). The middle ear and NP lavage samples were cultured overnight on Columbia agar plates at 37°C with 5% CO2, and the number of CFU per milliliter was determined by standard dilution and plate count.
For each chinchilla, tympanic membrane inflammation was assessed in both ears by means of an otoscope by using the rating scale previously described (22). Animals with significant to severe erythema, retraction, and middle ear fluid (obtained by tympanocentesis) were considered to have developed OME.
Statistical analysis.
Data are expressed as the geometric means ± standard errors. Differences in S. pneumoniae concentrations in nasal and middle ear lavage samples from the cohorts immunized with neuraminidase or buffer alone were analyzed by use of the Mann-Whitney rank sum test. Differences in the incidence of OME were analyzed by use of chi-square analysis. For both the Mann-Whitney rank sum test and chi-square analysis, P of <0.05 was set as the level of significance.
Purification of native neuraminidase.
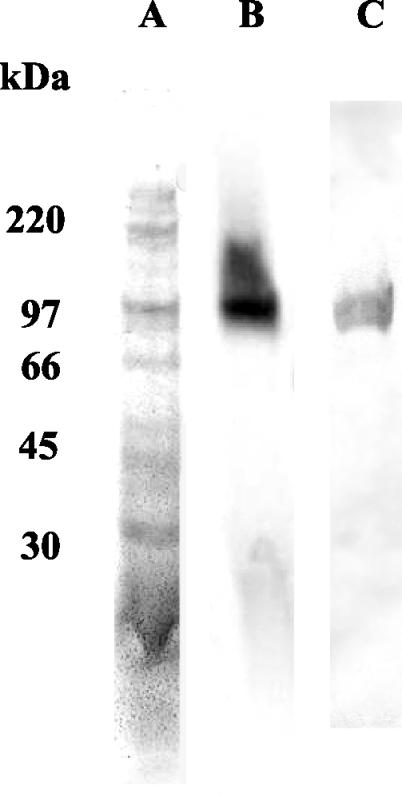
The column-purified neuraminidase had a specific activity of 439 U/mg of protein. An SDS-PAGE gel of a crude column-purified preparation can be seen in Fig. 1. An overlay assay using the fluorogenic neuraminidase substrate revealed that enzymatic activity was associated with the 100-kDa band, which is in accordance with previously reported molecular weights for neuraminidase (12) (Fig. 1).
FIG. 1.
Analysis of crude column-purified native S. pneumoniae neuraminidase (3 μg per lane) following SDS-PAGE. Lanes: A, column-purified native neuraminidase was subjected to SDS-PAGE and stained with Coomassie brilliant blue; B, the neuraminidase identified and visualized by a fluorimetric assay; C, Western blotting performed using sera from chinchillas immunized with gel-purified neuraminidase (1:10,000 dilution of serum). This figure represents a composite of lanes taken from separate gels.
Expression and purification of recombinant NanA.
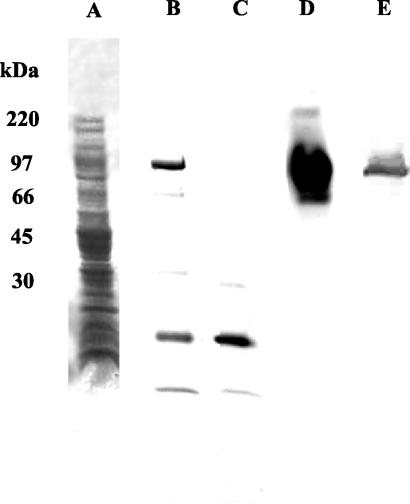
As can be seen in Fig. 2, the final product typically contained two specific protein bands at or above 100 kDa, as well as several low-molecular-mass bands. An overlay assay using the fluorogenic neuraminidase substrate revealed that enzymatic activity was associated with the two protein bands at or above 100 kDa. Enzymatic activity was not associated with the lower-molecular-mass bands (below 100 kDa). In contrast, SDS-PAGE analysis of the final product obtained following Ni-NTA agarose column purification of cell lysates from control cultures had no proteins in the 100-kDa range. The only protein bands obtained were below 50 kDa. No neuraminidase activity was obtained for control cultures, as determined by the overlay assay.
FIG. 2.
