Abstract
3,4-(±)-Methylenedioxymethamphetamine (MDMA, Ecstasy) is a ring-substituted amphetamine derivative with potent psychostimulant properties. The neuropharmacological effects of MDMA are biphasic in nature, initially causing synaptic monoamine release, primarily of serotonin (5-HT). Conversely, the long-term effects of MDMA manifest as prolonged depletions in 5-HT, and reductions in 5-HT reuptake transporter (SERT), indicative of serotonergic neurotoxicity. MDMA-induced 5-HT efflux relies upon disruption of vesicular monoamine storage, which increases cytosolic 5-HT concentrations available for release via a carrier-mediated mechanism. The vesicular monoamine transporter 2 (VMAT2) is responsible for packaging monoamine neurotransmitters into cytosolic vesicles. Thus, VMAT2 is a molecular target for a number of psychostimulant drugs, including methamphetamine and MDMA. We investigated the effects of depressed VMAT2 activity on the adverse responses to MDMA, via reversible inhibition of the VMAT2 protein with Ro4-1284. A single dose of MDMA (20 mg/kg, subcutaneous) induced significant hyperthermia in rats. Ro4-1284 (10 mg/kg, intraperitoneal) pretreatment prevented the thermogenic effects of MDMA, instead causing a transient decrease in body temperature. MDMA-treated rats exhibited marked increases in horizontal velocity and rearing behavior. In the presence of Ro4-1284, MDMA-mediated horizontal hyperlocomotion was delayed and attenuated, whereas rearing activity was abolished. Finally, Ro4-1284 prevented deficits in 5-HT content in rat cortex and striatum, and reduced depletions in striatal SERT staining, 7 days after MDMA administration. In summary, acute inhibition of VMAT2 by Ro4-1284 protected against MDMA-mediated hyperthermia, hyperactivity, and serotonergic neurotoxicity. The data suggest the involvement of VMAT2 in the thermoregulatory, behavioral, and neurotoxic effects of MDMA.
Keywords: MDMA, VMAT2, Ro4-1284, hyperthermia, hyperactivity, neurotoxicity
The recreational use of 3,4-(±)-methylenedioxymethamphetamine (MDMA, Ecstasy) among adolescents in the Unites States is widespread, despite its harmful effects to the peripheral and central nervous systems. Pharmacologically, MDMA acts as an indirect monoaminergic agonist, causing potent release of [3H]-serotonin (5-HT) and [3H]-norepinephrine (NE), and to a lesser extent, [3H]-dopamine (DA) from rat synaptosomes (Partilla et al., 2000; Rothman et al., 2001). Microdialysis studies corroborate in vitro findings, revealing dose-dependent increases in extracellular 5-HT and DA in rat striatum and prefrontal cortex following MDMA administration (Baumann et al., 2005; Gudelsky and Nash, 1996). MDMA also induces acute elevations in body temperature in rats, leading to hyperthermia (Mechan et al., 2002). Behavioral changes accompany MDMA dosing, most notably stimulation of locomotor activity and changes in exploratory rearing (Bubar et al., 2004; Walker et al., 2007). MDMA is also an established serotonergic neurotoxicant, producing long-lasting reductions in 5-HT and 5-hydroxyindole acetic acid (5-HIAA) in rat cortex, striatum, hippocampus, and hypothalamus, along with depletions in 5-HT uptake sites and serotonergic nerve-terminal density (for a review, see Capela et al., 2009), perhaps reflective of neurodegeneration.
The ability of MDMA and other substituted amphetamines to stimulate synaptic monoamine neurotransmitter release is attributed to the interference of monoamine uptake mechanisms at the plasma membrane and intra-neuronal storage vesicles (Rothman and Baumann, 2002; Rudnick and Clark, 1993). MDMA likely binds to 5-HT reuptake transporters (SERTs) located in the plasma membrane, inducing 5-HT release via a carrier-mediated exchange mechanism. Disruption of vesicular monoamine transporter 2 (VMAT2) function assists in MDMA-evoked monoamine release by elevating biogenic amine concentrations in the cytosol, permitting their rapid efflux into the synapse. MDMA competes with the vesicular inhibitor, reserpine, for the substrate-binding site in VMAT2, suggestive of a direct interaction between MDMA and VMAT2 (Schuldiner et al., 1993). Additionally, MDMA hinders VMAT2 function by dissipating the ATP-driven transmembrane pH gradient, which regulates the vesicular transport of biogenic amines (Schuldiner et al., 1993). MDMA-mediated outflow of tritiated monoamines, especially 5-HT, from rat brain slices is reduced by reserpine in a dose-dependent manner (Fitzgerald and Reid, 1993). Likewise, efflux of 5-HT is markedly attenuated in reserpine-treated raphe neurons (Gu and Azmitia, 1993). MDMA and other amphetamine-related compounds are established substrates of VMAT2; they exhibit low affinity for [3H]-dihydrotetrabenazine (DTBZ) binding but high potency at inhibiting vesicular [3H]-DA uptake and releasing preloaded [3H]-DA from rat vesicular fractions (Partilla et al., 2006). MDMA likely enters intra-neuronal vesicles and depletes monoamine storage by reversing VMAT2 function (Partilla et al., 2006).
VMAT2 stores DA, 5-HT, NE, epinephrine, and histamine into synaptic vesicles for exocytotic release. VMAT2 transports biogenic amines against a concentration gradient by exchanging two intra-vesicular protons for one substrate molecule (Parsons, 2000). Alterations in vesicular loading through VMAT2 modulation have profound effects on neurotransmission and neuronal health (Fumagalli et al., 1999; Pothos et al., 2000; Vergo et al., 2007). Disruption of VMAT2 function can cause DA mishandling, resulting in increased cytosolic DA levels available for oxidation, thereby compromising dopaminergic neurons and modulating their interactions with exogenous compounds. Low expression VMAT2 mice exhibit extensive nigral and extranigral neurodegeneration, and manifest Parkinsonian-like behaviors (Taylor et al., 2011). Furthermore, VMAT2 is implicated in the mechanism of psychostimulant abuse. Indeed, lobeline, an alkaloid found in “Indian tobacco,” perturbs monoamine storage by interfering with VMAT2 (Teng et al., 1998). Acute inhibition of VMAT2 by a lobeline analog, UKCP 110, prevents in vitro methamphetamine-mediated DA release and self-administration in rats without development of tolerance (Beckmann et al., 2010). Thus, lobeline analogs with an enhanced selectivity for VMAT2 are proposed for the treatment of psychostimulant abuse (Nickell et al., 2014).
Contributions of VMAT2 function to the mechanism of MDMA toxicity in rats are examined herein, employing a pharmacological model of diminished VMAT2 activity by administering Ro4-1284 prior to MDMA challenge. Ro4-1284 reversibly inhibits VMAT2, displaying faster and shorter-acting monoamine-depleting properties (Staal and Sonsalla, 2000), relative to the non-selective, irreversible VMAT1/2 inhibitor, reserpine. As such, Ro4-1284 analogs exert a less pronounced pharmacological response (Bernstein et al., 2014; Chen et al., 2012). Unlike MDMA, Ro4-1284 is not a substrate for VMAT2 but rather an uptake inhibitor, displaying similar potency at inhibiting [3H]-DTBZ binding and vesicular [3H]-DA uptake. Moreover, uptake inhibitors of VMAT2 exert partial release of [3H]-DA from synaptic vesicles (Partilla et al., 2006). We hypothesized that antagonism of VMAT2 with Ro4-1284 pretreatment would profoundly impact the acute and long-term response to MDMA due to the importance of this transporter to the neuropharmacological mechanism of MDMA and related psychostimulant drugs.
MATERIALS AND METHODS
Drugs and chemicals
(±)MDMA-HCl was obtained from Sigma-Aldrich (St Louis, Missouri). The VMAT2 inhibitor, Ro4-1284 (2-hydroxy-2-ethyl-3-isobutyl-9,10-dimethoxy-1,2,3,4,5,6,7-hexahydrobenzo[a]chinolizine), was also purchased from Sigma-Aldrich.
