Abstract
Rotenone, a common pesticide and inhibitor of mitochondrial complex I, induces loss of dopaminergic neurons and consequential aspects of Parkinson’s disease (PD). However, the exact mechanism of rotenone neurotoxicity is not fully elucidated. Here, we show that rotenone induced reactive oxygen species (ROS), leading to apoptotic cell death in PC12 cells and primary neurons. Pretreatment with catalase (CAT), a hydrogen peroxide-scavenging enzyme, attenuated rotenone-induced ROS and neuronal apoptosis, implying hydrogen peroxide (H2O2) involved, which was further verified by imaging intracellular H2O2 using a peroxide-selective probe H2DCFDA. Using thenoyltrifluoroacetone (TTFA), antimycin A, or Mito-TEMPO, we further demonstrated rotenone-induced mitochondrial H2O2-dependent neuronal apoptosis. Rotenone dramatically inhibited mTOR-mediated phosphorylation of S6K1 and 4E-BP1, which was also attenuated by CAT in the neuronal cells. Of interest, ectopic expression of wild-type mTOR or constitutively active S6K1, or downregulation of 4E-BP1 partially prevented rotenone-induced H2O2 and cell apoptosis. Furthermore, we noticed that rotenone-induced H2O2 was linked to the activation of caspase-3 pathway. This was evidenced by the finding that pretreatment with CAT partially blocked rotenone-induced cleavages of caspase-3 and poly (ADP-ribose) polymerase. Of note, zVAD-fmk, a pan caspase inhibitor, only partially prevented rotenone-induced apoptosis in PC12 cells and primary neurons. Expression of mTOR-wt, S6K1-ca, or silencing 4E-BP1 potentiated zVAD-fmk protection against rotenone-induced apoptosis in the cells. The results indicate that rotenone induction of H2O2 inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, resulting in caspase-dependent and -independent apoptosis in neuronal cells. Our findings suggest that rotenone-induced neuronal loss in PD may be prevented by activating mTOR signaling and/or administering antioxidants.
Keywords: rotenone, hydrogen peroxide, mammalian target of rapamycin, neuronal cells, apoptosis, Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by loss of midbrain dopaminergic neurons in the substantia nigra pars compacta (SNc) (Choi et al., 2004; Lin et al., 2012; Malagelada et al., 2008). Mounting data indicate that pesticide exposure is associated with an increased risk for developing PD (Dhillon et al., 2008; Taetzsch and Block, 2013; Tanner et al., 2011). As a broad-spectrum pesticide, rotenone is a naturally occurring lipophilic compound extracted from the roots and stems of certain plants species (Derris and Lonchocarpus) (Emmrich et al., 2013; Giordano et al., 2012; Wu et al., 2013; Xiong et al., 2012). It has been described that rotenone readily traverses the blood-brain barrier and cellular membranes without transporters (Blesa et al., 2012). Once inside cells, rotenone impedes mitochondrial function leading to intracellular oxidative stress (Drechsel and Patel, 2008; Taetzsch and Block, 2013). Particularly, it can mimic most clinical features of idiopathic PD and recapitulate the slow and progressive loss of dopaminergic neurons and the Lewy body formation in the nigral-striatal system, revealing that rotenone neurotoxicity is a possible etiological factor in neurodegenerative diseases (Cannon et al., 2009; Gao et al., 2003; Rodriguez-Rocha et al., 2013; Xiong et al., 2012). However, the exact mechanism(s) by which rotenone elicits its neurotoxic effects on brain is still incompletely understood.
Overwhelming evidence has demonstrated that oxidative stress and partial deficiency of mitochondrial complex I play a crucial role in the pathogenesis of PD (Adam-Vizi, 2005; Tretter et al., 2004). They are interconnected; complex I inhibition results in an increased production of reactive oxygen species (ROS), which, in turn, inhibits the function of complex I (Tretter et al., 2004). The vicious cycle in dopaminergic neurons leads over time to excessive oxidative stress and ATP deficit that eventually will result in cell death in the nigro-striatal pathway (Adam-Vizi, 2005; Tamilselvam et al., 2013; Tretter et al., 2004). It has been reported that hydrogen peroxide (H2O2), a major radical of ROS, in mitochondria in situ in isolated nerve terminals is sufficiently enhanced when complex I is inhibited at a small degree (Adam-Vizi, 2005). Rotenone is a powerful and selective inhibitor of mitochondrial complex I. This prompted us to study whether rotenone indeed elicits ROS/H2O2 generation contributing to neuronal apoptosis.
Excessive or sustained ROS can easily react with thiol groups of proteins, thereby disrupting the structure of cellular proteins and altering their functions, and also activate or inhibit related signaling pathways, leading to neuronal apoptosis or neurodegeneration (Chen et al., 2010, 2011; Franklin, 2011; Kim and Choi, 2010; Miller et al., 2009; Niizuma et al., 2010). For example, rotenone inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells (Shamoto-Nagai et al., 2003), and the inhibitory effects of rotenone on proteasome activity are involved in PD (Wang XF et al., 2006). A pivotal signaling pathway that is activated by ROS is the mitogen-activated protein kinase (MAPK) pathway. It has been reported that rotenone triggers cell apoptosis through ROS and c-Jun N-terminal kinase (JNK)/p38 MAPKs in MCF-7 human breast cancer cells, RGC-5 cells, SH-SY5Y cells (Deng et al., 2010; Kamalden et al., 2012; Newhouse et al., 2004). However, whether rotenone targets other signaling pathways responsible for neuronal cell survival via ROS is largely unknown. Mammalian target of rapamycin (mTOR), a serine/threonine (Ser/Thr) protein kinase, is a central controller for cell proliferation/growth and survival (Cornu et al., 2013; Laplante and Sabatini, 2012). Extensive studies have shown that mTOR regulates differentiation, development and survival in neurons, and exerts a crucial role in synaptic plasticity, learning and memory, and food uptake in adult brain (Jaworski and Sheng, 2006; Swiech et al., 2008). Especially, mTOR activity is modified in various pathologic states of the nervous system, including brain tumors, tuberous sclerosis, cortical dysplasia, and neurodegenerative disorders such as PD, Alzheimer’s disease (AD), and Huntington’s disease (HD) (Swiech et al., 2008). Our group has recently demonstrated that cadmium induction of ROS activates mTOR signaling contributing to neuronal cell death (Chen et al., 2011), whereas H2O2 induces neuronal cell death via suppression of mTOR pathway (Chen et al., 2010). We have also identified that PD mimetics (6-hydroxydopamine, N-methyl-4-phenylpyridine or rotenone) activate AMP-activated protein kinase (AMPK) and inactivate protein kinase B (PKB/Akt), causing neuronal cell death via inhibiting mTOR signaling pathway (Xu et al., 2014). Here we show that rotenone induced H2O2-dependent inhibition of mTOR-mediated ribosomal p70 S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) pathways, resulting in caspase-dependent and -independent apoptosis in neuronal cells.
MATERIALS AND METHODS
Chemicals and reagents
Rotenone, catalase (CAT), poly-d-lysine (PDL), 2′7′-dichlorodihydrofluorescein diacetate (H2DCFDA), 2′7′-dichlorofluorescein (DCF), protease inhibitor cocktail, thenoyltrifluoroacetone (TTFA), antimycin A, and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma (St Louis, MO). Dulbecco’s modified Eagle medium (DMEM), 0.05% Trypsin-EDTA, NEUROBASAL™ Media, and B27 Supplement were purchased from Invitrogen (Grand Island, NY). Horse serum and fetal bovine serum (FBS) were supplied by Hyclone (Logan, UT). Enhanced chemiluminescence solution was from Millipore (Billerica, MA). CellTiter 96 AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI). The 5 -(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was purchased from MP Biomedicals Inc (Solon, OH). Mitochondria-targeted antioxidant, Mito-TEMPO and the pan-caspase inhibitor, z-Val-Ala-Asp-CH2F (zVAD-fmk) were acquired from ALEXIS Biochemicals Corporation (San Diego, CA). The following antibodies were used: phospho-S6K1 (Thr389), phospho-4E-BP1 (Thr70), 4E-BP1, phospho-S6 ribosomal protein (Ser235/236), S6 ribosomal protein, caspase-3, cleaved-caspase-3, poly (ADP-ribose) polymerase (PARP) (Cell Signaling Technology, Beverly, MA); β-tubulin, phospho-mTOR (Ser2448), mTOR, HA, FLAG (Sigma); S6K1 (Santa Cruz Biotechnology, Santa Cruz, CA); Goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce, Rockford, IL). Other chemicals were purchased from local commercial sources and were of analytical grade.
Cell culture
Rat pheochromocytoma (PC12) cell line was from the American Type Culture Collection (Manassas, VA). Cells were grown in antibiotic-free DMEM supplemented with 10% horse serum and 5% FBS and maintained at 37°C in a humidified incubator containing 5% CO2. Primary cortical neurons were isolated from fetal mice at 16–18 days of gestation and cultured as described (Chen et al., 2010).
Recombinant adenoviral constructs and infection of cells
The recombinant adenoviral vectors encoding FLAG-tagged wild-type mTOR (Ad-mTOR-wt), HA-tagged constitutively active S6K1 (Ad-S6K1-ca), and the control virus encoding the green fluorescence protein (GFP) (Ad-GFP) were generated as described previously (Chen et al., 2010; Liu et al., 2006). The viruses were amplified, titrated, and used as described (Liu et al., 2008).
Lentiviral shRNA cloning, production, and infection
Lentiviral shRNAs to 4E-BP1 and GFP (as control) were generated and used as described (Chen et al., 2010). Monolayer PC12 cells or primary neurons, when grown to about 70% confluence, were infected with above lentivirus-containing supernatant in the presence of 8 µg/ml polybrene and exposed to 2 µg/ml puromycin after 24 h of infection. After 5 days, cells were used for experiments.
Analysis for cell viability and morphology
PC12 and primary neurons, seeded at a density of 1 × 104 cells/well in a flat-bottomed 96-well plate precoated with PDL (0.2 µg/ml for PC12 cells; 10 µg/ml for primary neurons), were treated with different concentrations of rotenone (0–1 µM) for 24 h, or with/without rotenone (1 µM) for different time (0–24 h), or with/without rotenone (0.5 and 1 μM) for 24 h following preincubation with/without Mito-TEMPO (10 μM) for 1 h, with 6 replicates of each treatment. In some cases, PC12 cells and primary neurons, infected with Ad-mTOR, Ad-S6K1-ca, or Ad-GFP (control), or infected with lentiviral shRNA to 4E-BP1 or GFP, respectively, were seeded in a 96-well plate (1 × 104 cells/well) or in a 6-well plate (5 × 105 cells/well). Next day, cells were exposed to rotenone (0.5 and/or 1 µM) for 24 h. Subsequently, the viability of the cells, after incubation with MTS reagent (one solution reagent) (20 μl/well) for 4 h, was determined by measuring the optical density (OD) at 490 nm using a Synergy™ 2 Multi-function Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT). In addition, for PC12 cells and primary neurons infected with Ad-S6K1-ca or Ad-GFP, after treatment with rotenone (1 µM) for 24 h, the images for morphological analysis were taken with a Nikon Eclipse TE2000-U inverted phase-contrast microscope (Nikon, Tokyo, Japan) (200 × ) equipped with a digital camera.
