Abstract
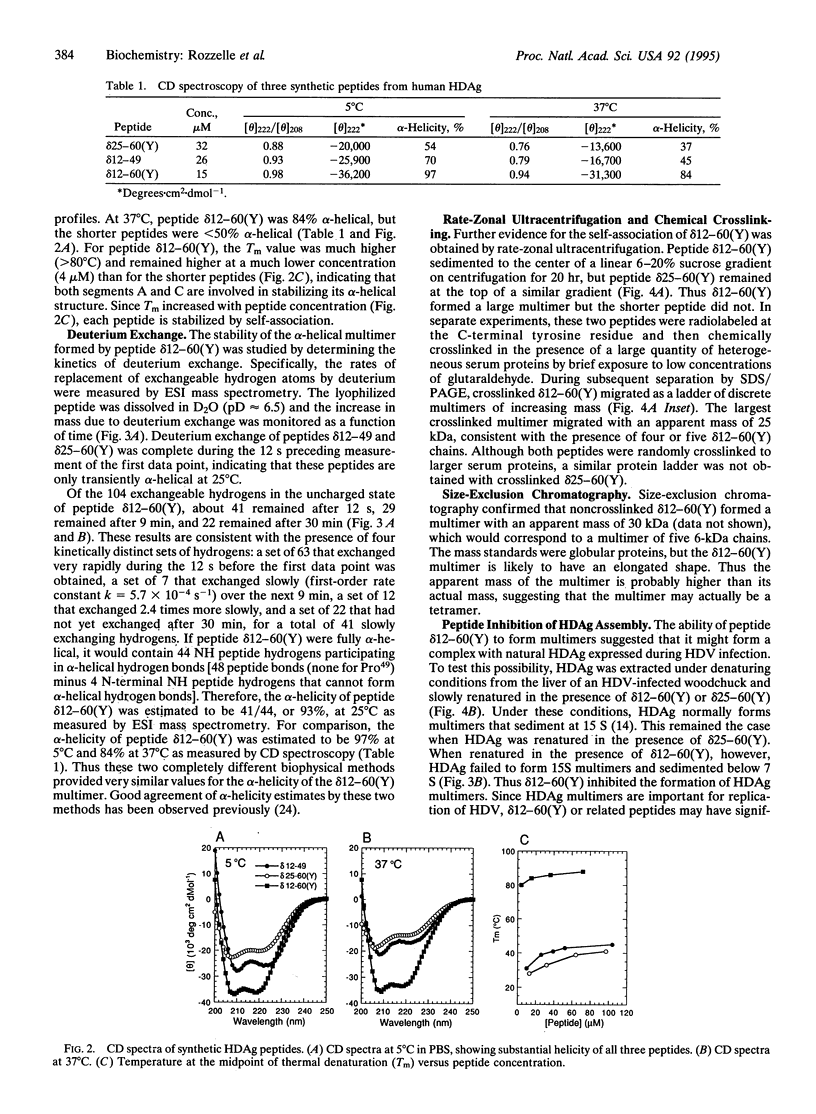
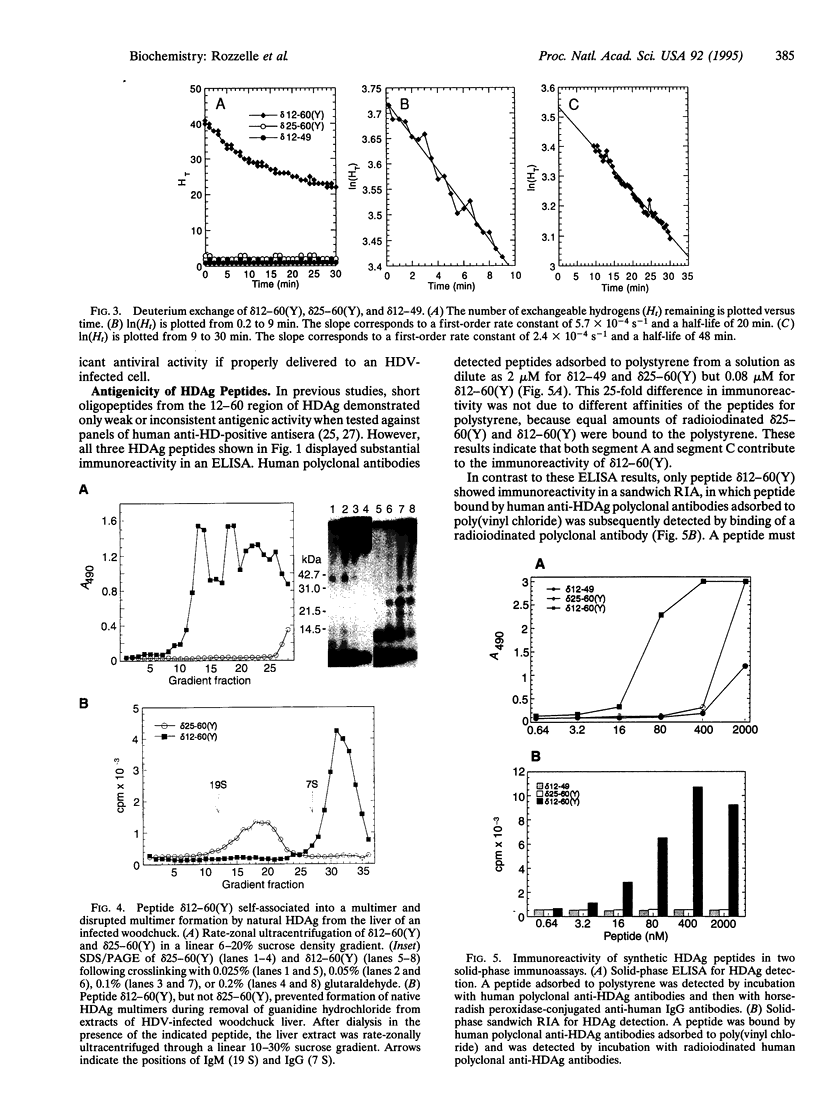
The formation of hepatitis delta antigen (HDAg) multimers is required for full biologic activity of this protein and for replication of the hepatitis delta virus. To determine the residues responsible for multimerization, three peptides [ delta 12-49, delta 25-60(Y), delta 12-60(Y)] from the putative coiled-coil multimer-forming domain of HDAg were chemically synthesized and biophysically characterized by circular dichroic spectroscopy, deuterium-exchange mass spectrometry, gel filtration, chemical crosslinking, and ultracentrifugation. By circular dichroism the 50-residue peptide delta 12-60(Y) was half-denatured above 80 degrees C and was 97% alpha-helical at 5 degrees C and 84% alpha-helical at 37 degrees C. By deuterium exchange, peptide delta 12-60(Y) was 93% alpha-helical at 25 degrees C. Its high alpha-helicity and melting temperature are due to the formation of an alpha-helical multimer consisting of four or more chains. All three synthetic peptides reacted with human anti-HDAg antibodies in an enzyme-linked immunosorbent assay, but only peptide delta 12-60(Y) was detected in a sandwich radioimmunoassay in which successful antigens must display at least two antibody-binding sites, which correlates with the ability of this peptide to form multimers. Peptide delta 12-60(Y) also interfered with the self-association of natural HDAg into multimers. These results have significant practical implications for development of improved diagnostic tests, antiviral agents, and possibly even vaccines for prevention of hepatitis delta virus disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann K. F., Cote P. J., Moriarty A., Gerin J. L. Hepatitis delta antigen. Antigenic structure and humoral immune response. J Immunol. 1989 Dec 1;143(11):3714–3721. [PubMed] [Google Scholar]