Subjects
Male Sprague-Dawley (SD) rats, weighing approximately 150 g, were obtained from Harlan Laboratories (Indianapolis, Indiana). Animals were housed in groups of 3 per cage and maintained on a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were given a week to acclimate prior to initiating experiments. Procedures were carried out in accordance with the University of Arizona Institutional Animal Care and Use Committee.
Dosing
Pharmacological inhibition of VMAT2 was achieved by Ro4-1284 pretreatment. Ro4-1284 was dissolved in dimethyl sulfoxide (DMSO) and administered to rats (10 mg/kg, intraperitoneal [ip]). Animals dosed with either Ro4-1284 or DMSO received a single injection of MDMA (20 mg/kg, subcutaneous [sc]) or 0.9% saline 1 h after pretreatment. Principles of interspecies scaling predict a dose of 1.28 mg/kg (96 mg/75 kg) in humans to be equivalent to a neurotoxic dose of MDMA of 20 mg/kg in rats (Ricaurte et al., 2000). Recreational doses of MDMA have been found to range from 75 to 125 mg of (±)-MDMA for a single dose, which are found within the neurotoxic threshold dictated by interspecies scaling (Ricaurte et al., 2000).
Core body temperature
Mini subcue data-loggers (SubCue, Calgary, Canada) were implanted into the peritoneal cavity of rats. Animals were anesthetized with isoflurane (5% induction, 2% maintenance) for the duration of the surgery. Body temperature was monitored for 24 h prior to any treatment to establish a baseline, and 48 h post-treatment in intervals of 3 min. Ambient temperature was kept at 22 ± 1°C prior and during the entire treatment period.
Locomotor activity
Animals were placed inside open-field activity cages (Coulbourn Instruments, Whitehall, Pennsylvania) consisting of a clear Plexiglas square box (3.25 × 10 × 7 × in) crisscrossed by 3 photobeam sensor rings. Rats were kept in these cages for 30 min prior to any data recording, allowing them to acclimate to the new environment. Computer software supplied by the manufacturer recorded locomotor activity for 3.5 h in 5 min bins, which included a 30-min baseline period, a 1-h pretreatment period, and 2-h post-treatment period. Two locomotor activity parameters were analyzed: horizontal velocity (cm/min) and vertical activity (rearing).
Neurotransmitter isolation
Animals were euthanized 7 days after MDMA dosing via CO2 asphyxiation followed by decapitation. Brains were excised and the cortex and striatum were dissected free of surrounding tissue. Samples were weighed and suspended in 10 volumes of ice-cold 0.1 M perchloric acid (134 μM EDTA and 263 μM octane-sulfonic acid sodium salt). The tissue was then sonicated for 15 s followed by centrifugation at 16 000× g (4°C) for 20 min. Supernatant was filtered at 0.45 μm and used for monoamine detection via high-performance liquid chromatography coupled to a coulometric electrode array system (HPLC-CEAS).
HPLC-CEAS
Neurotransmitter content was assayed using a Shimadzu 10ADvp system equipped with an ESA C-18, 3 μm, 4.6 × 80 mm column (Dionex, Massachusetts) coupled to an ESA Model 5600A CoulArray system (Dionex). Mobile phase consisted of 35 mM citric acid, 54 mM sodium acetate, 324 μM octane-sulfonic acid sodium salt, 171 μM EDTA, 3% (v/v) methanol, 3% (v/v) acetonitrile, pH 4.0. Flow rate was 0.8 ml/min. Potentials were set at +50, +150, +300, and +450 mV. 50 μl of tissue preparation were used per injection and peak areas were compared with a standard curve of DA, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-HIAA to achieve quantitation.
Immunohistochemical staining
Animals were given a lethal dose of pentobarbital (200 mg/kg, ip) and transcardially perfused with 4% paraformaldehyde (w/v) in 0.1 M phosphate-buffered saline (PBS, pH = 7.4) 7 days following MDMA administration. Brains were extracted and post-fixed overnight at 4°C in the same solution. They were subsequently cryoprotected in 30% sucrose (w/v) in 0.1 M PBS. Coronal sections of 40 µm were cut using a freezing microtome and stored in cryoprotectant solution at −20°C until further analysis. Tissue sections were washed with 0.1 M PBS, followed by antigen retrieval with citrate buffer (pH = 6.0) and 0.03% Triton X-100 for 20 min at 80°C. Endogenous peroxide activity was blocked by incubation with 0.03% hydrogen peroxide in 0.1 M PBS and 0.03% Triton X-100 (PBS-T) for 30 min. Tissue sections were then incubated in 5% normal goat serum (NGS) in PBS-T for 90 min. Primary antibody incubation with a polyclonal anti-SERT antibody (AB9726, 1:40 000; Millipore, Billerica, Massachusetts) in antiserum diluent (3% NGS in PBST) was performed for 48 h at 4°C. Following rinses with 0.1 M PBS, sections were incubated with biotinylated secondary antibody (goat anti-rabbit) diluted 1:300 in antiserum diluent. Tissue was visualized using avidin-biotin-HRP conjugate solution (VECTASTAIN Elite ABC kit, VectorLabs, Burlingame, California). Staining was enhanced by incubation in a 3, 3′-diaminobenzidine (DAB) solution (20 mg of DAB, 2.5 g of nickel (II) sulfate, and 0.04% hydrogen peroxide in 100 ml of 0.175 M sodium acetate) for 10 min. Sections were mounted on collagen-coated slides, dehydrated with increasing concentrations of ethanol and xylenes. Sections were coverslipped and analyzed under a light microscope.
Statistics
Results are presented as absolute values and expressed as the mean ± SE (n = 4–10). Effects of treatment on core body temperature and behavioral activity were analyzed via two-way ANOVA (treatment group × time interval) with time as a repeated measure. Significant interactions between treatment groups at different time intervals were examined by post hoc Bonferroni multiple comparisons. Total horizontal and vertical activity and neurotransmitter concentrations were analyzed by one-way ANOVA followed by Fisher-Hayter post hoc tests. All analyzes were performed using Stata 11.0 (College Station, Texas) and GraphPad Prism 5 software (La Jolla, California).
RESULTS
Inhibition of VMAT2 Induces Acute Decreases in Brain Monoamine Levels
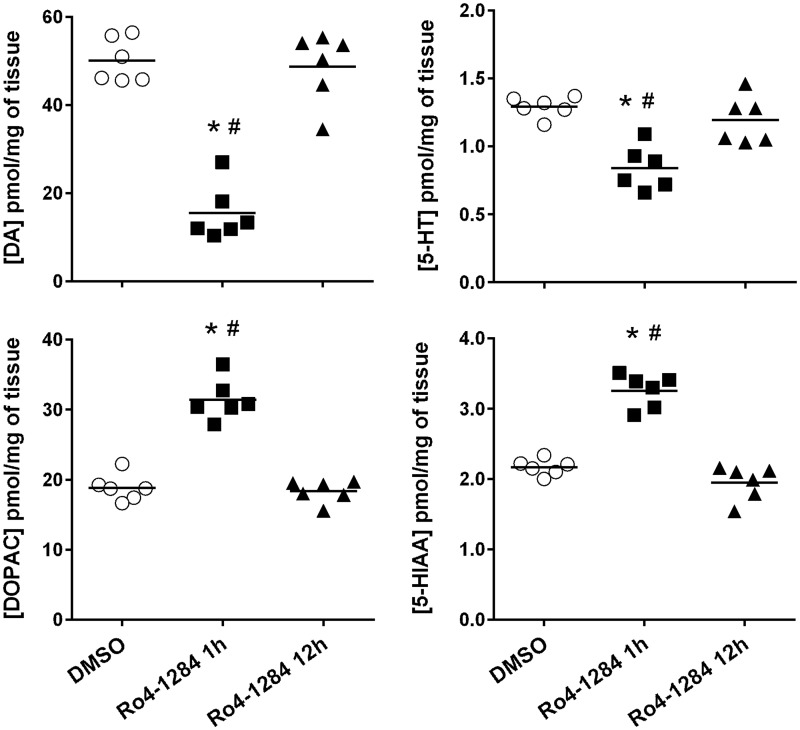
Pharmacological antagonism of VMAT2 depletes brain monoamine concentrations (Staal and Sonsalla, 2000). Thus, indoleamine and catecholamine content in the striatum of SD rats were assessed at 1 h and 12 h following Ro4-1284 (10 mg/kg, ip) administration (Fig. 1). One-way ANOVA displayed significant differences in tissue concentrations of 5-HT [F (2, 15) = 16.5, P < 0.0002)], DA [F (2, 15) = 54.0, P < 0.0001] and their respective metabolites, 5-HIAA [F (2, 15) = 68.7, P < 0.0001) and DOPAC [F (2, 15) = 67.6, P < 0.0001]. Post hoc analysis revealed marked depletions in DA content of 69% (P < 0.05) 1 h after Ro4-1284 exposure. Although to a lesser extent, significant decreases in striatal 5-HT (35%, P < 0.05) were also noted in Ro4-1284-treated animals compared with DMSO controls. These findings are consistent with previous reports, demonstrating that brain DA is most sensitive to the effects of the acute VMAT2 inhibitor, tetrabenazine, compared with other monoamines (Pearson and Reynolds, 1988; Pettibone et al., 1984). Conversely, tissue concentrations of monoamine metabolites 5-HIAA and DOPAC were notably elevated (50% and 67%, respectively; P < 0.05) from controls at 1 h following Ro4-1284 treatment, reflecting increased monoamine turnover. Indeed, DA and 5-HT deficits resulting from VMAT2 antagonism by tetrabenazine are reversed by inhibition of monoamine oxidases (Pettibone et al., 1984), which likely contribute to the increase in deaminated metabolites observed with Ro4-1284 treatment. Importantly, changes in brain monoamine content were absent in SD rats at 12 h post-treatment, indicating dissipation of the depleting activity of Ro4-1284. Overall, Ro4-1284 induced rapid and short-lived reductions in striatal DA and 5-HT concentrations, confirming disruption of VMAT2 function at the time of MDMA administration.