DAPI staining
PC12 and primary neurons, seeded at a density of 5 × 105 cells/well in a 6-well plate containing a PDL-coated glass coverslip per well, were treated with rotenone (0, 0.1, 0.5, and 1 μM) for 24 h, or with/without rotenone (0.5 and 1 μM) for 24 h following preincubation with/without CAT (350 U/ml) or Mito-TEMPO (10 μM) for 1 h or zVAD-fmk (100 μM) for 2 h, with 6 replicates of each treatment. In addition, PC12 cells, infected with Ad-mTOR, Ad-S6K1-ca or Ad-GFP, or infected with lentiviral shRNA to 4E-BP1 or GFP, respectively, were treated with/without rotenone (1 µM) for 24 h, or pre-treated with zVAD-fmk (100 μM) for 2 h and then exposed to rotenone (1 μM) for 24 h. Afterwards, the cells with fragmented and condensed nuclei were determined using DAPI staining as described (Chen et al., 2008b). Finally, slides were mounted in glycerol/PBS (1:1, v/v) containing 2.5% 1,4-diazabiclo-(2,2,2)octane. Photographs were taken under a fluorescence microscope (Nikon 80i, Tokyo, Japan) equipped with a digital camera.
TUNEL staining
PC12 cells and primary neurons, seeded at a density of 5 × 105 cells/well in a 6-well plate containing a PDL-coated glass coverslip per well, treated with rotenone (0, 0.1, 0.5, and 1 μM) for 24 h, or with/without rotenone (0.5 and 1 μM) for 24 h following preincubation with/without CAT (350 U/ml) for 1 h. After that, cells were fixed with 4% paraformaldehyde prepared in PBS for 2 h at 4°C, followed by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) staining, according to the manufacturer’s instructions of In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany). Finally, all stained samples were analyzed by fluorescence microscopy (Nikon 80i) equipped with digital camera. For quantitative analysis of the fluorescence staining, the integral optical density (IOD) was measured by Image-Pro Plus 6.0 software (Media Cybernetics Inc., Newburyport, MA).
Cell ROS assay and H2O2 imaging
The production of ROS was measured by detecting the fluorescent intensity of oxidant-sensitive probe CM-H2DCFDA, as described (Chen et al., 2008a). Briefly, PC12 and primary neurons, seeded in a 96-well plate (1 × 104 cells/well), were treated with rotenone (0–1 µM) for 24 h, or with/without rotenone (1 µM) for 0–24 h, or with/without rotenone (0.5 and 1 µM) for 24 h following preincubation with/without CAT (350 U/ml) for 1 h. The cells were then loaded with CM-H2DCFDA (10 µM) for 40 min. Fluorescence intensity was recorded by excitation at 485 nm and emission at 535 nm using a Synergy™ 2 Multi-function Microplate Reader (Bio-Tek Instruments, Inc.).
Imaging intracellular H2O2 was recorded by using a nonfluorescent probe, H2DCFDA. This peroxide-selective dye can penetrate into the intracellular matrix of cells, where it is cleaved by intracellular esterases and oxidized by H2O2 to form fluorescent DCF (Bao et al., 2005). In brief, PC12 and primary neurons, or PC12 cells and primary neurons infected with lentiviral shRNA to 4E-BP1 or GFP, or with Ad-mTOR, Ad-S6K1-ca and Ad-GFP, seeded at a density of 5 × 105 cells/well in a 6-well plate containing a glass coverslip per well, were treated with/without rotenone (0.5 and/or 1 µM) for 24 h, with/without rotenone (0.5 and/or 1 µM) in the presence or absence of TTFA (10 μM) or antimycin A (50 μM) for 24 h, or with/without rotenone (0.5 and 1 μM) for 24 h following pre-incubation with/without CAT (350 U/ml) or Mito-TEMPO (10 μM) for 1 h. The cells were then loaded with H2DCFDA (20 µM) for 1 h. Subsequently, all stained specimens were rinsed three times with PBS, followed by imaging under a fluorescence microscope (Nikon 80i) equipped with a digital camera. For quantitative analysis of the fluorescence intensity, IOD was measured by Image-Pro Plus 6.0 software as described above.
Western blot analysis
After treatment, cells were briefly washed with cold PBS, and then on ice, lysed in the radioimmunoprecipitation assay buffer. Afterwards, Western blotting was performed as described previously (Chen et al., 2010).
Statistical analysis
All quantified data were expressed as means ± standard error of the mean (means ± SEM). Student’s t-test for nonpaired replicates was used to identify statistically significant differences between treatment means. Group variability and interaction were compared using either one-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means. A level of P < .05 was considered to be significant.
RESULTS
Rotenone-induced Cell Apoptosis is Mitochondrial ROS/H2O2-dependent in Neuronal Cells
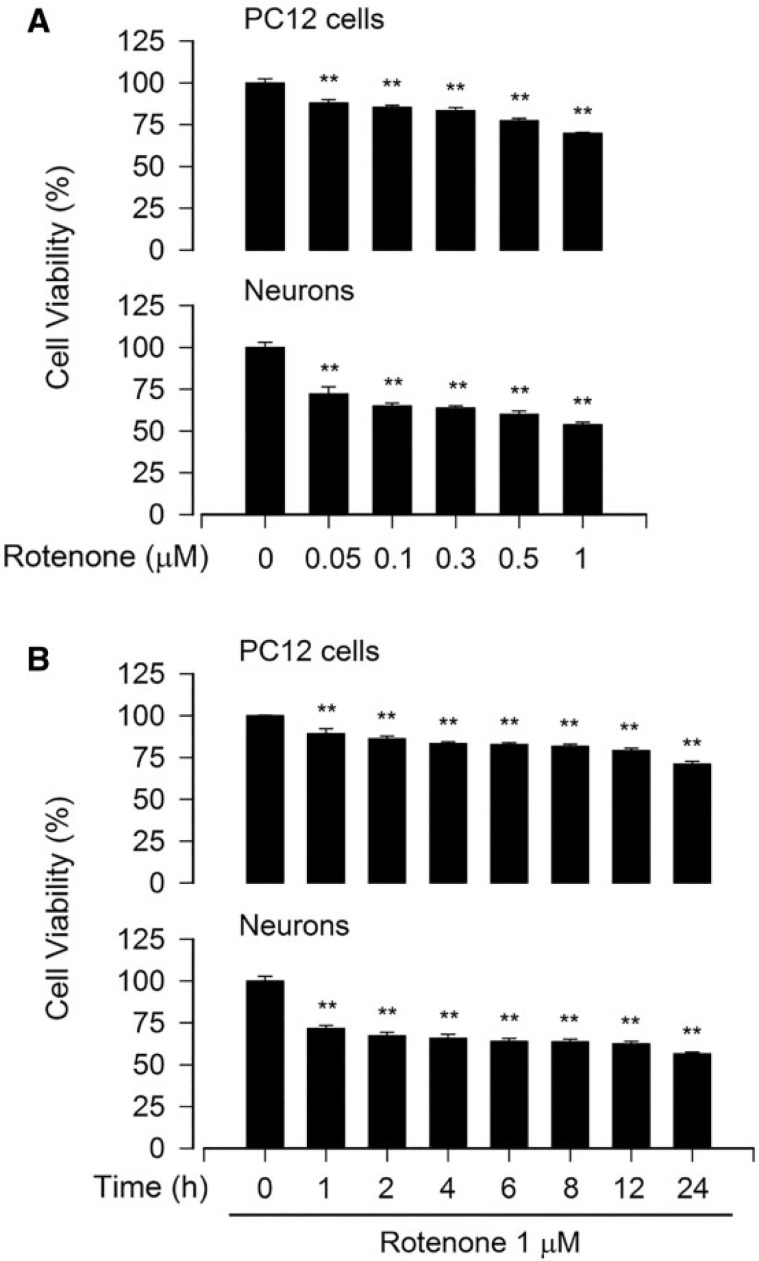
To find an appropriate concentration and treatment time of rotenone for the mechanism studies on rotenone-induced neuronal apoptosis, we first performed cell viability assay. As shown in Figure 1A, treatment with rotenone for 24 h resulted in a concentration-dependent reduction of viability in PC12 cells and primary neurons. At 1 μM, rotenone reduced the cell viability by 40%–50%, compared with the vehicle control. A time-dependent decline in cell viability occurred during the 24 h period (Fig. 1B). Starting from 1 h treatment, rotenone (1 μM) markedly decreased cell viability. Interestingly, in comparison with PC12 cells, primary neurons appeared to be more sensitive to rotenone. When exposed to 0.5 μM of rotenone for 24 h, 77% of PC12 cells remained viable, whereas only about 60% of primary neurons were viable (Fig. 1A). The data suggest that rotenone may induce remarkable cell death in the neuronal cells at concentrations of > 0.5 μM within 24 h. Therefore, up to 1 μM of rotenone was used for all the following experiments.
FIG. 1.
Rotenone reduces cell viability in neuronal cells. The indicated cells were treated with rotenone (0–1 μM) for 24 h (A), or rotenone (1 μM) for 0–24 h (B), followed by cell viability evaluation using the MTS assay. All data were expressed as means ± SEM (n = 6). **p < .01, difference with control group.
