Abstract
Objective
To establish a score threshold that constitutes a clinically relevant change for each domain of the Expanded Prostate Cancer Index Composite - Short Form (EPIC-26). While its use in clinical practice and clinical trials has increased worldwide, the clinical interpretation of this 26-item disease-specific patient-reported quality of life questionnaire for men with localized prostate cancer would be facilitated by characterization of score thresholds for clinically relevant change (the minimally important differences, or MID).
Methods
We used distribution- and anchor-based approaches to establish the MID range for each EPIC-26 domain (urinary, sexual, bowel, hormonal) based on a prospective, multi-institutional cohort of 1,201 men treated for prostate cancer between 2003 and 2006 and followed for 3 years after treatment. For the anchor-based approach, we compared within/between subject score changes for each domain to an external “anchor” measure of overall cancer treatment satisfaction.
Results
We found the bowel and vitality/hormonal domains to have the lowest MID range (a 4–6 point change should be considered clinically relevant), while the sexual domain had the greatest MID values (10–12). Urinary incontinence appeared to have a greater MID range (6–9) compared with the urinary irritation/obstruction domain (5–7).
Conclusions
Using two independent approaches, we established the minimally important differences for each EPIC-26 domain. Definition of these MID values is essential for the researcher or clinician to understand when changes in symptom burden among prostate cancer survivors are clinically relevant.
Keywords: Prostate cancer, quality of life, instrument, patient-reported outcomes, EPIC
INTRODUCTION
Many of the nearly 3 million prostate cancer survivors in the US deal with the side effects of prostate cancer treatment.1,2 Even in the midst of advanced technologies to treat the disease (i.e., robotic-assisted surgery, proton beam therapy), urinary, sexual, bowel and hormonal side effects remain common.1–6 The Expanded Prostate Cancer Index-Composite (EPIC) is a well-established patient-reported outcome (PRO) questionnaire developed to monitor health-related quality of life outcomes among prostate cancer survivors.7,8 The 26-item version of EPIC, also known as EPIC Short Form or EPIC-26, contains five symptom domains (urinary incontinence, urinary irritative/obstructive, sexual, bowel, hormonal), scored from 0 (worst) to 100 (best), that can be tracked over time to understand symptom burden, functional outcomes and the impact of side effect management strategies.1,8,9
While EPIC-26 has proven to be a powerful research tool1 with its use increasing worldwide, there exists a longstanding challenge in its interpretation; the domain score thresholds that should be considered clinically relevant have not yet been defined. In other words, if a patient’s sexual domain score changes from 96 pre-treatment to 90 post-treatment, should this be considered clinically significant, or is it simply statistical noise?
An NCI-sponsored working group charged with recommending a core set of symptoms to be assessed using PROs in prostate cancer clinical trials cited the definition of these score thresholds, also known as minimally important differences (MID), as an essential methodological step in confronting the interpretability challenges of PRO data.10 In particular, the group questioned whether the commonly used distribution-based statistical threshold of one-half standard deviation is entirely adequate for inferring clinically meaningful change.
Our objective was to use two independent approaches (distribution-based and anchor-based methods) to define the MID for each EPIC-26 domain. Our findings provide the necessary context for determining when changes in patient-reported symptoms are likely to be clinically meaningful to patients, providers, researchers and payers.
METHODS
Study Population
We identified a longitudinal cohort of 1,201 men with stage T1 or T2 prostate cancer based on a previously reported multi-institutional study.1 The men in our study received primary treatment between March 2003 and March 2006 with radical prostatectomy, brachytherapy, or external-beam radiotherapy at one of nine university-affiliated hospitals. We examined their longitudinal EPIC-26 data prior to treatment and for 3 years post-treatment. The institutional review boards at each site approved the parent study and all patients provided written informed consent.
Outcomes
The primary outcome for this study was the minimally important range for each of the five EPIC-26 domains (urinary incontinence, urinary irritative/obstructive, sexual, bowel, hormonal). We determined pre-treatment, one, two and three year post-treatment EPIC-26 values for each patient. We based the final MID values on a combination of two well-described methods from the survey literature.11–13
Distribution-based approach
First, we used a distribution-based approach to compare changes in EPIC-26 scores to corresponding standard deviations (SD) for each domain. Previous studies have found that half of a standard deviation and one-third of a standard deviation are appropriate choices for a distribution-based minimally important difference cutpoint, with ½ SD representing a medium-sized effect and ⅓ SD representing a small effect.11–13 For this study, we based each domain’s SD on the entire cohort’s EPIC-26 scores for that domain.