FIG. 1.
Acute effects of Ro4-1284 on striatal indoleamine and catecholamine content. Concentrations of 5-HT, DA, and corresponding metabolites, 5HIAA and DOPAC were assessed in rat striatum 1 and 12 h following Ro4-1284 (10 mg/kg, ip) treatment by HPLC-CEAS. Results show individual concentrations of monoamines in each treatment group. Values are different from the DMSO control group at (*) P < 0.05 and from the Ro4-1284 (12 h) group at (#) P < 0.05.
Decreased VMAT2 Activity Protects Against MDMA-Mediated Hyperthermia
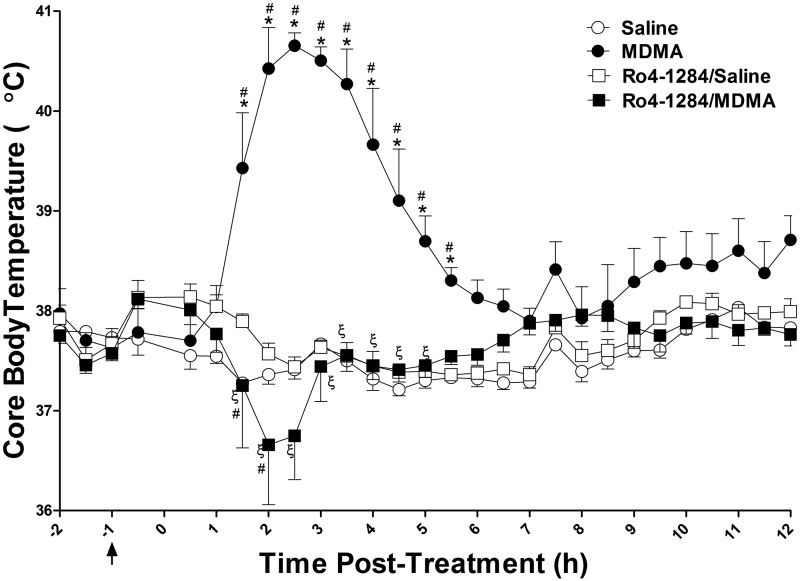
As expected, a single dose of MDMA (20 mg/kg, sc) induced a thermogenic response in rats, causing elevations in core body temperature that reached a maximum of 40.7 ± 0.1°C, 2.5 h after drug administration; body temperatures remained above those of saline controls for up to 12 h (Fig. 2). Pretreatment with the VMAT2 inhibitor, Ro4-1284 (10 mg/kg, ip), attenuated MDMA-mediated hyperthermia in rats. Following MDMA administration, the Ro4-1284/MDMA treatment group displayed a transient drop in body temperature to 36.7 ± 0.6°C, returning to baseline levels 3 h post-treatment. Two-way repeated measures ANOVA of core body temperature revealed significant effects of treatment group (F (3, 675) = 25.7, P < 0.0001), significant effects of time interval (F (27, 675) = 5.6, P < 0.0001), and a significant treatment × time interaction (F (81, 675) = 9.1, P < 0.0001). Bonferroni post hoc tests confirmed a significant hyperthermic effect in the MDMA-treated rats when compared with their saline counterparts between 1.5 and 5.5 h post-treatment (P < 0.001). Notably, Ro4-1284 treatment alone had no profound effects on body temperature. Body temperatures recorded in the Ro4-1284/MDMA treatment group were significantly different from both the Ro4-1284/saline group at the 2 h time interval (P < 0.01), and the MDMA group between 1.5 and 5 h post-treatment (P < 0.001). In summary, Ro4-1284 pretreatment abolished hyperthermia in MDMA-exposed rats, instead inducing an acute decrease in body temperature.
FIG. 2.
MDMA-mediated hyperthermia is absent in Ro4-1284-treated rats. Temperature probes implanted into the peritoneal cavity of Sprague-Dawley rats recorded core body temperature. Animals were given Ro4-1284 (10 mg/kg, ip) 1 h prior to MDMA (20 mg/kg, sc) or saline (control) treatment. The black arrow represents the time of Ro4-1284 pretreatment and zero indicates the time of MDMA treatment. Results are expressed as means ± SE (n = 5–8) and represent temperature measurements collected from all four treatment groups. Values are different from the saline group at (*) P < 0.05, from the Ro4-1284/saline group at (#) P < 0.05 and from the MDMA group at (ξ) P < 0.05.
Inhibition of VMAT2 Attenuates Induction of Locomotor Activity in MDMA-Treated Rats
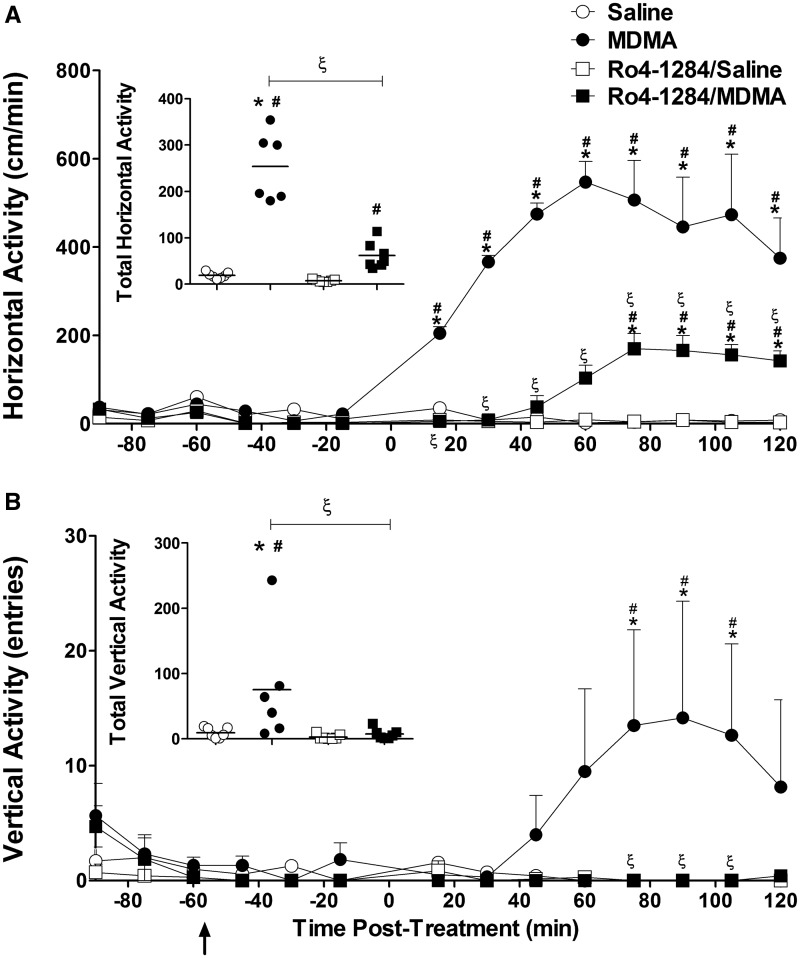
The behavioral response to MDMA was measured using activity chambers that recorded various aspects of locomotion. Two specific parameters were selected for this analysis: horizontal velocity (hyperactivity) and vertical activity (rearing; exploratory behavior). MDMA (20 mg/kg, sc) stimulated horizontal locomotor activity in SD rats. Animals attained maximal activity (547 ± 46 cm/min), 60 min post-MDMA treatment (Fig. 3A). In combination with Ro4-1284, MDMA also induced horizontal locomotor activity. Horizontal velocity in the Ro4-1284/MDMA treatment group reached maximal activity (170 ± 35cm), 75 min following MDMA dosing. Two-way repeated measures ANOVA of horizontal hyperactivity revealed significant effects of treatment group (F (3, 299) = 57.8, P < 0.0001), significant effects of time interval (F (13, 299) = 26.1, P < 0.0001), and a significant treatment × time interaction (F (39, 299) = 16.9, P < 0.0001). Further Bonferroni post hoc analysis confirmed significant increases in horizontal velocity 15–120 min after MDMA administration compared with saline controls (P < 0.001). Horizontal activity was unaffected by Ro4-1284 treatment alone. Moreover, post hoc tests revealed significant inductions in MDMA-mediated horizontal locomotion in the presence of Ro4-1284, displaying marked elevations in movement velocity over controls 75–120 min following drug exposure (P < 0.01). However, when compared with MDMA-only treatment, increased horizontal velocity was significantly reduced in the Ro4-1284/MDMA group throughout the post-treatment period (P < 0.001).