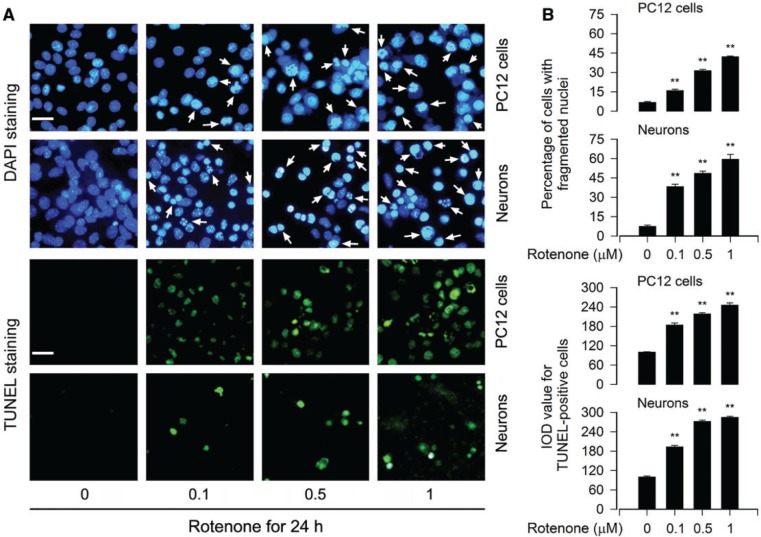
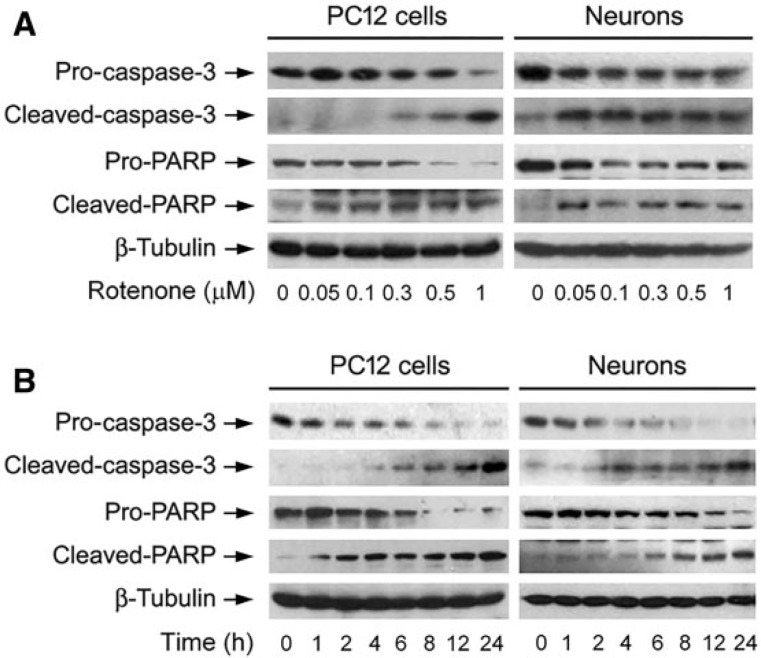
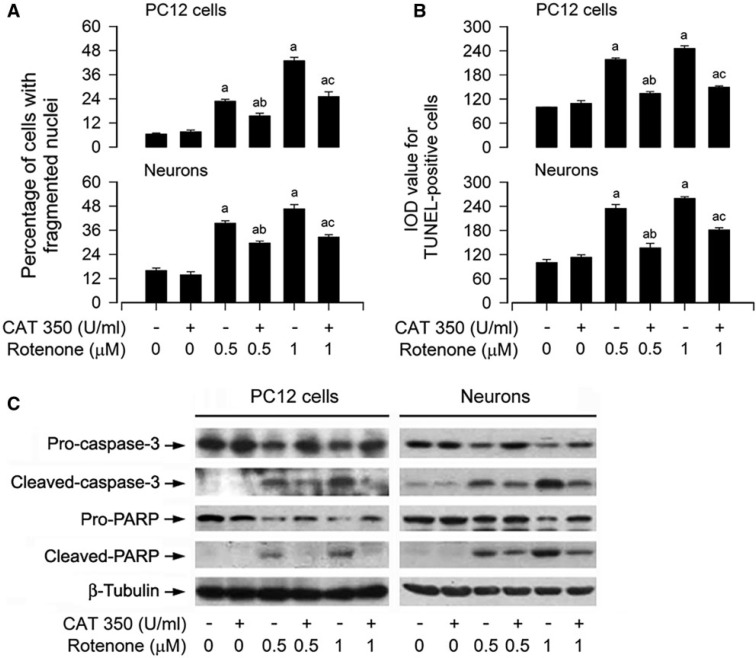
To elucidate whether the fall of cell viability by rotenone was because of rotenone-induced apoptosis, DAPI staining was used, showing that exposure to rotenone for 24 h markedly increased nuclear fragmentation and condensation (arrows), a hallmark of apoptosis (Hao et al., 2013), concentration-dependently in PC12 cells and primary neurons (Fig. 2A and B). To confirm the event, we subsequently detected DNA strand breaks in the cells by TUNEL staining. Indeed, rotenone increased TUNEL-positive cells (highlighted by fluorescence staining) concentration-dependently (Fig. 2A and B). Furthermore, we also examined proteolytic cleavages of caspase-3 and PARP in PC12 and primary neurons. The results revealed that rotenone resulted in robust cleavages of caspase-3 and PARP in a concentration- (Fig. 3A) and time-dependent manner in the cells (Fig. 3B). Taken together, these findings strongly imply that apoptosis is the major cause for rotenone-reduced cell viability in the neuronal cells.
FIG. 2.
Rotenone induces apoptotic cell death in neuronal cells. The indicated cells were treated with rotenone (0–1 μM) for 24 h, followed by cell apoptosis analysis using DAPI and TUNEL staining. A, The cells with nuclear fragmentation and condensation (arrows) and TUNEL-positive cells (highlighted by fluorescence staining) with DNA strand breaks are shown, respectively. Scale bar: 20 µm. B, Quantitative data for (A) were expressed as means ± SEM (n = 6). **p < .01, difference with control group.
FIG. 3.
Rotenone induces activation of caspase cascade in neuronal cells. The indicated cells were treated with rotenone (0–1 μM) for 24 h (A), or rotenone (1 μM) for 0–24 h (B), followed by Western blotting using the indicated antibodies. Blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments.
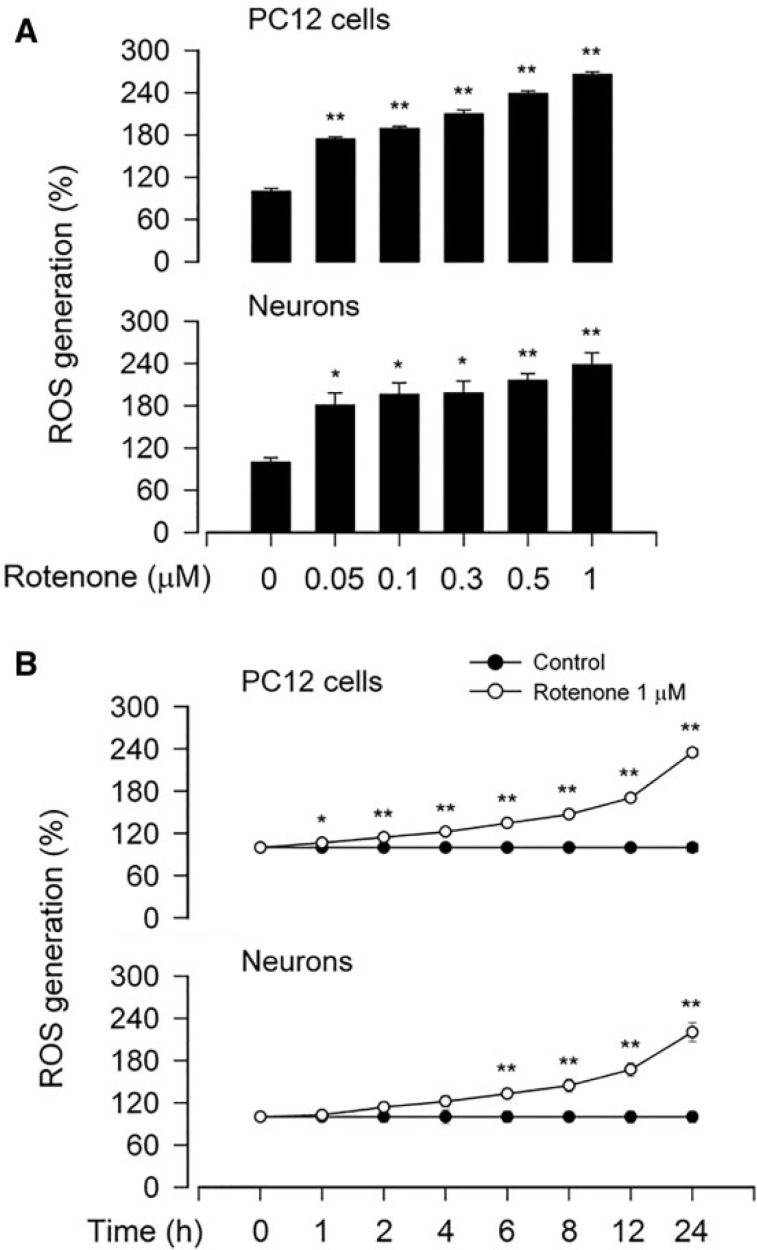
To determine whether rotenone-induced apoptotic cell death is associated with its induction of ROS, we measured intracellular ROS level using an oxidant-sensitive probe CM-H2DCFDA. As shown in Figure 4A, treatment with rotenone (0–1 μM) for 24 h resulted in a concentration-dependent increase of ROS production in PC12 and primary neurons. Rotenone also induced a time-dependent elevation of cellular ROS within 24 h (Fig. 4B). This was consistent with the findings of decreased viability and increased apoptosis observed by cell viability assay as well as by DAPI and TUNEL staining (Figs. 1–3), respectively.
FIG. 4.
Rotenone induces ROS production in neuronal cells. The indicated cells were treated with (A) rotenone (0–1 μM) for 24 h, or (B) rotenone (1 μM) for 0–24 h, followed by ROS assay using an oxidant-sensitive probe CM-H2DCFDA. All data were expressed as means ± SEM (n = 6). *p < .05, **p < .01, difference with control group.
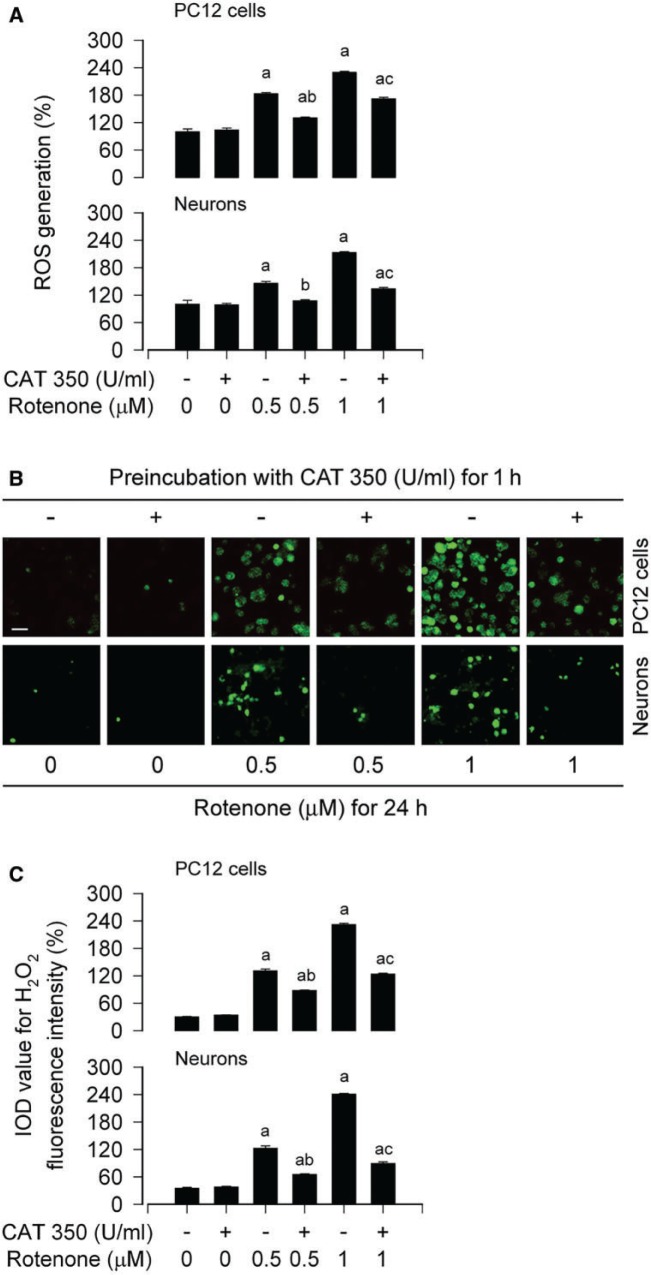
It has been reported that H2O2 in mitochondria in situ in isolated nerve terminals is sufficiently enhanced when mitochondrial complex I is inhibited at a small degree (Adam-Vizi, 2005). Therefore, we ask whether rotenone, as a selective inhibitor of complex I, indeed elicits H2O2 generation contributing to neuronal apoptosis. For this, PC12 cells and primary neurons were pretreated with hydrogen peroxide-scavenging enzyme CAT (350 U/ml) for 1 h, and then exposed to rotenone (0.5 and 1 μM) for 24 h. We found that CAT dramatically blocked rotenone-induced ROS in the cells (Fig. 5A). To corroborate the finding, we extended our studies using H2DCFDA, a peroxide-selective probe (Bao et al., 2005). The results revealed that rotenone elicited a robust level of H2O2 (highlighted by fluorescence staining) in PC12 and primary neurons, which was abrogated by CAT (Fig. 5B and C). Interestingly, DAPI and TUNEL staining showed that CAT potently prevented the number of fragmented nuclear cells and TUNEL-positive cells (Fig. 6A and B). Moreover, our Western blotting exhibited that CAT obviously attenuated rotenone-induced cleavages of caspase-3 and PARP in PC12 cells and primary neurons (Fig. 6C).