Anchor-based approach
Second, we used an anchor-based approach to examine within/between-patient EPIC-26 scores corresponding to an external criterion. In general, anchor-based studies compare patient-reported outcome scores to another subjective assessment, often a global assessment of an external criterion, in order to detect meaningful differences in patient-reported scores.11 For this reason, we selected the following anchor item from the Service Satisfaction Scale for Cancer Care (SCA) scale,14 (derived from the Service Satisfaction Scale15) which was included in the parent study: “In an overall general sense, how satisfied are you with the cancer treatment you received?” The corresponding responses included: Completely Satisfied, Very Satisfied, Somewhat Satisfied, Mixed, Somewhat Unsatisfied, Very Unsatisfied, and Completely Unsatisfied. We combined the Mixed and Unsatisfied groups due to low response levels for those options. The selected global satisfaction item has the highest corrected item-total correlations with the overall 16-item SCA scale (0.82) and the Outcome of Care Satisfaction subscale (0.80).
Statistical analysis
For the distribution-based approach, we calculated the mean and SD of the EPIC-26 values for each domain at our selected time points (pre-treatment, 1, 2, and 3 years), using these to calculate the ⅓ SD and ½ SD values corresponding to MID values. For the anchor-based approach, examining changes in response levels to the aforementioned anchor question, we used unadjusted and age-adjusted linear regression to model EPIC-26 changes from pre-treatment and cross-sectional results from 1, 2, and 3 years of follow-up. We calculated changes in model-adjusted scores between adjacent anchor-item levels. Last, we used the distribution- and anchor-based EPIC-26 MID to recommend ranges for clinically meaningful differences in EPIC-26 scores for each domain.11 For both distribution-based and anchor-based methods, the values derived from the methods above were averaged over the time points, with MID values chosen as the predominant range across methods.
All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC) and all testing was two-sided. The probability of a Type I error was set at 0.05.
RESULTS
The standard deviation in EPIC-26 domain scores ranged from 8.8–27.7 pre-treatment and from 12.6–31.9 at 3 years. Sexual domain scores tended to have the highest SD at each time point. The corresponding distribution-based ⅓ and ½ SD values, analogous to MID values, demonstrated similar variability over time as shown in Table 1. The differences between using a ⅓ or ½ SD approach to define MIDs ranged from roughly 2–5 EPIC-26 points, with the greatest differences in the sexual domain.
Table 1.
Distribution-based Expanded Prostate Cancer Index Composite - Short Form (EPIC-26) standard deviation values corresponding to minimally important differences over time since primary prostate cancer treatment by domain*
| EPIC-26 Domain | Pre-treatment | 1-year | 2-year | 3-year | ||||
|---|---|---|---|---|---|---|---|---|
| ⅓ SD | ½ SD | ⅓ SD | ½ SD | ⅓ SD | ½ SD | ⅓ SD | ½ SD | |
| Urinary Incontinence | 4.3 | 6.4 | 7.0 | 10.5 | 7.0 | 10.5 | 7.0 | 10.5 |
| Urinary Irritative/Obstructive | 4.6 | 7.0 | 5.0 | 7.5 | 4.7 | 7.0 | 4.2 | 6.3 |
| Bowel | 2.9 | 4.4 | 4.7 | 7.0 | 4.7 | 7.1 | 4.2 | 6.4 |
| Sexual | 9.2 | 13.8 | 10.3 | 15.4 | 10.5 | 15.8 | 10.6 | 15.9 |
| Hormonal | 3.8 | 5.7 | 4.5 | 6.8 | 4.8 | 7.2 | 3.9 | 5.9 |
For the distribution-based approach, we calculated the mean and standard deviation (SD) of the EPIC-26 values for each domain at our selected time points since primary prostate cancer treatment, using these to calculate the ⅓ SD and ½ SD corresponding to minimally important difference (MID) values.
As shown in Table 2, responses varied over time for the cross-sectional SCA Anchor item: “In an overall general sense, how satisfied are you with the cancer treatment you received?” Most patients were Completely or Very Satisfied over the study duration. The age-adjusted changes from pre-treatment and cross-sectional EPIC-26 domain values between adjacent anchor-level responses over time from primary prostate cancer treatment are shown in the online Appendix Table. The Sexual domain again had the highest values.