FIG. 3.
Ro4-1284 pretreatment attenuates horizontal velocity and vertical activity in MDMA-treated rats. Locomotor activity was monitored by placing animals into open-field activity chambers. Baseline levels of locomotor activity were measured for 30 min, followed by a 60-min pretreatment period with either DMSO or Ro4-1284 (10 mg/kg, ip) and a 120-min post-treatment period after MDMA (20 mg/kg, sc) or saline administration. Graphs show the mean (SE ± 6–7) horizontal (A) and vertical (B) activity at each time interval. The black arrow represents the time of Ro4-1284 pretreatment and zero indicates the time of MDMA dosing. Scatter plots display total horizontal and vertical locomotion over the entire testing period for each treatment group. Values are different from the saline group at (*) P < 0.05, from the Ro4-1284/saline group at (#) P < 0.05 and from the MDMA group at (ξ) P < 0.05.
MDMA represses exploratory behaviors immediately following drug dosing, however, high-dose treatment can augment late-phase rearing activity (Bubar et al., 2004; Walker et al., 2007). Consistent with these observations, MDMA-mediated increases in vertical activity occurred more gradually compared with horizontal locomotion, reaching maximal activity (14.2 ± 10.2) 90 min post-treatment (Fig. 3B). In contrast, induction of vertical activity with MDMA was absent in animals pretreated with Ro4-1284. Rearing activity displayed significant treatment group effects (F (3, 299) = 4.5, P = 0.013) and a significant time × treatment interaction (F (39, 299) = 1.6, P = 0.013) but no significant time interval effects (F (13, 299) = 1.4, P = 0.15). Enhanced vertical activity was noted in the MDMA-treated group between 75 and 105 min post-treatment (P < 0.01). Stimulation of rearing behavior mediated by MDMA was completely suppressed with Ro4-1284 pretreatment between 75 and 105 min after drug administration (P < 0.01). At last, Ro4-1284 treatment alone had no effects on rearing activity.
Locomotor activity results were also analyzed as the total horizontal and vertical activity over the entire measurement period (Figs. 3A and 3B insets). One-way ANOVA showed significant differences in total horizontal velocity (F (3, 23) = 57.7, P < 0.0001) and total vertical activity (F (3, 23) = 4.5, P < 0.01). Fisher-Hayter pairwise comparisons confirmed significant stimulation in total locomotor activity with MDMA treatment (P < 0.05). Ro4-1284/MDMA-treated animals also displayed significant elevations in total horizontal velocity (P < 0.05) but no marked increases in total vertical activity. Furthermore, total activity levels in the Ro4-1284/MDMA group were lower compared with MDMA-only treatment (P < 0.05). In summary, Ro4-1284 pretreatment delayed and attenuated MDMA-induced horizontal locomotion, while completely abolishing stimulation in rearing behavior.
Prevention of MDMA-Mediated Indoleamine Depletion with VMAT2 Inhibition
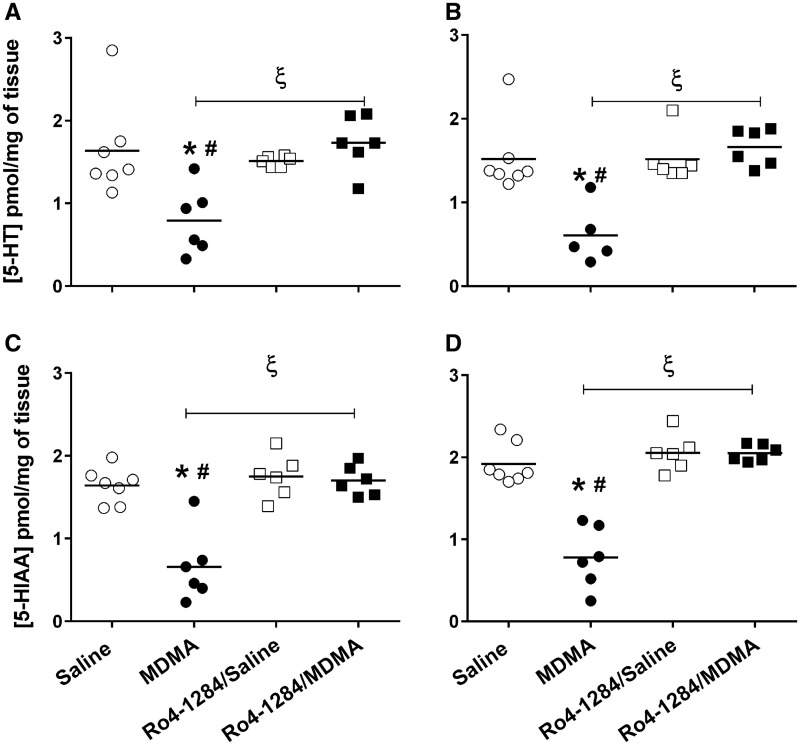
In order to determine the effect of VMAT2 inhibition on MDMA neurotoxicity, tissue neurotransmitter concentrations were assessed in the cortex (Cx) and striatum (St), 7 days after MDMA (20 mg/kg, sc) exposure. Although tissue 5-HT depletions are indirect markers of MDMA-induced neurotoxicity, these adverse changes persist for months after cessation of drug treatment (Sabol et al., 1996) and correlate positively with long-term cognitive deficits (Marston et al., 1999). Basal indoleamine levels in saline-treated animals were as follows: [5-HTCx = 1.64 ± 0.22, 5-HTSt = 1.52 ± 0.16, 5-HIAACx = 1.64 ± 0.081, 5-HIAASt = 1.92 ± 0.095 pmol/mg of tissue) (Fig. 4). One-way ANOVA revealed significant differences in neurotransmitter levels among the treatment groups [5-HTCx (F (3, 21) = 7.0, P < 0.0019), 5-HTSt (F (3, 20) = 10.9, P < 0.0002), 5-HIAACx (F (3, 21) = 20.2, P < 0.0001), 5-HIAASt (F (3, 21) = 34.9, P < 0.0001)]. Fisher-Hayter pairwise comparisons showed significant reductions in 5-HT concentrations in both cortex (approximately 50%, P < 0.05) and striatum (approximately 60%, P < 0.05) of MDMA-treated rats when compared with saline controls. Similarly, decreases in cortical (62%, P < 0.05) and striatal (65%, P < 0.05) 5-HIAA levels were apparent after MDMA administration. Tissue indoleamine content was unaffected by Ro4-1284 treatment, as evidenced by similar neurotransmitter concentrations observed in the Ro4-1284/saline and saline-only treatment groups. Furthermore, 5-HT and 5-HIAA levels in Ro4-1284/MDMA-exposed rats were comparable to those of saline controls, but significantly higher from those of the MDMA treatment group in both brain regions analyzed (P < 0.05). Thus, pretreatment with Ro4-1284 prevented MDMA-induced 5-HT deficits.