FIG. 5.
Rotenone induces H2O2 in neuronal cells. The indicated cells were pretreated with/without CAT (350 U/ml) for 1 h and then exposed to rotenone (0.5 and 1 μM) for 24 h, followed by (A) ROS assay using CM-H2DCFDA, (B and C) H2O2 imaging using a peroxide-selective probe H2DCFDA. For (B), the cells with higher H2O2 (highlighted by fluorescence staining) are shown. Scale bar: 20 µm. For (A) and (C), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, difference with 0.5 μM rotenone group; cp < .05, difference with 1 μM rotenone group.
FIG. 6.
Rotenone-induced H2O2 mediates apoptosis in neuronal cells. The indicated cells were pretreated with/without CAT (350 U/ml) for 1 h and then exposed to rotenone (0.5 and 1 μM) for 24 h, followed by cell apoptosis analysis using DAPI (A) and TUNEL staining (B), or Western blotting using the indicated antibodies (C). For (C), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (A) and (B), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, difference with 0.5 μM rotenone group; cp < .05, difference with 1 μM rotenone group.
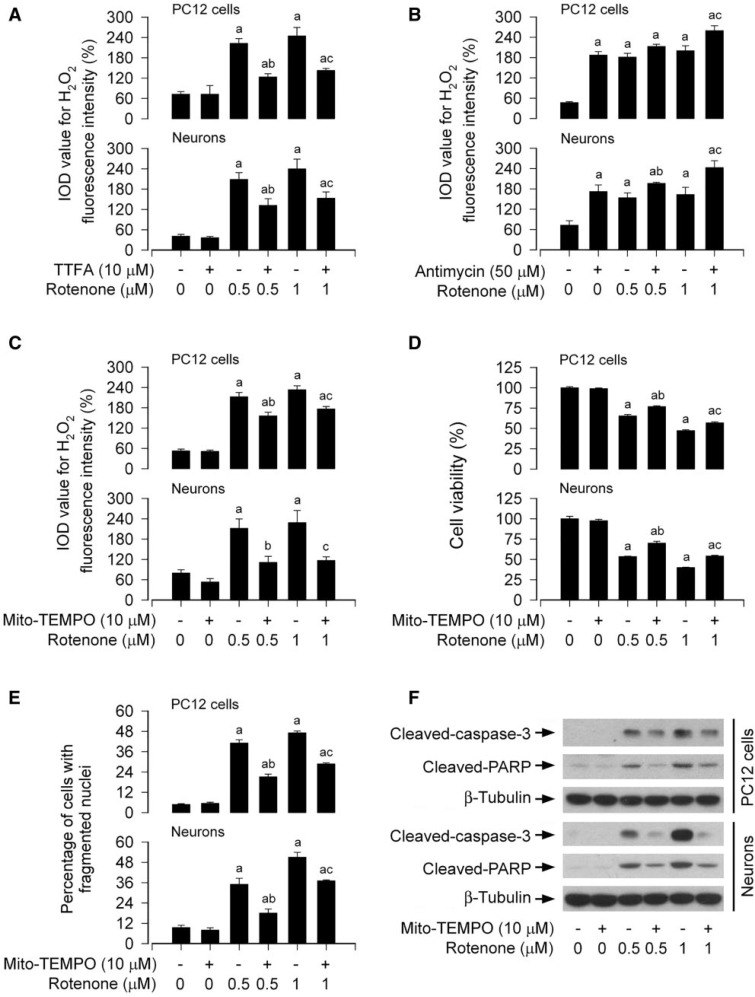
Because rotenone is a selective inhibitor of mitochondrial complex I, we next examined the association of rotenone-induced apoptosis with mitochondrial H2O2 generation in neuronal cells. When PC12 cells and primary neurons were treated with rotenone (0.5 and 1 μM) in the presence or absence of TTFA (10 μM), a mitochondrial complex II ubiquinone site inhibitor with blockage of electron supply to ubiquinol and consequential limiting the formation of ubisemiquinone (Moreno-Sanchez et al., 2013), for 24 h, this produced an obvious decrease in H2O2 fluorescence during cotreatment with rotenone and TTFA in the cells (Fig. 7A). In contrast, when PC12 cells and primary neurons were exposed to rotenone in the presence of antimycin A (50 μM), a mitochondrial complex III inhibitor that increases the lifetime of ubisemiquinone (Lanju et al., 2014), for 24 h, a further increase in H2O2 levels was observed in the cells (Fig. 7B). Of note, pretreatment with Mito-TEMPO (10 μM), a mitochondria-targeted antioxidant (Yeh et al., 2014), significantly reduced rotneone-induced H2O2 levels (Fig. 7C) and prevented rotenone-induced cell death (Fig. 7D and E) in PC12 cells and primary neurons. Consistently, Mito-TEMPO also diminished cleavages of caspase-3 and PARP in the cells in response to rotenone (Fig. 7F). Collectively, our findings verify that rotenone-induced neuronal apoptosis is mitochondrial ROS/H2O2-dependent.
FIG. 7.
Mitochondrial H2O2 elicits apoptosis in rotenone-exposed neuronal cells. The indicated cells were treated with (A and B) rotenone (0.5 and 1 μM) in the presence or absence of TTFA (10 μM) or antimycin A (50 μM) for 24 h, or (C, D, E, and F) pretreated with/without Mito-TEMPO (10 μM) for 1 h and then exposed to rotenone (0.5 and 1 μM) for 24 h, followed by (A, B, and C) H2O2 imaging using a peroxide-selective probe H2DCFDA, (D) cell viability evaluation using the MTS assay, (E) cell apoptosis analysis using DAPI staining, or (F) Western blotting using the indicated antibodies. For (F), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (A), (B), (C), (D), and (E), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; b p < .05, difference with 0.5 μM rotenone group; cp < .05, difference with 1 μM rotenone group.
Rotenone Induction of H2O2 Inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E Pathways in Neuronal Cells
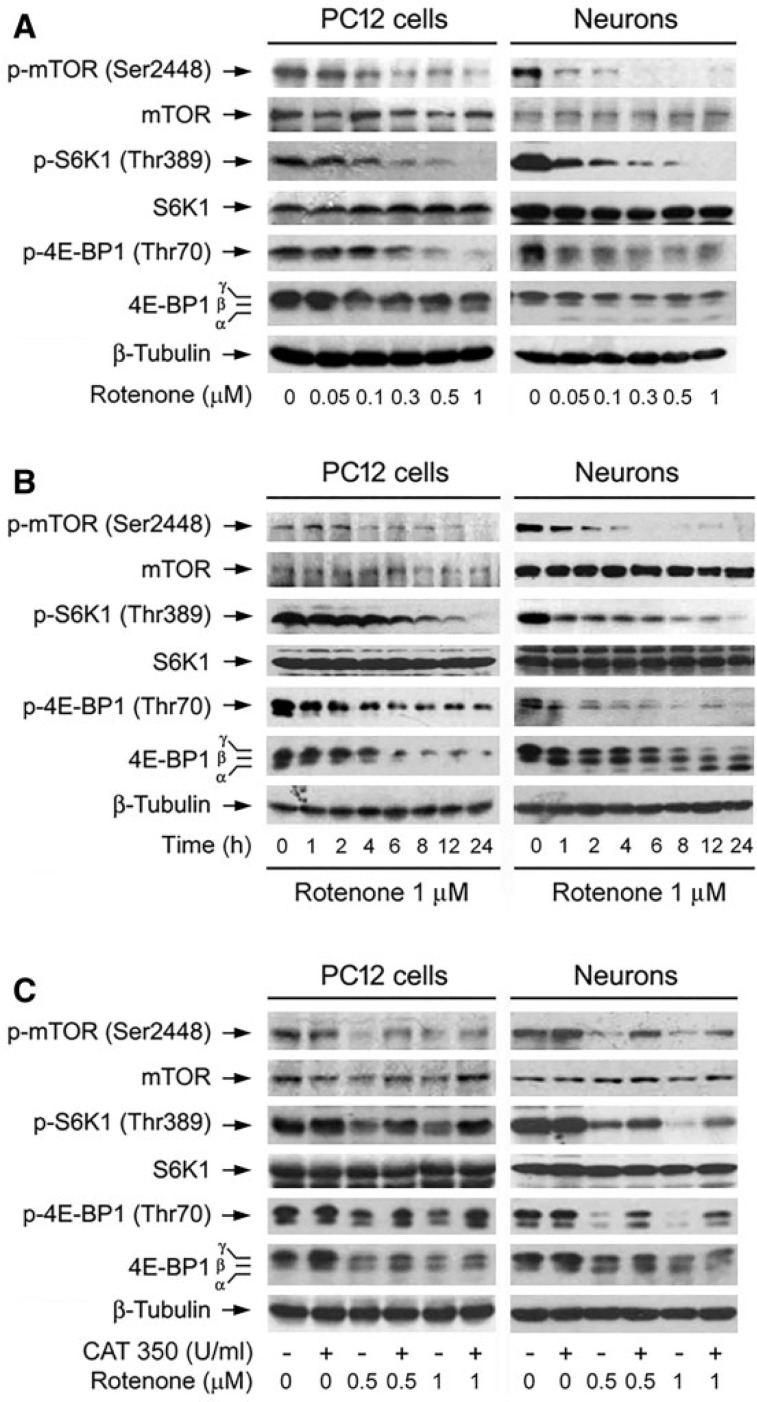
Studies have demonstrated that overactivation or inhibition of mTOR activity in the brain may impair neuronal functions, and have also detrimental consequences on neuronal regeneration after damage (Dello Russo et al., 2013; Malagelada et al., 2006; Swiech et al., 2008). Therefore, we queried how rotenone affects mTOR signaling pathway by ROS/H2O2. To this end, first of all, PC12 cells and primary neurons were treated with 0–1 µM of rotenone for 24 h or with 1 µM of rotenone for different time (0–24 h), followed by Western blotting. The results showed that treatment with 0.05–1 μM of rotenone for 24 h (Fig. 8A) or with 1 μM of rotenone for 1–24 h (Fig. 8B) obviously decreased phosphorylation of mTOR and its downstream effector molecules S6K1 and 4E-BP1 in a concentration- and time-dependent manner, clearly indicating that mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways are inhibited in neuronal cells exposed to rotenone.