Analysis of recombinant S. pneumoniae neuraminidase following SDS-PAGE. Lanes: A, lysate from the recombinant E. coli expression construct (prior to purification with Ni-NTA agarose) stained with Coomassie brilliant blue; B, the final purified NanA after elution from Ni-NTA agarose stained with Coomassie brilliant blue; C, the final product from E. coli without insertion of nanA stained with Coomassie brilliant blue; D, the final purified NanA identified and visualized by a fluorimetric assay; E, crude column-purified native neuraminidase (3 μg per lane) was probed with antisera from chinchillas immunized with gel-purified recombinant neuraminidase (1:10,000 dilution of serum). This figure represents a composite of lanes taken from separate gels.
A Western blot with Penta-His antibody against the final recombinant protein product recognized exclusively the two protein bands associated with neuraminidase enzymatic activity (data not shown).
Western blot analysis to determine serum antibody response.
Antiserum generated in chinchillas immunized with gel-purified native neuraminidase recognized the 100-kDa protein band associated with neuraminidase enzymatic activity (Fig. 1).
When antiserum from chinchillas immunized with the recombinant neuraminidase was tested against column-purified native neuraminidase in a Western blot, the antiserum recognized the protein band associated with neuraminidase enzymatic activity, as well as a smear of protein above and below 100 kDa (Fig. 2).
Titers for antiserum raised against either native or recombinant neuraminidase were typically in excess of 1:100,000. Serum collected from animals prior to the start of the experiment (prebleeds) as well as serum from sham-inoculated controls contained no detectable activity versus native neuraminidase when tested at a 1:1,000 dilution.
Effect of native neuraminidase immunization on NP colonization, middle ear invasion, and the development of OME.
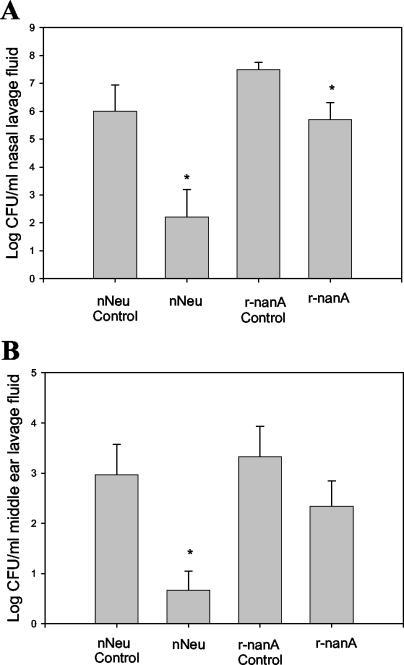
Immunization with native neuraminidase resulted in a 32% reduction in the incidence rate of OME for neuraminidase-immunized chinchillas (P = 0.031). In the cohort receiving the gel-purified native neuraminidase, 3 out of 22 ears (14%) developed OME. In contrast, for sham-immunized animals, 13 of 28 (46%) ears developed OME. There was also a significant reduction in NP colonization and middle ear invasion by S. pneumoniae following immunization with the native neuraminidase (Fig. 3).
FIG. 3.
Immunization with native neuraminidase (nNeu) or recombinant neuraminidase (r-nanA) reduces NP colonization (A) and middle ear invasion (B). Data shown represent the geometric mean number of CFU of S. pneumoniae ± the standard errors of the means per milliliter of nasal lavage fluid. *, P of <0.05 for the comparison.
Effect of recombinant neuraminidase NanA immunization on NP colonization, middle ear invasion, and the development of OME.
In order to rule out the possibility of contamination with capsular polysaccharide or other pneumococcal antigens, a protection study was conducted using recombinant neuraminidase NanA expressed in E. coli. Immunization with gel-purified recombinant neuraminidase NanA resulted in a 33% reduction in the incidence of OME relative to sham-immunized controls (P = 0.047). In the cohort receiving the recombinant neuraminidase, only 8 out of 26 ears (31%) developed OME. In contrast, for sham-immunized animals, 14 of 22 (64%) ears developed OME.
Immunization with recombinant neuraminidase NanA also resulted in a significant reduction in NP colonization (Fig. 3). There was an almost 10-fold reduction in the number of viable S. pneumoniae in the middle ear lavage samples of the experimental cohort compared to that of the control group; however, this difference was not statistically significant.