Table 2.
Service Satisfaction Scale for Cancer Care (SCA) Anchor item responses over time since primary prostate cancer treatment from a cohort of 1201 men
| SCA Anchor item: “In an overall general sense, how satisfied are you with the cancer treatment you received?” | Time since primary prostate cancer treatment | ||
|---|---|---|---|
| 1 year | 2 years | 3 years | |
| Completely Satisfied (%) | 48.9 | 44.2 | 48.2 |
| Very Satisfied (%) | 43.1 | 46.5 | 43.0 |
| Somewhat Satisfied (%) | 5.8 | 7.0 | 6.2 |
| Mixed (%) | 0.9 | 1.0 | 0.6 |
| Somewhat Unsatisfied (%) | 0.4 | 0.5 | 0.8 |
| Very Unsatisfied (%) | 0.3 | 0.5 | 0.8 |
| Completely Unsatisfied (%) | 0.6 | 0.3 | 0.4 |
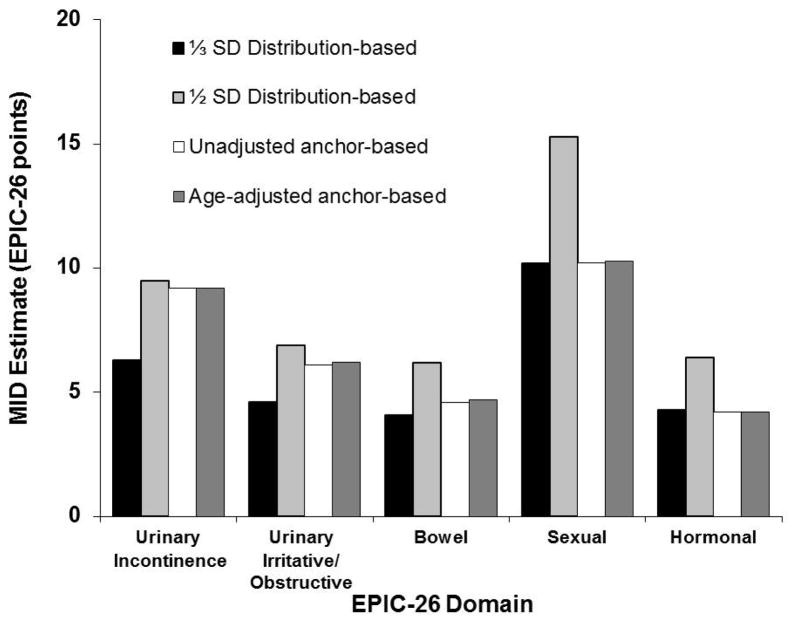
As illustrated in the Figure, our EPIC-26 MID estimates are derived from the ranges (recommended best practice11) provided by the two approaches used in this study. Based on pooled averages from distribution- and anchor-based approaches, we found that the bowel and hormonal domains had the lowest MID values (both 4–6 EPIC-26 points), while the sexual domain had the greatest MID values, ranging from 10–12. Urinary incontinence appeared to have a larger and higher MID range (6–9) compared with the urinary irritative/obstructive domain (5–7) (Table 3).
Figure.
Average Expanded Prostate Cancer Index Composite - Short Form (EPIC-26) Minimally Important Difference (MID) values using distribution- and anchor-based approaches by domain.
Table 3.
Recommended Expanded Prostate Cancer Index Composite - Short Form (EPIC-26) Minimally Important Difference (MID) values by domain
| EPIC-26 Domain | Minimally Important Difference (EPIC-26 points)* |
|---|---|
| Urinary Incontinence | 6–9 |
| Urinary Irritative/Obstructive | 5–7 |
| Bowel | 4–6 |
| Sexual | 10–12 |
| Hormonal | 4–6 |
DISCUSSION
We used distribution- and anchor-based approaches to establish minimally important (i.e., clinically-relevant) differences for each EPIC-26 domain. In general, MID estimates were consistent between methods for most domains. Clinically meaningful changes in EPIC-26 scores ranged from 4 to 12 points depending on the domain. We believe the EPIC-26 MID values provided in this study offer the necessary context for determining when changes in symptom burden among prostate cancer survivors are significant. In addition, these findings provide useful endpoints for clinical trials, comparative effectiveness research and the clinical care of men with prostate cancer after treatment.