FIG. 8.
Rotenone induction of H2O2 inhibits mTOR-mediated phosphorylation of S6K1 and 4E-BP1 in neuronal cells. The indicated cells were treated with (A) rotenone (0–1 μM) for 24 h, (B) rotenone (1 μM) for 0–24 h, or (C) pretreated with/without CAT (350 U/ml) for 1 h and then exposed to rotenone (0.5 and 1 μM) for 24 h, followed by Western blotting using the indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments.
Next we sought to validate whether rotenone-induced inhibition of mTOR signaling is due to its induction of intracellular H2O2. PC12 and primary neurons were pretreated with CAT (350 U/ml) for 1 h and then exposed to rotenone (0.5 and 1 μM) for 24 h. Western blot analysis revealed that rotenone-inhibited phosphorylation of mTOR, S6K1, and 4E-BP1 was remarkably attenuated by CAT (Fig. 8C). The data imply that rotenone inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways by induction of H2O2 in the neuronal cells.
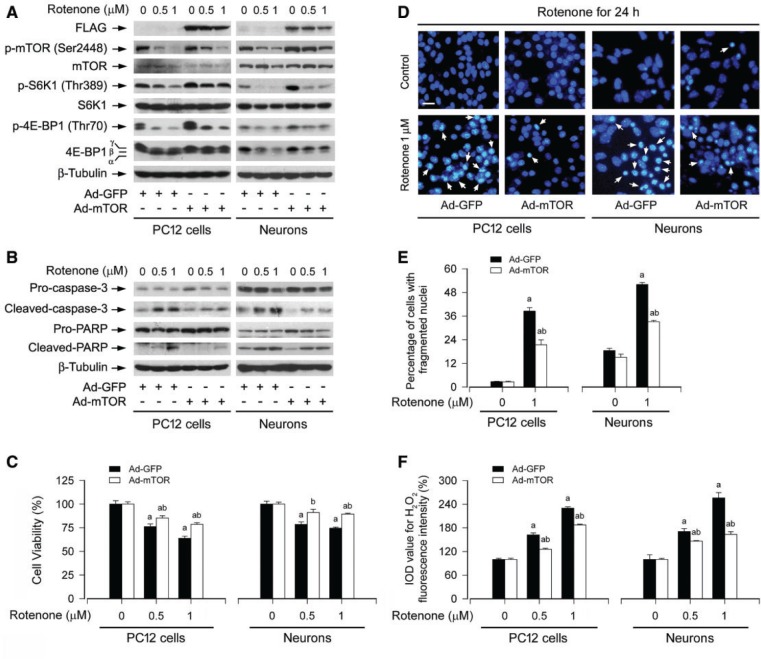
Overexpression of mTOR Partially Prevents Rotenone-induced H2O2 and Apoptosis in Neuronal Cells
To pinpoint the role and significance of mTOR activity in rotenone-induced neuronal apoptosis, PC12 cells and primary neurons, infected with Ad-mTOR or Ad-GFP (control), were exposed to rotenone (0.5 and/or 1 µM) for 24 h. We observed that ectopic expression of FLAG-tagged wt-mTOR conferred substantial resistance to rotenone-induced inhibition of S6K1/4E-BP1 phosphorylation in the cells (Fig. 9A). Consistently, rotenone-induced cleavages of caspase-3 and PARP were apparently attenuated by overexpression of mTOR (Fig. 9B). Furthermore, overexpression of mTOR also in part attenuated the cytotoxic effect of rotenone on PC12 cells and primary neurons, as detected by cell viability assay (Fig. 9C), morphological analysis (data not shown), and DAPI staining (Fig. 9D and E). However, interestingly, we found that ectopic overexpression of mTOR did not obviously alter the intracellular level of H2O2, but substantially attenuated rotenone-induced H2O2 in PC12 cells and primary neurons (Fig. 9F), as determined by imaging intracellular H2O2 using H2DCFDA. These findings suggest that mTOR may negatively regulate rotenone induction of H2O2 and apoptosis in neuronal cells.
FIG. 9.
Overexpression of wt-mTOR partially prevents rotenone-induced H2O2 and apoptosis in neuronal cells. PC12 cells and primary neurons, infected with replication-defective recombinant adenovirus expressing FLAG-tagged wt-mTOR (Ad-mTOR) or the control adenovirus (Ad-GFP), were exposed to rotenone (0.5 and/or 1 µM) for 24 h, followed by (A and B) Western blotting using the indicated antibodies, (C) cell viability evaluation using the MTS assay, (D and E) cell apoptosis analysis using DAPI staining, or (F) H2O2 imaging using a peroxide-selective probe H2DCFDA. For (A) and (B), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (D), the cells with nuclear fragmentation and condensation (arrows) are shown. Scale bar: 20 µm. For (C), (E), and (F), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, Ad-mTOR group versus Ad-GFP group.
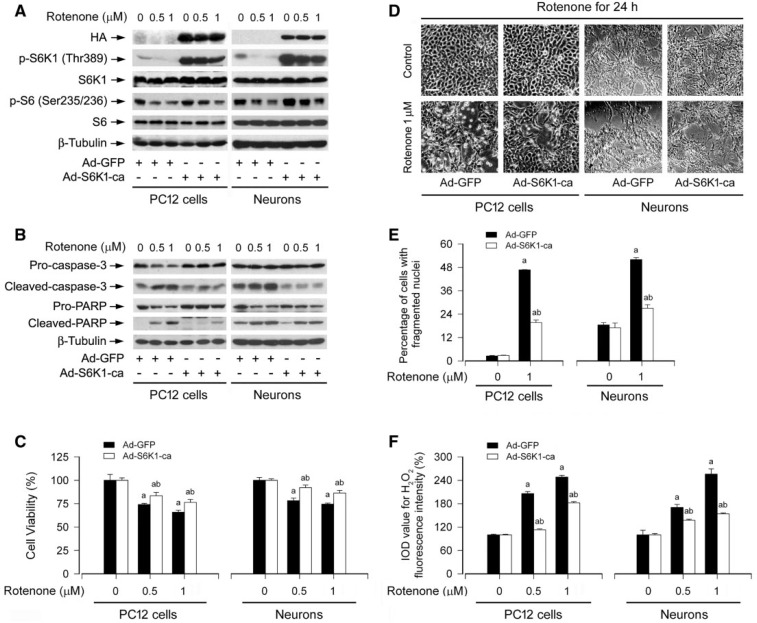
Ectopic Expression of Constitutively Active S6K1 or Downregulation of 4E-BP1 Partially Blocks Rotenone-induced H2O2 and Apoptosis in Neuronal Cells
Both S6K1 and 4E-BP1 are the two best-characterized downstream effector molecules of mTOR (Cornu et al., 2013; Laplante and Sabatini, 2012). In the present study, we have shown that rotenone inhibits mTOR-mediated phosphorylation of S6K1 and 4E-BP1 by induction of H2O2. Next, we further investigated the importance of the two mTOR downstream targets in rotenone induction of H2O2-mediated neuronal apoptosis. First of all, a recombinant adenovirus expressing HA-tagged constitutively active S6K1 (Ad-S6K1-ca) was employed. We observed a high level of recombinant S6K1-ca in PC12 cells and primary neurons infected with Ad-S6K1-ca, but not in those infected with Ad-GFP (as control), as determined by Western blotting (Fig. 10A). Of note, after PC12 cells and primary neurons, infected with Ad-S6K1-ca and Ad-GFP, were exposed to rotenone (0.5 and 1 µM) for 24 h, cells expressing S6K1-ca, but not GFP, were highly resistant to rotenone inhibition of phosphorylation of S6K1 and S6 (Fig. 10A). Consistently, expression of S6K1-ca also rendered a potent blockage of rotenone-induced cleavage of caspase-3/PARP (Fig. 10B). Interestingly, expression of S6K1-ca also partially rescued the cells from rotenone-induced cell death (Fig. 10C and D) and ameliorated apoptosis in the cells in response to rotenone (Fig. 10E). Subsequently, we found that there existed a lower level of H2O2 induced by rotenone in PC12 cells and primary neurons infected with Ad-S6K1-ca than in those infected with Ad-GFP (Fig. 10F).
FIG. 10.
Ectopic expression of constitutively active S6K1 partially blocks rotenone-induced H2O2 and apoptosis in neuronal cells. PC12 cells and primary neurons, infected with replication-defective recombinant adenovirus expressing constitutively active S6K1 (Ad-S6K1-ca) or Ad-GFP (as control), were exposed to rotenone (0.5 and/or 1 µM) for 24 h, followed by (A and B) Western blotting using the indicated antibodies, (C) cell viability evaluation using the MTS assay, (D) morphological analysis using a Nikon Eclipse TE2000-U inverted phase-contrast microscope (200 × ) equipped with a digital camera, (E) cell apoptosis analysis using DAPI staining, or (F) H2O2 imaging using a peroxide-selective probe H2DCFDA. For (A) and (B), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (D), cell morphology was visualized under an Olympus inverted phase-contrast microscope (200×) equipped with Quick Imaging system. Scale bar: 50 µm. For (C), (E), and (F), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, Ad-S6K1-ca group versus Ad-GFP group.
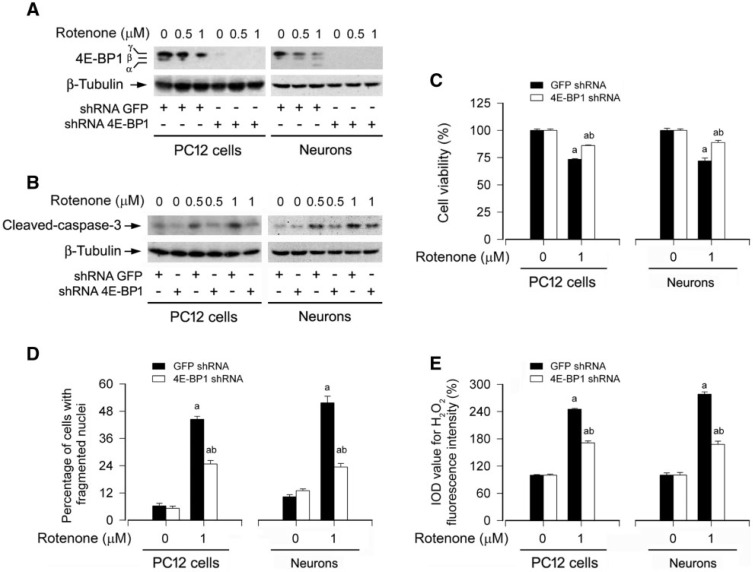
Next, we dissected the role of 4E-BP1/eIF4E pathway in rotenone induction of ROS-mediated neuronal apoptosis. As 4E-BP1 functions as a suppressor of eIF4E (Martorell et al., 2009), downregulation of 4E-BP1 would lead to the loss of suppression of eIF4E. As shown in Figure 11A, infection of PC12 cells and primary neurons with lentiviral shRNA to 4E-BP1, but not to GFP (control), silenced 4E-BP1 protein expression by >90%. Downregulation of 4E-BP1 remarkably attenuated the activation of caspase-3 (Fig. 11B) and the cytotoxicity (Fig. 11C and D) induced by rotenone (0.5 and/or 1 μM). Furthermore, rotenone induction of H2O2 was also significantly diminished by silencing 4E-BP1 (Fig. 11E). Collectively, our findings support the notion that rotenone induction of H2O2 inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis.