The data presented here demonstrate that immunization of chinchillas with S. pneumoniae neuraminidase confers a degree of protection against pneumococcal OM. Two distinct pneumococcal neuraminidases, NanA and NanB, have been cloned and sequenced (2, 5). The use of neuraminidase as a possible protective antigen was initially assessed in an S. pneumoniae intraperitoneal (i.p.) infection model in mice (13). In that study, a comparison between neuraminidase and pneumolysin revealed that although inoculation with purified neuraminidase afforded significant protection against systemic infection and death relative to sham-inoculated controls, it was not as effective as pneumolysin. Furthermore, immunization with neuraminidase did not prevent mortality following i.p. inoculation with S. pneumoniae. These prior assessments did not take into consideration the special role neuraminidase plays in the host-parasite interaction of S. pneumoniae within the respiratory epithelium. As noted in the introduction, a body of evidence suggests that neuraminidase plays a crucial role in NP colonization by S. pneumoniae and the development of OM. In this regard, neuraminidase as a protective antigen appears to be anatomically niche specific and plays a significant role in the prevention of pneumococcal diseases involving the respiratory epithelium.
Since whole-cell lysates were used to prepare the native neuraminidase, a more important and/or relevant question is whether it was neuraminidase or other pneumococcal antigens that were responsible for the protective effect of the gel-purified native neuraminidase preparation. In order to answer this question, we conducted immunization studies utilizing a recombinant S. pneumoniae neuraminidase A (NanA). We found that immunization with the recombinant NanA resulted in a significant reduction in the incidence of OME and NP colonization. In fact, the reductions in the incidence rates of OME relative to controls for the animals immunized with either native or recombinant neuraminidase were very similar (32 and 33%, respectively). However, immunization with recombinant NanA resulted in an effect on NP colonization and concentration of S. pneumoniae in the middle ear fluid that was less than with the native neuraminidase. There are two possible explanations for this. First, the native and recombinant NanA experiments were performed separately, resulting in different baseline NP colonization levels. Second, the native neuraminidase preparations used for immunization could have also contained NanB or NanC, thereby resulting in a higher level of protection. Although it has not been determined, it is reasonable to assume that all three pneumococcal neuraminidases may play a role in the pathogenesis of OM. The separate recombinant NanA expression and purification performed as part of this study ensured that the observed protection was not due to contaminating S. pneumoniae antigens such as capsular polysaccharide or other surface proteins. These results dispel and allay concerns regarding the purity of the native neuraminidase preparations and clearly demonstrate the potential of neuraminidase as a protective immunogen.
The threat of rapid emergence and dissemination of strains which can circumvent polysaccharide capsular-based vaccines points to the need for the development of S. pneumoniae protein-based vaccines (3, 15, 19). Noncapsular antigens currently being evaluated as potential vaccine candidates include pneumococcal surface protein A, pneumolysin, and permease PsaA (17). The results of this study suggest that neuraminidase should also be evaluated as a vaccine candidate for the prevention of pneumococcal infections of the respiratory epithelium in general and OM in particular.
Acknowledgments
This study was supported, in part, by a grant from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (RO1 DC3105-06).