The use of EPIC in clinical practice and clinical trials has, in large part, been limited to the realm of clinical epidemiology. At least 2 barriers to its use in real-world practice have been identified. First, the length of EPIC has been cited as a barrier to widespread clinical adoption.7,16,17 This concern was reduced with the shorter versions of EPIC, including its Short Form (i.e., EPIC-26) included in this study, and most recently with the EPIC for clinical practice (EPIC-CP).17 The EPIC-26 is the most widely version used in clinical studies due to its comprehensive and rigorous delineation of function and bother for each of the relevant domains.1,7 The subsequent clinical practice version of EPIC, EPIC-CP, further reduced the instrument to a one-page format with 16 items to facilitate measuring HRQOL in the routine practice setting, and is highly correlated to the original and Short Forms.17 Its 16-item, one-page structure makes ease of use in routine practice straightforward. In addition, our findings are relevant for its scoring given the high correlation among the EPIC instruments.16
A second longstanding barrier is what represents a clinically-relevant (i.e., minimally important) difference for each domain of EPIC. Comparisons to other quality of life instruments have revealed cutoffs for symptom severity in different domains,18,19 although there have never been thresholds for symptom improvement or worsening developed for the EPIC-26 instrument. Our findings, along with recent work in this area using the UCLA-Prostate Cancer Index (UCLA-PCI)20 and EPIC-CP,16 builds a strong foundation for understanding meaningful differences in patient-reported outcomes among survivors. For example, comparable MID values for the UCLA Prostate Cancer Index,20 a precursor of EPIC also scored from 0–100, were found for urinary function (8) and bother (9), bowel function (7) and bother (8), and sexual function (8) and bother (11). That the upper bounds of thresholds in our study closely match these values supports construct validity and robustness of the recommended MID levels.
As patient-centered cancer care increases in demand, understanding how best to alleviate symptom burden among prostate cancer survivors has important implications for patients, providers, researchers and payers.21 The Patient-Centered Outcomes Research Institute (PCORI) methodology core is currently investigating strategies for increasing the use of patient-reported outcomes into electronic health records.22 Just as normal and abnormal laboratory values are routinely examined in clinical care, patient-reported outcomes should be easily accessed in clinically meaningful contexts to patients and providers. In particular, following EPIC outcomes over time and understanding clinically meaningful differences in their values could facilitate more effective and comprehensive communication between patients and providers regarding clinically meaningful outcomes and thereby improve care quality.
This study identifies thresholds for clinically meaningful differences in patient-reported prostate cancer quality of life outcomes; however, there are several limitations to keep in mind. First, the study did not include patients on active surveillance, an increasingly popular treatment option among men at low risk of dying of their disease, as there would not have been sufficient changes in EPIC scores to examine MID.23 Caution in applying this to men on active surveillance is warranted; nevertheless, knowing which side effect management strategies work best in terms of meaningful and measureable differences in EPIC-26 outcomes will remain relevant for men treated for the disease. Second, we only used two approaches to estimate the EPIC-26 MID values for each domain;11,12,20 however, the consistency between these well-vetted approaches is reassuring.11 Third, the estimates derived in this study are from a non-randomized, prospective study raising generalizability concerns in terms of pre-treatment function and treatment selection. This limitation applies to all observational studies where a subset of the population is enrolled in a non-random fashion. The parent study attempted to address this through enrollment at multiple institutions using a standardized protocol. Fourth, there may be some concern that a 10 point improvement in the lower range of a domain score (e.g., 30 to 40) represents a larger proportional increase compared with that in the upper range of a domain score (e.g., 80 to 90). Fortunately, the approaches used to estimate the EPIC-26 MID values provide thresholds across the entire range of domain scores from 0–100. We feel the MIDs in this manuscript and based on raw differences indicate when a clinically meaningful change in the patient’s state has taken place thereby adding a helpful threshold for interpreting EPIC-26 results. However, we recognize that for individuals, the perception of whether the change is truly important may differ based on where on the scale they fall.