FIG. 11.
Downregulation of 4E-BP1 partially attenuates rotenone-induced H2O2 and apoptosis in neuronal cells. PC12 cells and primary neurons, infected with lentiviral shRNA to 4E-BP1 or GFP (as control), were exposed to rotenone (0.5 and/or 1 µM) for 24 h, followed by (A and B) Western blotting using the indicated antibodies, (C) cell viability evaluation using the MTS assay, (D) cell apoptosis analysis using DAPI staining, or (E) H2O2 imaging using a peroxide-selective probe H2DCFDA. For (A) and (B), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (C), (D), and (E), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, 4E-BP1 shRNA group versus GFP shRNA group.
Rotenone Induction of H2O2 Contributes to Caspase-dependent and -independent Apoptosis in Neuronal Cells
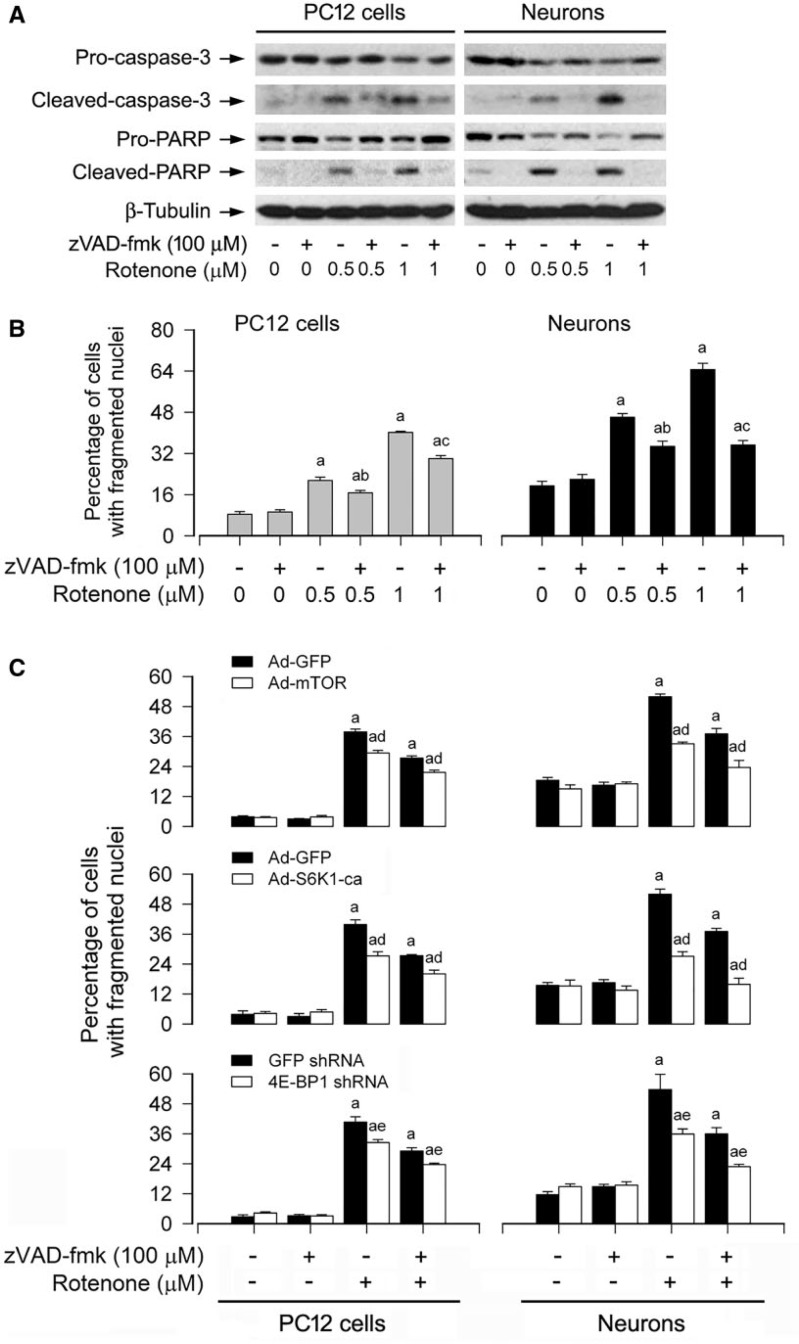
Studies have shown that rotenone may trigger cell death by caspase-dependent and/or -independent apoptotic mechanisms, depending on cell types (Chen et al. 2006; Kamalden et al., 2012; Kang et al., 2012; Lim et al., 2007; Newhouse et al., 2004; Sherer et al., 2002). We found that rotenone induced cleavages of caspase-3 and PARP as well as neuronal apoptosis in a concentration- and time-dependent fashion (Fig. 3), and CAT obviously attenuated rotenone-induced cleavages of caspase-3 and PARP in PC12 and primary neurons (Fig. 6C), suggesting that rotenone induction of H2O2 triggers caspase-dependent neuronal apoptosis. To unveil whether there exists a caspase-independent mechanism involved in rotenone-induced neuronal apoptosis, PC12 cells and primary neurons were exposed to rotenone (0.5 and 1 μM) for 24 h after pretreatment with zVAD-fmk (100 μM), a pan caspase inhibitor, for 2 h. As shown in Figure 12A, rotenone-induced cleavages of caspase-3 and PARP were almost completely blocked by zVAD-fmk. However, zVAD-fmk itself did not obviously alter cell viability, but only partially rescued cells from rotenone-induced apoptosis (Fig. 12B). Similar data were observed using morphological analysis in PC12 cells and primary neurons (data not shown).
FIG. 12.
Rotenone elicits caspase-dependent and -independent apoptosis in neuronal cells. The indicated cells were pretreated with/without zVAD-fmk (100 μM) for 2 h and then exposed to rotenone (0.5 and/or 1 μM) for 24 h, followed by (A) Western blotting using the indicated antibodies or (B and C) cell apoptosis analysis using DAPI staining. For (A), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. For (B) and (C), all data were expressed as means ± SEM (n = 6). ap < .05, difference with control group; bp < .05, difference with 0.5 μM rotenone group; cp < .05, difference with 1 μM rotenone group; dp < .05, Ad-mTOR group or Ad-S6K1-ca group versus Ad-GFP group; ep < .05, 4E-BP1 shRNA group versus GFP shRNA group.
As ectopic expression of mTOR-wt or S6K1-ca, or downregulation of 4E-BP1 only partially protected against rotenone-mediated neuronal apoptosis (Figs. 9–11), we next determined whether combination of zVAD-fmk with genetic manipulating mTOR, S6K1, or 4E-BP1, respectively, has synergistic protective effects. To this end, PC12 cells and primary neurons were infected with Ad-mTOR, Ad-S6K1-ca or Ad-GFP (as control), or with lentiviral shRNA to 4E-BP1 or GFP, and then exposed to rotenone (1 µM) for 24 h post preincubation with/without zVAD-fmk (100 µM) for 2 h. As expected, expression of mTOR-wt, S6K1-ca, or silencing 4E-BP1 potentiated zVAD-fmk protection against rotenone-induced apoptosis in the cells (Fig. 12C). Collectively, the findings support the notion that rotenone induces H2O2 leading to caspase-dependent and -independent apoptosis in neuronal cells.
DISCUSSION
Rotenone, an extensively used plant-derived pesticide, has been known to possess highly selective toxicity on dopaminergic neurons in vitro and in vivo contributing to neurodegeneration (Taetzsch and Block, 2013; Tamilselvam et al., 2013; Xiong et al., 2012). Accumulating evidence has pointed to rotenone contributing to decreased ATP synthesis, mitochondrial depolarization, and ROS generation via inhibiting mitochondrial complex I (Drechsel and Patel, 2008; Taetzsch and Block, 2013). Excessive ROS in turn will further inhibit complex I (Tretter et al., 2004). The vicious cycle in dopaminergic neurons eventually leads to cell death as crucial factors in the pathogenesis of PD (Adam-Vizi, 2005; Tamilselvam et al., 2013; Tretter et al., 2004). However, the contribution and role of ROS in rotenone-induced neuronal cell death have not been fully elucidated. Especially, it is unclear what signaling molecules are critical for rotenone-induced dopaminergic cell death related to PD. Multiple studies have demonstrated that sufficient activation of Bax and Bak facilitates mitochondrial outer membrane permeabilization, which releases death-inducing factors and causes apoptotic and nonapoptotic cell death (Ethell and Fei, 2009). Oxidative modification by rotenone exerts neurotoxicity by inhibiting proteasome activities and increasing oxidized proteins (Shamoto-Nagai et al., 2003; Wang XF et al., 2006). Rotenone triggers apoptosis by its inducing elevation of intracellular-free Ca2+ and activation of JNK and p38 pathways in SH-SY5Y cells (Newhouse et al., 2004; Wang and Xu, 2005). Our group recently found that PD mimetics (6-hydroxydopamine, N-methyl-4-phenylpyridine or rotenone) activate AMPK and inactivate Akt, causing neuronal cell death via inhibiting mTOR signaling pathway (Xu et al., 2014). Here, for the first time, we provide evidence that rotenone induced H2O2, which inhibited mTOR-mediated S6K1 and 4E-BP1 pathways, resulting in caspase-dependent and -independent apoptosis in neuronal cells. Overexpression of mTOR or manipulation of mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways was able to partially inhibit rotenone-induced H2O2 and apoptosis in the cells.