Editor: J. N. Weiser
REFERENCES
- 1.Andersson, B., J. Dahmen, T. Frejd, H. Leffler, G. Magnusson, G. Noori, and C. Svanborg-Eden. 1983. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J. Exp. Med. 158:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, A. M., R. A. Lock, and J. C. Paton. 1996. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J. Bacteriol. 178:4854-4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler, J. C., R. F. Breiman, J. F. Campbell, H. B. Lipman, C. V. Broome, and R. R. Facklam. 1993. Pneumococcal vaccine efficacy: an evaluation of current recommendations. JAMA 270:1826-1831. [PubMed] [Google Scholar]
- 4.Byers, H. L., E. Tarelli, K. A. Homer, and D. Beighton. 2000. Isolation and characterisation of sialidase from a strain of Streptococcus oralis. J. Med. Microbiol. 49:235-244. [DOI] [PubMed] [Google Scholar]
- 5.Camara, M., G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1994. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 62:3688-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung, M. H., S. R. Griffith, K. H. Park, D. J. Lim, and T. F. DeMaria. 1993. Cytological and histological changes in the middle ear after inoculation of influenza A virus. Acta Otolaryngol. (Stockholm) 113:81-87. [DOI] [PubMed] [Google Scholar]
- 7.Diven, W. F., W. J. Doyle, and B. Vietmeier. 1988. Hydrolytic enzymes in otitis media pathogenesis. Ann. Otol. Rhinol. Laryngol. Suppl. 132:6-9. [DOI] [PubMed] [Google Scholar]
- 8.Giebink, G. S. 1989. The microbiology of otitis media. Pediatr. Infect. Dis. 8:S18-S20. [PubMed] [Google Scholar]
- 9.Jedrzejas, M. J. 2001. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 65:187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder, T. E., R. L. Daniels, D. J. Lim, and T. F. DeMaria. 1994. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb. Pathog. 16:435-441. [DOI] [PubMed] [Google Scholar]
- 11.Linder, T. E., D. J. Lim, and T. F. DeMaria. 1992. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb. Pathog. 13:293-303. [DOI] [PubMed] [Google Scholar]
- 12.Lock, R. A., J. C. Paton, and D. Hansman. 1988. Purification and immunological characterization of neuraminidase produced by Streptococcus pneumoniae. Microb. Pathog. 4:33-43. [DOI] [PubMed] [Google Scholar]
- 13.Lock, R. A., J. C. Paton, and D. Hansman. 1988. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb. Pathog. 5:461-467. [DOI] [PubMed] [Google Scholar]
- 14.Luotonen, J., E. Herva, P. Karma, M. Timonen, M. Leinonen, and P. H. Makela. 1981. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand. J. Infect. Dis. 13:177-183. [DOI] [PubMed] [Google Scholar]
- 15.Nesin, M., M. Ramirez, and A. Tomasz. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J. Infect. Dis. 177:707-713. [DOI] [PubMed] [Google Scholar]
- 16.Park, K., L. O. Bakaletz, J. M. Coticchia, and D. J. Lim. 1993. Effect of influenza A virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann. Otol. Rhinol. Laryngol. 102:551-558. [DOI] [PubMed] [Google Scholar]
- 17.Patton, J. C. 1998. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 6:85-87. [DOI] [PubMed] [Google Scholar]
- 18.Scanlon, K. L., W. F. Diven, and R. Glew. 1989. Purification and properties of Streptococcus pneumoniae neuraminidase. Enzyme 41:143-150. [DOI] [PubMed] [Google Scholar]
- 19.Siber, G. R. 1994. Pneumococcal disease: prospects for a new generation of vaccines. Science 265:1385-1387. [DOI] [PubMed] [Google Scholar]
- 20.Tanenbaum, S. W., and S. C. Sun. 1971. Some molecular properties of pneumococcal neuraminidase isoenzymes. Biochim. Biophys. Acta 229:824-828. [DOI] [PubMed] [Google Scholar]
- 21.Tong, H. H., L. E. Blue, M. A. James, and T. F. DeMaria. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong, H. H., L. M. Fisher, G. M. Kosunick, and T. F. DeMaria. 2000. Effect of adenovirus type 1 and influenza A virus on Streptococcus pneumoniae nasopharyngeal colonization and otitis media in the chinchilla. Ann. Otol. Rhinol. Laryngol. 109:1021-1027. [DOI] [PubMed] [Google Scholar]
- 23.Tong, H. H., X. Liu, Y. Chen, M. James, and T. F. DeMaria. 2002. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta Otolaryngol. 122:413-419. [DOI] [PubMed] [Google Scholar]
- 24.Tong, H. H., M. A. McIver, L. M. Fisher, and T. F. DeMaria. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb. Pathog. 26:111-119. [DOI] [PubMed] [Google Scholar]
- 25.Tong, H. H., J. N. Weiser, M. A. James, and T. F. DeMaria. 2001. Effect of influenza A virus infection on nasopharyngeal colonization and otitis media induced by transparent or opaque phenotype variants of Streptococcus pneumoniae in the chinchilla model. Infect. Immun. 69:602-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter, A. J., S. D. Comis, M. P. Osborne, M. J. Tarlow, J. Stephen, P. W. Andrew, J. Hill, and T. J. Mitchell. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]