In conclusion, our findings provide the necessary context for determining when changes in patient-reported symptoms using the EPIC-26 are likely to be clinically meaningful to prostate cancer patients, their providers, researchers and payers. In doing so, this study provides useful endpoints for clinical trials, comparative effectiveness research and the clinical care of men with prostate cancer.
Supplementary Material
Appendix Table.
Age-adjusted changes from pre-treatment and cross-sectional Expanded Prostate Cancer Index Composite - Short Form (EPIC-26) domain values between adjacent anchor-level responses over time since primary prostate cancer treatment
| EPIC-26 Domain | Anchor-level comparison Anchor item: “In an overall general sense, how satisfied are you with the cancer treatment you received?” | Change between adjacent anchor-level responses based on pre-treatment EPIC-26 domain values (within patient) | Change between adjacent anchor-level responses based on cross-sectional EPIC-26 domain values (between patient) | ||||
|---|---|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 1 year | 2 years | 3 years | ||
| Urinary Incontinence | Completely vs Very Satisfied | 3.2 | 2.4 | 0.1 | 3.0 | 3.6 | −0.7 |
| Very vs Somewhat Satisfied | 4.8 | 11.5 | 10.9 | 8.2 | 12.1 | 13.0 | |
| Somewhat Satisfied vs. Mixed or Unsatisfied | 7.3 | 5.4 | 35.7 | 7.1 | 6.0 | 31.7 | |
| Urinary Irritative/Obstructive | Completely vs Very Satisfied | 0.4 | 0.9 | −1.6 | 2.3 | 2.9 | 1.1 |
| Very vs Somewhat Satisfied | 5.7 | 7.8 | 6.0 | 11.2 | 12.5 | 5.4 | |
| Somewhat Satisfied vs. Mixed or Unsatisfied | 13.2 | 5.0 | 13.1 | 4.9 | 4.4 | 15.9 | |
| Bowel | Completely vs Very Satisfied | 1.9 | 0.4 | −0.6 | 2.7 | 1.5 | 1.6 |
| Very vs Somewhat Satisfied | 3.8 | 5.5 | 2.7 | 5.5 | 7.9 | 3.6 | |
| Somewhat Satisfied vs. Mixed or Unsatisfied | 6.1 | 13.5 | 3.0 | 6.2 | 15.4 | 4.5 | |
| Sexual | Completely vs Very Satisfied | 8.5 | 6.4 | 2.4 | 8.1 | 9.0 | 6.6 |
| Very vs Somewhat Satisfied | 3.1 | 8.7 | 19.9 | 9.8 | 19.3 | 20.3 | |
| Somewhat Satisfied vs. Mixed or Unsatisfied | 16.2 | 21.5 | 9.1 | 8.3 | 6.2 | 2.2 | |
| Hormonal | Completely vs Very Satisfied | 1.4 | 0.9 | 0.6 | 3.3 | 1.7 | 0.9 |
| Very vs Somewhat Satisfied | 6.4 | 5.3 | 1.5 | 8.0 | 11.1 | 4.7 | |
| Somewhat Satisfied vs. Mixed or Unsatisfied | 3.4 | 9.3 | 4.1 | 1.3 | 9.8 | 2.0 | |
Acknowledgments
Dr. Skolarus is supported by a VA HSR&D Career Development Award - 2 (CDA 12-171). Dr. Greenfield is supported by a National Institute on Alcohol Abuse and Alcoholism (NIAAA Center grant P50 AA005595). Dr. Chang is supported by a grant from the Urology Care Foundation Research Scholar Program and Dornier Medtech entitled “Measuring Prostate Cancer Patient Reported Outcomes at the Point of Care.” PROST-QA Consortium supported by National Institutes of Health Grants R01 CA95662 and RC1 CA146596*
We acknowledge PROSTQA Data Coordinating Center Project Management by Jill Hardy, MS (Michigan State University, East Lansing, MI), Erin Najuch and Jonathan Chipman (Dana Farber Cancer Institute, Boston, MA) and Catrina Crociani, MPH (Beth Israel Deaconess Medical Center, Boston, MA), grant administration by Beth Doiron, BA (Beth Israel Deaconess Medical Center, Boston, MA), and technical support from coordinators at each clinical site. We would like to thank the study participants. Without them this study would not be possible.