FIG. 4.
Ro4-1284 pretreatment affords protection against long-lasting MDMA-induced tissue 5-HT depletions. 5-HT and 5-HIAA levels were measured from cortex (A, C) and striatum (B, D) 7 days after MDMA exposure by HPLC-CEAS. Ro4-1284 (10 mg/kg, ip) was administered 1 h prior to MDMA treatment (20 mg/kg, sc). Results show individual concentrations of tissue indoleamines for each treatment group. Values are different from the saline group at (*) P < 0.05, from the Ro4-1284/saline group at (#) P < 0.05 and from the MDMA group at (ξ) P < 0.05.
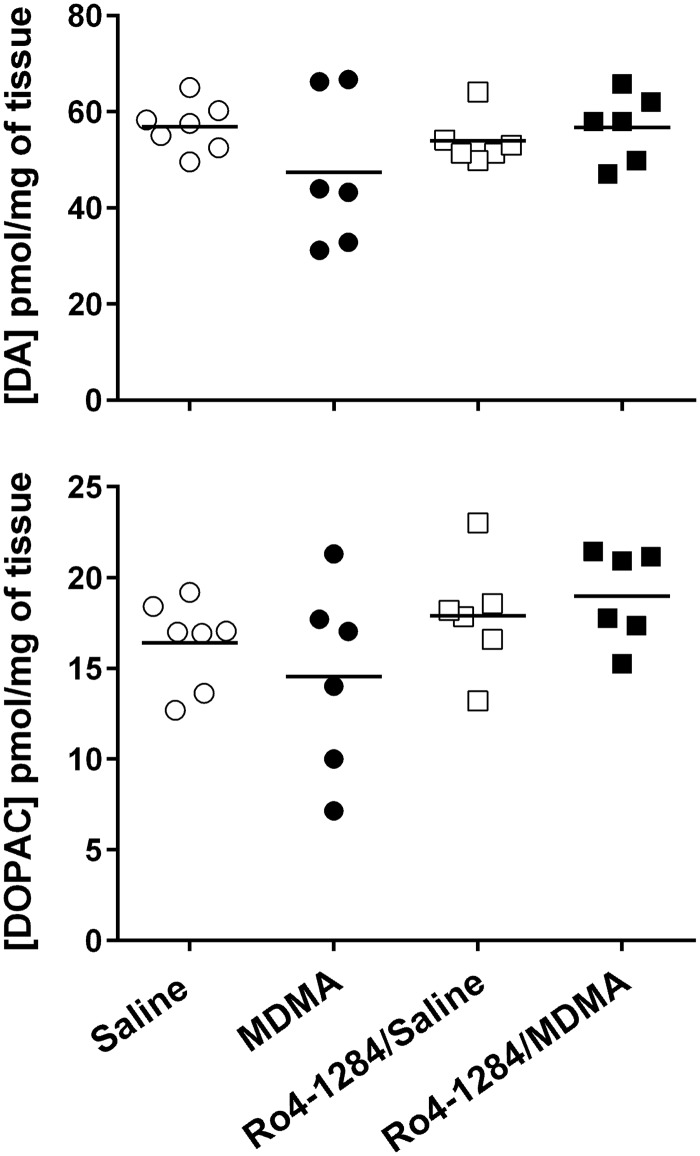
The selectivity of MDMA toward the serotonergic system was confirmed by evaluating changes in tissue catecholamine levels in SD rats 7 days following peripheral MDMA administration (20 mg/kg, sc). Saline-treated animals displayed basal DA and DOPAC levels in rat striatum: [DA = 56.88 ± 1.92, DOPAC = 16.41 ± 0.91 pmol/mg of tissue] (Fig. 5). One-way ANOVA indicated no significant differences in catecholamine content among the treatment groups [DA (F (3, 21) = 1.5, P = 0.25; DOPAC (F (3, 21) = 1.8, P = 0.17]. As expected, MDMA failed to induce damage to the dopaminergic system in rats. More importantly, the data asserts that Ro4-1284 treatment alone or in combination with MDMA has no enduring effects on brain catecholamine content.
FIG. 5.
Long-term effects of Ro4-1284 and MDMA on striatal catecholamine content. DA and DOPAC concentrations were measured from striatum 7 days after MDMA exposure by HPLC-CEAS. Ro4-1284 (10 mg/kg, ip) was administered 1 h prior to MDMA treatment (20 mg/kg, sc). Results show individual catecholamine concentrations in each treatment group.
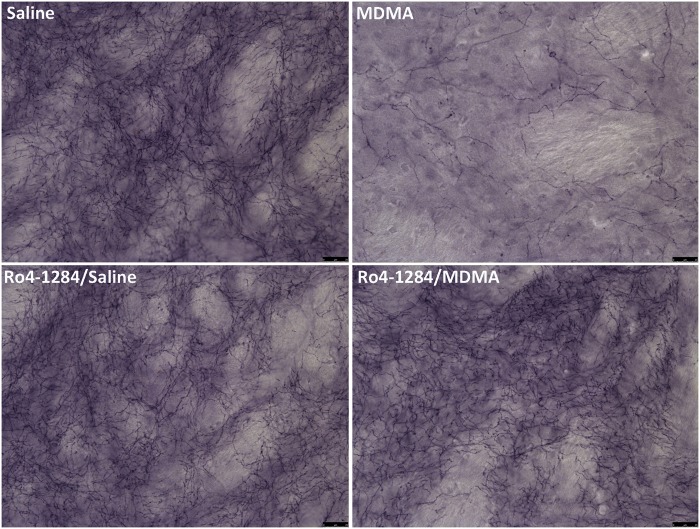
VMAT2 Inhibition Suppresses Serotonergic Nerve-Terminal Damage in MDMA-Exposed Rats
Immunohistochemical staining of SERT is considered a sensitive marker of 5-HT nerve-terminal viability and MDMA induces similar reductions in SERT immunoreactivity to the serotonergic neurotoxic analog, 5,7-dihydroxytryptamine (5,7-DHT) (Xie et al., 2006). Animals showed widespread changes in SERT staining pattern in the striatum upon MDMA treatment. Saline controls displayed basal levels of SERT protein in this brain region (Fig. 6). High-magnification images revealed staining of 5-HT nerve endings (fibrous appearance) and terminal buttons (granular pattern). MDMA induced severe reductions in SERT protein expression, with decreased axonal and terminal button staining 7 days post-treatment. Furthermore, the loss in SERT protein reflects a decrease in striatal serotonergic nerve-terminal density. In contrast, treatment with Ro4-1284 alone had no discernable effects on SERT staining. Similarly, Ro4-1284/MDMA-treated rats exhibited minimal to no changes in nerve-terminal marker expression from saline controls. Individual variability in SERT staining patterns, in response to the different treatments, paralleled the results obtained in the neurochemical assay. Overall, these findings suggest that Ro4-1284 pretreatment attenuated long-term reductions in serotonergic nerve-terminal density in rat striatum associated with MDMA neurotoxicity.
FIG. 6.
Loss of striatal serotonergic nerve-terminal density following MDMA dosing is prevented with Ro4-1284 treatment. Representative images of rat striatum were captured by light microscopy. Coronal sections were stained for SERT in animals treated with saline, MDMA, Ro4-1284/saline, and Ro4-1284/MDMA, 7 days post-drug administration. Panels illustrate high-magnification images with scale bars denoting 25 µm.