The primary ROS starts with the formation of a superoxide () as a result of reduced O2 and free radicals, afterwards superoxide dismutase (SOD) quickly decomposes and produces H2O2 (Droge, 2002). It is well known that both SOD1 and SOD2 can catalyze the dismutation of into O2 and H2O2 (Fernandez et al., 2011). To cope with the H2O2-mediated injuries and maintain the cellular redox homeostasis, H2O2 can be catalyzed to water by CAT in the cells (Droge, 2002; Tretter et al., 2004). Of note, H2O2 takes place mainly at the most vulnerable mitochondrial complex I (Bao et al., 2005). It has been reported that H2O2 in mitochondria in situ in isolated nerve terminals is sufficiently enhanced when complex I is inhibited at a small degree (Adam-Vizi, 2005). In the present study, to clearly unveil the fact that ROS/H2O2 contributes to the apoptotic consequences of rotenone-exposed neuronal cells, an oxidant-sensitive probe CM-H2DCFDA and a peroxide-selective probe H2DCFDA were employed for ROS assay and H2O2 imaging (Bao et al., 2005; Chen et al., 2008a). We showed that rotenone exposure triggered a concentration- and time-dependent elevation of cellular ROS in PC12 and primary neurons. Importantly, we validated that CAT, a hydrogen peroxide-scavenging enzyme, markedly abrogated rotenone-induced ROS, hinting that rotenone may primarily induce H2O2. This is in line with the previous findings that rotenone is involved in striatal dopamine release suppression and medium spiny neuron depolarization via enhancing H2O2 generation (Bao et al., 2005). Furthermore, we also found that pretreatment with CAT (350 U/ml) for 1 h was able to prevent rotenone (0.5–1 µM, 24 h)-induced apoptotic cell death in PC12 and primary neurons. In addition, to assess the association of rotenone-induced mitochondrial H2O2 generation with neuronal apoptosis, mitochondrial complex II inhibitor TTFA (Moreno-Sanchez et al., 2013), complex III inhibitor antimycin A (Lanju et al., 2014) and mitochondria-targeted antioxidant Mito-TEMPO (Yeh et al., 2014) were employed. We found that TTFA attenuated rotenone-induced H2O2 elevation, whereas antimycin A strengthened rotenone-induced H2O2 levels in PC12 cells and primary neurons. Administration of Mito-TEMPO dramatically diminished H2O2 and cell apoptosis in the cells triggered by rotenone. Collectively, our data support the notion that rotenone induces mitochondrial ROS/H2O2-dependent neuronal cell apoptosis in the context of PD.
A number of studies have shown that mTOR is able to sense and integrate a variety of environmental cues, which is involved in regulating differentiation and survival in neurons, as well as synaptic plasticity, learning and memory, and food uptake in adult brain (Dello Russo et al., 2013; Jaworski and Sheng, 2006; Swiech et al., 2008). However, the role of mTOR in the regulation of neuronal cell survival remains enigmatic. For example, both activation and inactivation of mTOR signaling have been found to contribute to the progression of neurodegenerative diseases including AD and PD (Chong et al., 2012; Wang et al., 2013). Recently, our group has demonstrated that cadmium, a heavy metal polluted in the environment, induces ROS-dependent activation of mTOR signaling leading to neuronal cell death (Chen et al., 2011), which can be reversed by pretreatment with rapamycin, a selective mTORC1 inhibitor (Chen et al., 2008a, 2011); whereas H2O2 induces neuronal cell death via suppression of mTOR, which can be rescued by overexpression of mTOR (Chen et al., 2010). It is well known that ROS comprise oxygen radicals, including , hydroxyl (•OH), peroxyl (), alkoxyl (RO•), and certain nonradicals that are oxidizing agents and/or are readily converted into radicals, such as hypochlorous acid (HOCl), ozone (O3), singlet oxygen (1O2), and H2O2 (Bedard and Krause, 2007). We do not know what kinds of ROS are induced by cadmium. Because H2O2 has been found to inhibit mTOR in neuronal cells (Chen et al., 2010), we therefore speculated that rotenone-dependent H2O2 induction might contribute to mTOR inhibition and the cytotoxicity in neuronal cells. As expected, we found that rotenone induction of ROS dramatically inhibited mTOR-mediated phosphorylation of S6K1 and 4E-BP1. Treatment with CAT was able to prevent rotenone-induced mTOR inhibition and cell death, confirming a critical role of rotenone-dependent H2O2 induction. This finding may be instructive to develop new therapeutic interventions that reduce H2O2-dependent inhibition of mTOR signaling and the progression of PD pathogenesis. In addition, it is worthy mentioning that the increase of high molecular ubiquitinated proteins caused by rotenone in SH-SY5Y cells may be attenuated by rapamycin (Pan et al., 2009). Rapamycin protects against rotenone-induced apoptosis through autophagy enhancement (Pan et al., 2009). Pan et al. have shown that there exists an upregulation of autophagy in the early stage of rotenone treatment, but the rotenone-exposed cells were eventually undergoing apoptosis along with the reduction of autophagy. The enhanced autophagy by rapamycin is truly required to protect SH-SY5Y cells against the sustained oxidative stress insults, including the aggregated proteins and dysfunctional mitochondria (Pan et al., 2009). The findings may represent neuronal cells’ responses to oxidative stress and autophagy modulation. Therefore, pharmacological induction of autophagy by rapamycin may be a useful therapeutic strategy as disease-modifiers in PD.
mTOR lies downstream of phosphatidylinositol 3′-kinase (PI3K) and Akt (Cornu et al., 2013; Laplante and Sabatini, 2012; Yap et al., 2008). PI3K/Akt is well known to regulate mTOR pathway positively (Cornu et al., 2013; Laplante and Sabatini, 2012). It has been reported that rotenone-induced activation of ROS/PI3K/Akt pathway in THP-1 cells leads to the release of factors that are toxic to SH-SY5Y cells and have implications for the onset of PD (Hu and Zhu, 2007). Our recent studies have shown that rotenone inhibition of mTOR signaling contributes to neuronal cell death, which is attributed to the suppression of Akt (Xu et al., 2014). This is supported by the findings that ectopic expression of constitutively active Akt partially attenuated inhibition of phosphorylation of mTOR, S6K1 and 4E-BP1, activation of caspase-3, and neuronal cell death triggered by rotenone (Xu et al., 2014). In this study, we observed that rotenone-induced ROS inhibited mTOR-mediated S6K1 and 4E-BP1 pathways in the neuronal cells. Thus, we tentatively conclude that rotenone may inhibit PI3K/Akt and consequently mTOR signaling pathway leading to neuronal apoptosis through induction of ROS generation. Undoubtedly, more studies are needed to address this issue.
In the present study, we observed that ectopic expression of wild-type mTOR, constitutively active S6K1 or downregulation of 4E-BP1 rendered a remarkable protection against rotenone-induced apoptotic cell death in PC12 cells and primary neurons. In the meantime, surprisingly, we found that rotenone induction of H2O2 was also attenuated in the cells by overexpression of wild-type mTOR or constitutively active S6K1, or by downregulation of 4E-BP1 (Figs. 9F, 10F, and 11E). In line with the above results, downregulation of S6K1 or ectopic expression of 4EBP1-5A also diminished rotenone-induced H2O2 and cell apoptosis (data not shown). These findings suggest that mTOR negatively regulates rotenone induction of H2O2 in the neuronal cells. This is in great contrast to the findings that mTOR positively mediates globular adiponectin-induced generation of ROS in the murine macrophage cell line RAW264 (Fujimoto et al., 2010), and cadmium-induced ROS generation in PC12 and SH-SY5Y cells (Chen et al., 2011). It is not known how mTOR regulates globular adiponectin-induced ROS production in RAW264 cells. However, we have noticed that cadmium induction of ROS activates mTOR, which, in turn, upregulates expression of the ROS-producing enzyme NADPH oxidase 2 (NOX2) and the regulatory proteins (p22phox, p67phox, p40phox, p47phox, and Rac1), leading to more ROS production (Chen et al., 2011). It appears that different ROS may have different effects on mTOR signaling. For instance, cadmium-induced ROS activates mTOR signaling, in part by activating the positive regulators insulin-like growth factor 1 receptor and phosphatidylinositide 3-kinase, and in part by inhibiting the negative regulators phosphatase and tensin homolog and AMPK (Chen et al., 2011). In contrast, H2O2, a kind of ROS, inhibits mTOR signaling by the activation of AMPK (Chen et al., 2010). Here, we observed that rotenone-induced mTOR inhibition and cell death could be attenuated by CAT, an enzyme that catalyzes the decomposition of H2O2 to water and oxygen (Droge, 2002), implying that rotenone-induced ROS may be mainly H2O2. It remains to be determined whether rotenone induces H2O2 in the neuronal cells by increasing H2O2 production and/or decreasing H2O2 clearance. Understanding the underlying mechanisms may be helpful to unveil why overexpression of wild-type mTOR/constitutively active S6K1 or downregulation of 4E-BP1 suppresses rotenone-induced H2O2.
In this study, our results show that rotenone potently increased cleavages of caspase-3 and PARP in a concentration- and time-dependent manner in PC12 and primary neurons. Interestingly, pretreatment of the cells with CAT markedly attenuated the cleavages of caspase-3 and PARP induced by rotenone, suggesting that induction of H2O2 by rotenone is associated with activation of caspase-3, which contributes to neuronal cell death. This is further supported by the findings that pretreatment of the cells with a broad-spectrum caspase inhibitor, zVAD-fmk, did prevent cleavage of caspase-3 and cell death. However, we also noticed that zVAD-fmk only partially prevented rotenone-induced apoptosis, as detected by DAPI staining and morphological analysis, suggesting that there exists a caspase-independent mechanism involved in rotenone-induced neuronal apoptosis. To corroborate the above findings, combination of zVAD-fmk with genetic manipulating mTOR, S6K1, or 4E-BP1, respectively, was utilized. The results showed that expression of mTOR-wt, S6K1-ca, or silencing 4E-BP1 in PC12 cells and primary neurons strengthened zVAD-fmk protection against rotenone-induced neuronal apoptosis. These data clearly indicate that rotenone may induce neuronal apoptosis through caspase-dependent and -independent mechanisms.
In summary, here we identify that rotenone-induced apoptosis is ROS/H2O2-dependent in neuronal cells. Further, rotenone induces H2O2-dependent inhibition of mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, resulting in caspase-dependent and -independent apoptosis in neuronal cells. The results highlight mTOR as a crucial intermediary between rotenone-induced oxidative stress and neuronal loss related to PD. Our findings suggest that activating mTOR signaling and/or administering antioxidants may be explored as a promising strategy for prevention and treatment of PD.
FUNDING
National Natural Science Foundation of China (81271416 to L.C.); Project for the Priority Academic Program Development and Natural Science Foundation of Jiangsu Higher Education Institutions of China (10KJA180027 to L.C.); National Institutes of Health (CA115414 to S.H.); American Cancer Society (RSG-08-135-01-CNE to S.H.); Louisiana Board of Regents (NSF-2009-PFUND-144 to S.H.); Innovative Research Program of Jiangsu College Graduate of China (CXLX12_0406 to Q.Z.).
ACKNOWLEDGMENTS
The authors declare no conflict of interest.