Abbreviation Key
- EPIC
Expanded Prostate Cancer Index-Composite
- EPIC-26
Expanded Prostate Cancer Index-Composite Short Form
- PRO
Patient-reported outcome
- MID
Minimally important difference
- SD
Standard deviation
- SCA
Service Satisfaction Scale for Cancer Care
- PCORI
Patient Centered Outcomes Research Institute
- UCLA-PCI
UCLA-Prostate Cancer Index
References
- 1.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England journal of medicine. 2008 Mar 20;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 2.Darwish-Yassine M, Berenji M, Wing D, et al. Evaluating long-term patient-centered outcomes following prostate cancer treatment: findings from the Michigan Prostate Cancer Survivor study. J Cancer Surviv. 2014 Mar;8(1):121–130. doi: 10.1007/s11764-013-0312-8. [DOI] [PubMed] [Google Scholar]
- 3.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009 Jun 16;101(12):888–892. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Zhang Y, Skolarus TA, et al. Comparative Effectiveness of External-Beam Radiation Approaches for Prostate Cancer. Eur Urol. 2012 Jul 6; doi: 10.1016/j.eururo.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolarus TA, Weizer AZ, Hedgepeth RC, He C, Wood DP, Jr, Hollenbeck BK. Understanding early functional recovery after robotic prostatectomy. Surg Innov. 2012 Mar;19(1):5–10. doi: 10.1177/1553350611403770. [DOI] [PubMed] [Google Scholar]
- 6.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008 Oct;54(4):785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 7.Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010 Nov;76(5):1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000 Dec 20;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 9.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005 Apr 20;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 10.Chen RC, Chang P, Vetter RJ, et al. Recommended Patient-Reported Core Set of Symptoms to Measure in Prostate Cancer Treatment Trials. J Natl Cancer Inst. 2014 Jul 8;106(7) doi: 10.1093/jnci/dju132. Print 2014 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008 Feb;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005 Mar;14(2):285–295. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 13.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004 Sep;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Kamo N, Dandapani SV, Miksad RA, et al. Evaluation of the SCA instrument for measuring patient satisfaction with cancer care administered via paper or via the Internet. Ann Oncol. 2011 Mar;22(3):723–729. doi: 10.1093/annonc/mdq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenfield TK, Attkisson CC. The UCSF Client Satisfaction Scales: II. The Service Satisfaction Scale-30. In: Maruish M, editor. Psychological Testing: Treatment planning and outcome assessment. Vol 3. Instruments for Adults. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. pp. 813–837. [Google Scholar]
- 16.Chipman JJ, Sanda MG, Dunn RL, et al. Measuring and predicting prostate cancer related quality of life changes using EPIC for clinical practice. J Urol. 2014 Mar;191(3):638–645. doi: 10.1016/j.juro.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang P, Szymanski KM, Dunn RL, et al. Expanded prostate cancer index composite for clinical practice: development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J Urol. 2011 Sep;186(3):865–872. doi: 10.1016/j.juro.2011.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheat JC, Hedgepeth RC, He C, Zhang L, Wood DP., Jr Clinical interpretation of the Expanded Prostate Cancer Index Composite-Short Form sexual summary score. J Urol. 2009 Dec;182(6):2844–2849. doi: 10.1016/j.juro.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Hedgepeth RC, Labo J, Zhang L, Wood DP., Jr Expanded Prostate Cancer Index Composite versus Incontinence Symptom Index and Sexual Health Inventory for Men to measure functional outcomes after prostatectomy. J Urol. 2009 Jul;182(1):221–227. doi: 10.1016/j.juro.2009.02.155. discussion 227–228. [DOI] [PubMed] [Google Scholar]
- 20.Jayadevappa R, Malkowicz SB, Wittink M, Wein AJ, Chhatre S. Comparison of distribution- and anchor-based approaches to infer changes in health-related quality of life of prostate cancer survivors. Health Serv Res. 2012 Oct;47(5):1902–1925. doi: 10.1111/j.1475-6773.2012.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012 Apr 18;307(15):1583–1584. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 22.Wu AW, Jensen RE, Salzberg C, Snyder C. Advances in the Use of Patient Reported Outcome Measures in Electronic Health Records Including Case Studies. [Accessed 12/17/2013.];2013 Nov 7; http://www.pcori.org/assets/2013/11/PCORI-PRO-Workshop-EHR-Landscape-Review-111913.pdf.
- 23.Dall’Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008 Apr 15;112(8):1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.