DISCUSSION
The thermoregulatory response to MDMA is profoundly impacted by Ro4-1284 pretreatment. A single neurotoxic dose of MDMA (20 mg/kg, sc) induced prominent hyperthermia in rats that was completely abolished in the presence of Ro4-1284 (Fig. 2). Irreversible inhibition of VMAT2 with reserpine also attenuates MDMA-mediated hyperthermia (Sabol and Seiden, 1998). Interestingly, reserpine-only treatment causes significant hypothermia that persists beyond the administration of MDMA (Sabol and Seiden, 1998) and beyond the presence of the drug in the brain and plasma (Brodie et al., 1956). Thus, it is difficult to discern whether the protective effects of reserpine against the thermogenic effects of MDMA are directly correlated to its vesicular depleting activity or the consequence of an induction of hypothermia. In the present study, and despite Ro4-1284-induced reductions in tissue 5-HT and DA levels (Fig. 1), body temperature was unaffected by Ro4-1284 treatment alone. Acute and chronic exposure to Ro4-1284 (40 mg/kg, ip) can cause intense hypothermia in rats due to its proficiency at inhibiting VMAT2 function and reducing tissue monoamine levels (Yehuda et al., 1999). However, the lower dose of Ro4-1284 administered in this study was insufficient to elicit hypothermia. Hyperthermia produced by MDMA involves activation of central post-synaptic 5-HT2A and D1 receptors (Mechan et al., 2002; Shioda et al., 2008). Therefore, we speculate that Ro4-1284 prevents MDMA-induced hyperthermia by stimulating monoamine depletion; however, additional studies are needed to investigate this hypothesis. Moreover, MDMA in conjunction with Ro4-1284 decreases body temperature, perhaps by further enhancing the ability of Ro4-1284 to diminish brain DA and 5-HT levels.
Depression of VMAT2 function reduced horizontal velocity in MDMA-treated rats (Fig. 3). DA neurotransmission is a critical mediator of the extrapyramidal motor system, and suppression of locomotor activity in reserpine-treated mice has been attributed to disturbances in DA homeostasis (Fisher et al., 2000; Liu et al., 2014). MDMA-induced elevation of locomotor activity is associated with enhanced striatal neuronal activity and modulation of D1/2 receptors (Ball et al., 2003). Thus, attenuation in horizontal hyperactivity mediated by MDMA is perhaps related to the robust DA-depleting activity of Ro4-1284 treatment (Fig. 1). Rats with deficiencies in SERT (Lizarraga et al., 2014) and mice with 5-HT1B receptor deficiencies (Scearce-Levie et al., 1999) exhibit delayed onset of horizontal hyperactivity following peripheral MDMA administration. In light of the fact that 5-HT pathways are seemingly important to the early-phase locomotor effects of MDMA, decreases in brain 5-HT following Ro4-1284 administration may be responsible for the late onset of MDMA-induced horizontal activity. As such, the data support a role for VMAT2 in the initiation and maintenance of horizontal hyperlocomotion upon MDMA dosing. In contrast, abolishment of rearing behavior is most likely the consequence of prominent striatal DA depression with Ro4-1284 treatment, given that antagonism of D1/2 receptors prevents induction of vertical activity following MDMA administration in rats (Bubar et al., 2004). Indeed, genetic ablation of the 5-HT1B receptor, and 5-HT uptake inhibition by fluoxetine, block stimulation of floor-plane locomotion without affecting vertical activity, which suggests that exploratory behaviors, such as rearing, are minimally impacted by 5-HT pathways stimulated by MDMA (Callaway et al., 1990; Scearce-Levie et al., 1999).
Overall, reversible antagonism of VMAT2 with Ro4-1284 attenuated MDMA-induced hyperthermia and hyperactivity. Compelling evidence exists for the involvement of enhanced monoamine release and neurotransmission in the acute responses to MDMA. Acute VMAT2 inhibition caused alterations in brain monoamine concentrations evident by decreases in striatal 5-HT and DA with Ro4-1284 treatment. Similarly, VMAT2 deficiency in mice elicits reductions in brain catecholamines and indoleamines (Taylor et al., 2014; Wang et al., 1997), and the appearance of depressive-like behaviors, including locomotor retardation (Fukui et al., 2007), and enhanced sensitivity to the locomotor effects of psychostimulants, such as amphetamine and cocaine (Wang et al., 1997). Furthermore, selective VMAT2 inhibition with the lobeline derivative, GZ-793 A, attenuates methamphetamine-evoked DA release in the nucleus accumbens shell of rats in a time-dependent manner (Meyer et al., 2013) and antagonizes the reinforcing effects of this psychostimulant (Beckmann et al., 2012). Collectively, these findings suggest that modulation of vesicular storage influences the pharmacology of amphetamine-like drugs; therefore, it should be anticipated that VMAT2 blockade by Ro4-1284 would have such antagonistic effects on the acute toxicities of MDMA, most likely via disruption of monoamine storage and release. Indeed, inhibition of VMAT2 with reserpine reduces the MDMA-mediated release of monoamines (Fitzgerald and Reid, 1993; Gu and Azmitia, 1993; Sabol and Seiden, 1998). In vivo microdialysis experiments are necessary to confirm putative alterations in MDMA-induced monoamine efflux by Ro4-1284 pretreatment.
Pharmacological inhibition of VMAT2 with Ro4-1284 afforded protection against the long-term neurotoxicity of MDMA. Deficits in tissue 5-HT (Fig. 4) and serotonergic nerve-terminal density (Fig. 6) 7 days after MDMA exposure were prevented in the presence of Ro4-1284. Reserpine pretreatment yields inconclusive results with respect to the effect of VMAT2 inhibition on MDMA neurotoxicity. Hekmatpanah et al. (1989) confirmed depletion of brain DA and 5-HT content with reserpine (5 mg/kg, ip) administration, but noted no changes in MDMA-induced reduction of 5-HT uptake sites. Conversely, Schmidt et al. (1990) reported a protective effect of reserpine (5 mg/kg, sc) on MDMA (20 mg/kg, sc) neurotoxicity. The latter study measured decreases in TPH activity, since tissue 5-HT levels remained depressed 1 week after reserpine-only treatment. The utilization of distinct endpoint markers of neurotoxicity may, in part, account for such discrepancies in the results. In addition, the persistent monoamine depleting activity of reserpine could be a confounding factor when evaluating the neurotoxic effects of MDMA. 5-HT deficits gradually appear 1 to 7 days after MDMA exposure (Schmidt, 1987), a time window seemingly important for the manifestation of lasting biochemical and structural damage to 5-HT nerve-terminals caused by the serotonergic neurotoxicant, 5,7-DHT (Capela et al., 2008). Unlike reserpine, the monoamine depleting properties of Ro4-1284 were short-acting, as evident by the full recovery of DA and 5-HT concentrations at 12 h post-treatment (Fig. 1) and the lack of alterations in basal tissue monoamine content (Figs. 4 and 5) and SERT staining (Fig. 6) in Ro4-1284-challenged rats, 7 days after saline treatment.
Our findings provide convincing evidence for the participation of VMAT2 in MDMA neurotoxicity. In support of this view, pharmacological inhibition of VMAT2 with lobeline attenuates long-term DA depletions after methamphetamine treatment (Eyerman and Yamamoto, 2005). In contrast, genetic ablation of VMAT2 enhances susceptibility of low VMAT2 expressing mice to methamphetamine, due to changes in DA compartmentalization that increase DA oxidation in the cytosol and presumably potentiate nerve-terminal damage (Guillot and Miller, 2009; Guillot et al., 2008). The etiology of MDMA neurotoxicity remains largely unknown, complicating the interpretation of the mechanisms driving Ro4-1284 neuroprotection against MDMA. Evidence suggests that VMAT2 immunoreactivity declines following acute methamphetamine treatment, supporting the prevailing hypothesis that decreases in VMAT2 protein precede the development of neurotoxicity mediated by amphetamines (Riddle et al., 2002). Similarly, enduring loss of VMAT2 ligand binding in non-human primates (Ricaurte et al., 2000), and reductions in VMAT2 protein expression in older mice (10 week old) (Reveron et al., 2005) have been reported after repeated MDMA administration.