REFERENCES
- Adam-Vizi V. (2005). Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxid. Redox Signal. 7, 1140–1149. [DOI] [PubMed] [Google Scholar]
- Bao L., Avshalumov M. V., Rice M. E. (2005). Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J. Neurosci. 25, 10029–10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K., Krause K. H. (2007). The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Blesa J., Phani S., Jackson-Lewis V., Przedborski S. (2012). Classic and new animal models of Parkinson's disease. J. Biomed. Biotechnol. 2012, 845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Tapias V., Na H. M., Honick A. S., Drolet R. E., Greenamyre J. T. (2009). A highly reproducible rotenone model of Parkinson's disease. Neurobiol. Dis. 34, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu L., Huang S. (2008a). Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic. Biol. Med. 45, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu L., Luo Y., Huang S. (2008b). MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J. Neurochem. 105, 251–261. [DOI] [PubMed] [Google Scholar]
- Chen L., Xu B., Liu L., Luo Y., Yin J., Zhou H., Chen W., Shen T., Han X., Huang S. (2010). Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab. Invest. 90, 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xu B., Liu L., Luo Y., Zhou H., Chen W., Shen T., Han X., Kontos C. D., Huang S. (2011). Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 50, 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Yap Y. W., Choy M. S., Koh C. H., Seet S. J., Duan W., Whiteman M., Cheung N. S. (2006). Early induction of calpains in rotenone-mediated neuronal apoptosis. Neurosci. Lett. 397, 69–73. [DOI] [PubMed] [Google Scholar]
- Choi W. S., Eom D. S., Han B. S., Kim W. K., Han B. H., Choi E. J., Oh T. H., Markelonis G. J., Cho J. W., Oh Y. J. (2004). Phosphorylation of p38 MAPK induced by oxidative stress is linked to activation of both caspase-8- and -9-mediated apoptotic pathways in dopaminergic neurons. J. Biol. Chem. 279, 20451–20460. [DOI] [PubMed] [Google Scholar]
- Chong Z. Z., Shang Y. C., Wang S., Maiese K. (2012). Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog. Neurobiol. 99, 128–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M., Albert V., Hall M. N. (2013). mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 23, 53–62. [DOI] [PubMed] [Google Scholar]
- Dello Russo C., Lisi L., Feinstein D. L., Navarra P. (2013). mTOR kinase, a key player in the regulation of glial functions: Relevance for the therapy of multiple sclerosis. Glia 61, 301–311. [DOI] [PubMed] [Google Scholar]
- Deng Y. T., Huang H. C., Lin J. K. (2010). Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 49, 141–151. [DOI] [PubMed] [Google Scholar]
- Dhillon A. S., Tarbutton G. L., Levin J. L., Plotkin G. M., Lowry L. K., Nalbone J. T., Shepherd S. (2008). Pesticide/environmental exposures and Parkinson's disease in East Texas. J. Agromedicine 13, 37–48. [DOI] [PubMed] [Google Scholar]
- Drechsel D. A., Patel M. (2008). Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic. Biol. Med. 44, 1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95. [DOI] [PubMed] [Google Scholar]
- Emmrich J. V., Hornik T. C., Neher J. J., Brown G. C. (2013). Rotenone induces neuronal death by microglial phagocytosis of neurons. FEBS J. 280, 5030–5038. [DOI] [PubMed] [Google Scholar]
- Fernandez M., Negro S., Slowing K., Fernandez-Carballido A., Barcia E. (2011). An effective novel delivery strategy of rasagiline for Parkinson's disease. Int. J. Pharm. 419, 271–280. [DOI] [PubMed] [Google Scholar]
- Franklin J. L. (2011). Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid. Redox Signal. 14, 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A., Akifusa S., Kamio N., Hirofuji T., Nonaka K., Yamashita Y. (2010). Involvement of mTOR in globular adiponectin-induced generation of reactive oxygen species. Free Radic. Res. 44, 128–134. [DOI] [PubMed] [Google Scholar]
- Gao H. M., Hong J. S., Zhang W., Liu B. (2003). Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: Relevance to the etiology of Parkinson's disease. J. Neurosci. 23, 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S., Lee J., Darley-Usmar V. M., Zhang J. (2012). Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS One 7, e44610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao B., Cheng S., Clancy C. J., Nguyen M. H. (2013). Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 57, 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. H., Zhu X. Z. (2007). Rotenone-induced neurotoxicity of THP-1 cells requires production of reactive oxygen species and activation of phosphatidylinositol 3-kinase. Brain Res. 1153, 12–19. [DOI] [PubMed] [Google Scholar]
- Jaworski J., Sheng M. (2006). The growing role of mTOR in neuronal development and plasticity. Mol. Neurobiol. 34, 205–219. [DOI] [PubMed] [Google Scholar]
- Kamalden T. A., Ji D., Osborne N. N. (2012). Rotenone-induced death of RGC-5 cells is caspase independent, involves the JNK and p38 pathways and is attenuated by specific green tea flavonoids. Neurochem. Res. 37, 1091–1101. [DOI] [PubMed] [Google Scholar]
- Kang H., Han B. S., Kim S. J., Oh Y. J. (2012). Mechanisms to prevent caspase activation in rotenone-induced dopaminergic neurodegeneration: Role of ATP depletion and procaspase-9 degradation. Apoptosis 17, 449–462. [DOI] [PubMed] [Google Scholar]
- Kim E. K., Choi E. J. (2010). Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 1802, 396–405. [DOI] [PubMed] [Google Scholar]
- Lanju X., Jing X., Shichang L., Zhuo Y. (2014). Induction of apoptosis by antimycin A in differentiated PC12 cell line. J. Appl. Toxicol. 34, 651–657. [DOI] [PubMed] [Google Scholar]
- Laplante M., Sabatini D. M. (2012). mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M. L., Mercer L. D., Nagley P., Beart P. M. (2007). Rotenone and MPP+ preferentially redistribute apoptosis-inducing factor in apoptotic dopamine neurons. Neuroreport 18, 307–312. [DOI] [PubMed] [Google Scholar]
- Lin X., Parisiadou L., Sgobio C., Liu G., Yu J., Sun L., Shim H., Gu X. L., Luo J., Long C. X., et al. (2012). Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 32, 9248–9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Chen L., Chung J., Huang S. (2008). Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 27, 4998–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li F., Cardelli J. A., Martin K. A., Blenis J., Huang S. (2006). Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 25, 7029–7040. [DOI] [PubMed] [Google Scholar]
- Malagelada C., Jin Z. H., Greene L. A. (2008). RTP801 is induced in Parkinson's disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J. Neurosci. 28, 14363–14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C., Ryu E. J., Biswas S. C., Jackson-Lewis V., Greene L. A. (2006). RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson's disease by a mechanism involving mammalian target of rapamycin inactivation. J. Neurosci. 26, 9996–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell L., Gentile M., Rius J., Rodriguez C., Crespo J., Badimon L., Martinez-Gonzalez J. (2009). The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol. Cell. Biol. 29, 5828–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., James-Kracke M., Sun G. Y., Sun A. Y. (2009). Oxidative and inflammatory pathways in Parkinson's disease. Neurochem. Res. 34, 55–65. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R., Hernandez-Esquivel L., Rivero-Segura N. A., Marin-Hernandez A., Neuzil J., Ralph S. J., Rodriguez-Enriquez S. (2013). Reactive oxygen species are generated by the respiratory complex II-evidence for lack of contribution of the reverse electron flow in complex I. FEBS J. 280, 927–938. [DOI] [PubMed] [Google Scholar]
- Newhouse K., Hsuan S. L., Chang S. H., Cai B., Wang Y., Xia Z. (2004). Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol. Sci. 79, 137–146. [DOI] [PubMed] [Google Scholar]
- Niizuma K., Yoshioka H., Chen H., Kim G. S., Jung J. E., Katsu M., Okami N., Chan P. H. (2010). Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta 1802, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Rawal P., Wu Y., Xie W., Jankovic J., Le W. (2009). Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164, 541–551. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Rocha H., Garcia-Garcia A., Pickett C., Li S., Jones J., Chen H., Webb B., Choi J., Zhou Y., Zimmerman M. C., et al. (2013). Compartmentalized oxidative stress in dopaminergic cell death induced by pesticides and complex I inhibitors: Distinct roles of superoxide anion and superoxide dismutases. Free Radic. Biol. Med. 61C, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoto-Nagai M., Maruyama W., Kato Y., Isobe K., Tanaka M., Naoi M., Osawa T. (2003). An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J. Neurosci. Res. 74, 589–597. [DOI] [PubMed] [Google Scholar]
- Sherer T. B., Betarbet R., Stout A. K., Lund S., Baptista M., Panov A. V., Cookson M. R., Greenamyre J. T. (2002). An in vitro model of Parkinson's disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 22, 7006–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L., Perycz M., Malik A., Jaworski J. (2008). Role of mTOR in physiology and pathology of the nervous system. Biochim. Biophys. Acta 1784, 116–132. [DOI] [PubMed] [Google Scholar]
- Taetzsch T., Block M. L. (2013). Pesticides, microglial NOX2, and Parkinson's disease. J. Biochem. Mol. Toxicol. 27, 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamilselvam K., Braidy N., Manivasagam T., Essa M. M., Prasad N. R., Karthikeyan S., Thenmozhi A. J., Selvaraju S., Guillemin G. J. (2013). Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxid. Med. Cell. Longev. 2013, 102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L., Sipos I., Adam-Vizi V. (2004). Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson's disease. Neurochem. Res. 29, 569–577. [DOI] [PubMed] [Google Scholar]
- Wang S., Wu J., Nie S. D., Bereczki E., Pei J. J. (2013). Dysregulated mTOR-dependent signaling in neurodegeneration or carcinogenesis: Implication for Alzheimer's disease and brain tumors. J. Alzheimers Dis. 37, 495–505. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Li S., Chou A. P., Bronstein J. M. (2006). Inhibitory effects of pesticides on proteasome activity: Implication in Parkinson's disease. Neurobiol. Dis. 23, 198–205. [DOI] [PubMed] [Google Scholar]
- Wang X. J., Xu J. X. (2005). Possible involvement of Ca2+ signaling in rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurosci. Lett. 376, 127–132. [DOI] [PubMed] [Google Scholar]
- Wu F., Wang Z., Gu J. H., Ge J. B., Liang Z. Q., Qin Z. H. (2013). p38 (MAPK)/p53-Mediated Bax induction contributes to neurons degeneration in rotenone-induced cellular and rat models of Parkinson's disease. Neurochem. Int. 63, 133–140. [DOI] [PubMed] [Google Scholar]
- Xiong N., Long X., Xiong J., Jia M., Chen C., Huang J., Ghoorah D., Kong X., Lin Z., Wang T. (2012). Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson's disease models. Crit. Rev. Toxicol. 42, 613–632. [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu C., Chen S., Ye Y., Guo M., Ren Q., Liu L., Zhang H., Xu C., Zhou Q., et al. (2014). Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson's disease. Cell. Signal. 26, 1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T. A., Garrett M. D., Walton M. I., Raynaud F., de Bono J. S., Workman P. (2008). Targeting the PI3K-AKT-mTOR pathway: Progress, pitfalls, and promises. Curr. Opin. Pharmacol. 8, 393–412. [DOI] [PubMed] [Google Scholar]
- Yeh Y. T., Yeh H., Su S. H., Lin J. S., Lee K. J., Shyu H. W., Chen Z. F., Huang S. Y., Su S. J. (2014). Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic. Biol. Med. 74, 1–13. [DOI] [PubMed] [Google Scholar]