One possible explanation for the protective mechanism of Ro4-1284 against MDMA neurotoxicity is the prevention of acute hyperthermia. Hyperthermia may exacerbate the neurotoxic effects of MDMA by increasing free radical generation (Green et al., 2003), presumably assisting in the neurodegeneration of 5-HT terminals. However, hyperthermia is not a requirement for MDMA-mediated neurotoxicity. For example, 5-HT deficits occur in Dark Agouti rats exposed to a low-dose MDMA regimen in the absence of hyperthermia (O'Shea et al., 1998). Furthermore, a number of compounds afford neuroprotection without altering the thermoregulatory response to MDMA, including 5-HT reuptake inhibitors (Sanchez et al., 2001) and anti-oxidants (Aguirre et al., 1999; Yeh, 1999). Moreover, VMAT2 inhibition with lobeline post-treatment, after dissipation of methamphetamine-mediated hyperthermia attenuates long-lasting DA depletions, indicating that the neuroprotective effects of lobeline are partly independent of its ability to modulate the temperature response to methamphetamine (Eyerman and Yamamoto, 2005). These findings also highlight the importance of late-phase events, those that occur after the initial pharmacological response, in the manifestation of psychostimulant neurotoxicity (Eyerman and Yamamoto, 2005). Alternatively, the neuroprotective response elicited by Ro4-1284 pretreatment may be closely related to its tissue monoamine depleting activity, particularly to the profound depression of brain DA content. The pivotal role of DA in MDMA-mediated neurotoxicity is well appreciated. As such, attenuation of DA availability prevents serotonergic deficits, whereas stimulation of DA synthesis and neurotransmission aggravate injury to 5-HT neurons after MDMA treatment (Aguirre et al., 1998; Breier et al., 2006; Kanthasamy et al., 2002).
The present results reveal the importance of VMAT2 in the mechanism of action of MDMA. Specifically, the data emphasize that decreased VMAT2 function suppresses the acute and long-term response to MDMA. Compounds that selectively target VMAT2 disrupt monoamine storage and amphetamine-induced monoamine release, hindering the neurochemical and behavioral effects of psychostimulants, including amphetamine and methamphetamine. Our studies are consistent with such findings, suggesting that attenuation of MDMA-induced hyperthermia, hyperactivity, and serotonergic neurotoxicity with Ro4-1284 pretreatment is likely mediated by similar mechanisms.
FUNDING
The National Institute on Drug Abuse (grant number DA023525) and the National Institute of Environmental Health Sciences Training in Environmental Toxicology of Complex Diseases Training Grant (grant number T32ES007091 to L.E.L.); the Southwest Environmental Health Sciences Center (SWEHSC) (grant number P30 ES006694); and the Integrative Health Sciences Facility Core (grant number P30 ES006694) at the University of Arizona.
ACKNOWLEDGMENTS
The authors thank Dr Gregory O. Dussor from the Department of Medical Pharmacology, College of Pharmacy at the University of Arizona for providing the activity chambers used in the behavioral studies. Special thanks to Dr Dean Billheimer from the SWEHSC, College of Pharmacy at the University of Arizona for his help and advice with the biostatistical analysis. The authors also acknowledge Douglas W. Cromey, M.S., from the SWEHSC’s Cellular Imaging Facility Core at the University of Arizona for his help with the immunohistochemical analysis.
REFERENCES
- Aguirre N., Barrionuevo M., Lasheras B., Del Rio J. (1998). The role of dopaminergic systems in the perinatal sensitivity to 3, 4-methylenedioxymethamphetamine-induced neurotoxicity in rats. J. Pharmacol. Exp. Ther. 286, 1159–1165. [PubMed] [Google Scholar]
- Aguirre N., Barrionuevo M., Ramirez M., Del Rio J., Lasheras B. (1999). Alpha-lipoic acid prevents 3,4-methylenedioxy-methamphetamine (MDMA)-induced neurotoxicity. Neuroreport 10, 3675–80. [DOI] [PubMed] [Google Scholar]
- Ball K. T., Budreau D., Rebec G. V. (2003). Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res. 994, 203–215. [DOI] [PubMed] [Google Scholar]
- Baumann M. H., Clark R. D., Budzynski A. G., Partilla J. S., Blough B. E., Rothman R. B. (2005). N-substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or ‘Ecstasy’). Neuropsychopharmacology 30, 550–560. [DOI] [PubMed] [Google Scholar]
- Beckmann J. S., Denehy E. D., Zheng G., Crooks P. A., Dwoskin L. P., Bardo M. T. (2012). The effect of a novel VMAT2 inhibitor, GZ-793A, on methamphetamine reward in rats. Psychopharmacology (Berl) 220, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J. S., Siripurapu K. B., Nickell J. R., Horton D. B., Denehy E. D., Vartak A., Crooks P. A., Dwoskin L. P., Bardo M. T. (2010). The novel pyrrolidine nor-lobelane analog UKCP-110 [cis-2,5-di-(2-phenethyl)-pyrrolidine hydrochloride] inhibits VMAT2 function, methamphetamine-evoked dopamine release, and methamphetamine self-administration in rats. J. Pharmacol. Exp. Ther. 335, 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A. I., Stout K. A., Miller G. W. (2014). The vesicular monoamine transporter 2: an underexplored pharmacological target. Neurochem. Int. 73, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier J. M., Bankson M. G., Yamamoto B. K. (2006). L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J. Neurosci. 26, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie B. B., Hess S. M., Shore P. A. (1956). Persistence of reserpine action after the disappearance of drug from brain: effect on serotonin. J. Pharmacol. Exp. Ther. 118, 84–89. [PubMed] [Google Scholar]
- Bubar M. J., Pack K. M., Frankel P. S., Cunningham K. A. (2004). Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl) 173, 326–336. [DOI] [PubMed] [Google Scholar]
- Callaway C. W., Wing L. L., Geyer M. A. (1990). Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J. Pharmacol. Exp. Ther. 254, 456–464. [PubMed] [Google Scholar]
- Capela J. P., Carmo H., Remiao F., Bastos M. L., Meisel A., Carvalho F. (2009). Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol. Neurobiol. 39, 210–271. [DOI] [PubMed] [Google Scholar]
- Capela J. P., Lautenschlager M., Dirnagl U., Bastos M. L., Carvalho F., Meisel A. (2008). 5,7-Dihydroxitryptamine toxicity to serotonergic neurons in serum free raphe cultures. Eur. J. Pharmacol. 588, 232–238. [DOI] [PubMed] [Google Scholar]
- Chen J. J., Ondo W. G., Dashtipour K., Swope D. M. (2012). Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin. Ther. 34, 1487–1504. [DOI] [PubMed] [Google Scholar]
- Eyerman D. J., Yamamoto B. K. (2005). Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J. Pharmacol. Exp. Ther. 312, 160–169. [DOI] [PubMed] [Google Scholar]
- Fisher A., Biggs C. S., Eradiri O., Starr M. S. (2000). Dual effects of L-3,4-dihydroxyphenylalanine on aromatic L-amino acid decarboxylase, dopamine release and motor stimulation in the reserpine-treated rat: evidence that behaviour is dopamine independent. Neuroscience 95, 97–111. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. L., Reid J. J. (1993). Interactions of methylenedioxymethamphetamine with monoamine transmitter release mechanisms in rat brain slices. Naunyn. Schmiedebergs. Arch. Pharmacol. 347, 313–323. [DOI] [PubMed] [Google Scholar]
- Fukui M., Rodriguiz R. M., Zhou J., Jiang S. X., Phillips L. E., Caron M. G., Wetsel W. C. (2007). Vmat2 heterozygous mutant mice display a depressive-like phenotype. J. Neurosci. 27, 10520–10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F., Gainetdinov R. R., Wang Y. M., Valenzano K. J., Miller G. W., Caron M. G. (1999). Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J. Neurosci. 19, 2424–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Mechan A. O., Elliott J. M., O’Shea E., Colado M. I. (2003). The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol. Rev. 55, 463–508. [DOI] [PubMed] [Google Scholar]
- Gu X. F., Azmitia E. C. (1993). Integrative transporter-mediated release from cytoplasmic and vesicular 5-hydroxytryptamine stores in cultured neurons. Eur. J. Pharmacol. 235, 51–57. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. A., Nash J. F. (1996). Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J. Neurochem. 66, 243–249. [DOI] [PubMed] [Google Scholar]
- Guillot T. S., Miller G. W. (2009). Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 39, 149–170. [DOI] [PubMed] [Google Scholar]
- Guillot T. S., Shepherd K. R., Richardson J. R., Wang M. Z., Li Y., Emson P. C., Miller G. W. (2008). Reduced vesicular storage of dopamine exacerbates methamphetamine-induced neurodegeneration and astrogliosis. J. Neurochem. 106, 2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmatpanah C. R., McKenna D. J., Peroutka S. J. (1989). Reserpine does not prevent 3,4-methylenedioxymethamphetamine-induced neurotoxicity in the rat. Neurosci. Lett. 104, 178–182. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A., Sprague J. E., Shotwell J. R., Nichols D. E. (2002). Unilateral infusion of a dopamine transporter antisense into the substantia nigra protects against MDMA-induced serotonergic deficits in the ipsilateral striatum. Neuroscience 114, 917–924. [DOI] [PubMed] [Google Scholar]
- Liu H.-F., Lu S., Ho P. W.-L., Tse H.-M., Pang S. Y.-Y., Kung M. H.-W., Ho J. W.-M., Ramsden D. B., Zhou Z.-J., Ho S.-L. (2014). LRRK2 R1441G mice are more liable to dopamine depletion and locomotor inactivity. Ann. Clin. Transl. Neurol. 1, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga L. E., Phan A. V., Cholanians A. B., Herndon J. M., Lau S. S., Monks T. J. (2014). Serotonin reuptake transporter deficiency modulates the acute thermoregulatory and locomotor activity response to 3,4-(±)-methylenedioxymethamphetamine, and attenuates depletions in serotonin levels in SERT-KO rats. Toxicol. Sci. 139, 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston H. M., Reid M. E., Lawrence J. A., Olverman H. J., Butcher S. P. (1999). Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology (Berl) 144, 67–76. [DOI] [PubMed] [Google Scholar]
- Mechan A. O., Esteban B., O’Shea E., Elliott J. M., Colado M. I., Green A. R. (2002). The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br. J. Pharmacol. 135, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. C., Neugebauer N. M., Zheng G., Crooks P. A., Dwoskin L. P., Bardo M. T. (2013). Effects of VMAT2 inhibitors lobeline and GZ-793A on methamphetamine-induced changes in dopamine release, metabolism and synthesis in vivo. J. Neurochem. 127, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell J. R., Siripurapu K. B., Vartak A., Crooks P. A., Dwoskin L. P. (2014). The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse. Adv. Pharmacol. 69, 71–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea E., Granados R., Esteban B., Colado M. I., Green A. R. (1998). The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 37, 919–926. [DOI] [PubMed] [Google Scholar]
- Parsons S. M. (2000). Transport mechanisms in acetylcholine and monoamine storage. FASEB J. 14, 2423. [DOI] [PubMed] [Google Scholar]
- Partilla J. S., Dempsey A. G., Nagpal A. S., Blough B. E., Baumann M. H., Rothman R. B. (2006). Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 319, 237–246. [DOI] [PubMed] [Google Scholar]
- Partilla J. S., Dersch C. M., Yu H., Rice K. C., Rothman R. B. (2000). Neurochemical neutralization of amphetamine-type stimulants in rat brain by the indatraline analog (-)-HY038. Brain Res. Bull. 53, 821–826. [DOI] [PubMed] [Google Scholar]
- Pearson S. J., Reynolds G. P. (1988). Depletion of monoamine transmitters by tetrabenazine in brain tissue in Huntington’s disease. Neuropharmacology 27, 717–719. [DOI] [PubMed] [Google Scholar]
- Pettibone D. J., Totaro J. A., Pflueger A. B. (1984). Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants. Eur. J. Pharmacol. 102, 425–430. [DOI] [PubMed] [Google Scholar]
- Pothos E. N., Larsen K. E., Krantz D. E., Liu Y., Haycock J. W., Setlik W., Gershon M. D., Edwards R. H., Sulzer D. (2000). Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci. 20, 7297–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveron M. E., Monks T. J., Duvauchelle C. L. (2005). Age-dependent (+)MDMA-mediated neurotoxicity in mice. Neurotoxicology 26, 1031–1040. [DOI] [PubMed] [Google Scholar]
- Ricaurte G. A., Yuan J., McCann U. D. (2000). (±)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42, 5–10. [DOI] [PubMed] [Google Scholar]
- Riddle E. L., Topham M. K., Haycock J. W., Hanson G. R., Fleckenstein A. E. (2002). Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur. J. Pharmacol. 449, 71–74. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H. (2002). Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol. Ther. 95, 73–88. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H., Dersch C. M., Romero D. V., Rice K. C., Carroll F. I., Partilla J. S. (2001). Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39, 32–41. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Clark J. (1993). From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim. Biophys. Acta 1144, 249–263. [DOI] [PubMed] [Google Scholar]
- Sabol K. E., Lew R., Richards J. B., Vosmer G. L., Seiden L. S. (1996). Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part I: Synaptosomal uptake and tissue concentrations. J. Pharmacol. Exp. Ther. 276, 846–854. [PubMed] [Google Scholar]
- Sabol K. E., Seiden L. S. (1998). Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 806, 69–78. [DOI] [PubMed] [Google Scholar]
- Sanchez V., Camarero J., Esteban B., Peter M. J., Green A. R., Colado M. I. (2001). The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (‘ecstasy’)-induced degeneration of 5-HT nerve endings in rat brain. Br. J. Pharmacol. 134, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K., Viswanathan S. S., Hen R. (1999). Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology (Berl) 141, 154–161. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J. (1987). Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 240, 1–7. [PubMed] [Google Scholar]
- Schmidt C. J., Black C. K., Taylor V. L. (1990). Antagonism of the neurotoxicity due to a single administration of methylenedioxymethamphetamine. Eur. J. Pharmacol. 181, 59–70. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Steiner-Mordoch S., Yelin R., Wall S. C., Rudnick G. (1993). Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol. Pharmacol. 44, 1227–1231. [PubMed] [Google Scholar]
- Shioda K., Nisijima K., Yoshino T., Kuboshima K., Iwamura T., Yui K., Kato S. (2008). Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology 29, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Staal R. G., Sonsalla P. K. (2000). Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. J. Pharmacol. Exp. Ther. 293, 336–342. [PubMed] [Google Scholar]
- Taylor T. N., Alter S. P., Wang M., Goldstein D. S., Miller G. W. (2014). Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology 76(Pt A), 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T. N., Caudle W. M., Miller G. W. (2011). VMAT2-deficient mice display nigral and extranigral pathology and motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis. 2011, 124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng L., Crooks P. A., Dwoskin L. P. (1998). Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: comparison with d-amphetamine. J. Neurochem. 71, 258–265. [DOI] [PubMed] [Google Scholar]
- Vergo S., Johansen J. L., Leist M., Lotharius J. (2007). Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 1185, 18–32. [DOI] [PubMed] [Google Scholar]
- Walker Q. D., Williams C. N., Jotwani R. P., Waller S. T., Francis R., Kuhn C. M. (2007). Sex differences in the neurochemical and functional effects of MDMA in Sprague–Dawley rats. Psychopharmacology (Berl) 189, 435–445. [DOI] [PubMed] [Google Scholar]
- Wang Y. M., Gainetdinov R. R., Fumagalli F., Xu F., Jones S. R., Bock C. B., Miller G. W., Wightman R. M., Caron M. G. (1997). Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 19, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Xie T., Tong L., McLane M. W., Hatzidimitriou G., Yuan J., McCann U., Ricaurte G. (2006). Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology 31, 2639–2651. [DOI] [PubMed] [Google Scholar]
- Yeh S. Y. (1999). N-tert-butyl-alpha-phenylnitrone protects against 3,4-methylenedioxymethamphetamine-induced depletion of serotonin in rats. Synapse 31, 169–177. [DOI] [PubMed] [Google Scholar]
- Yehuda S., Rabinovitz S., Mostofsky D. I. (1999). Treatment with a polyunsaturated fatty acid prevents deleterious effects of Ro4-1284. Eur. J. Pharmacol. 365, 27–34. [DOI] [PubMed] [Google Scholar]