- Chang F. L., Chen P. J., Tu S. J., Wang C. J., Chen D. S. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8490–8494. doi: 10.1073/pnas.88.19.8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. F., Baker S. C., Soe L. H., Kamahora T., Keck J. G., Makino S., Govindarajan S., Lai M. M. Human hepatitis delta antigen is a nuclear phosphoprotein with RNA-binding activity. J Virol. 1988 Jul;62(7):2403–2410. doi: 10.1128/jvi.62.7.2403-2410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. F., Sun C. Y., Chen C. J., Chang S. C. Functional motifs of delta antigen essential for RNA binding and replication of hepatitis delta virus. J Virol. 1993 May;67(5):2529–2536. doi: 10.1128/jvi.67.5.2529-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M., Hsieh S. Y., Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol. 1990 Oct;64(10):5066–5069. doi: 10.1128/jvi.64.10.5066-5069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M., Hsieh S. Y., Taylor J. The antigen of hepatitis delta virus: examination of in vitro RNA-binding specificity. J Virol. 1991 Aug;65(8):4057–4062. doi: 10.1128/jvi.65.8.4057-4062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. J., Chang F. L., Wang C. J., Lin C. J., Sung S. Y., Chen D. S. Functional study of hepatitis delta virus large antigen in packaging and replication inhibition: role of the amino-terminal leucine zipper. J Virol. 1992 May;66(5):2853–2859. doi: 10.1128/jvi.66.5.2853-2859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Glenn J. S., White J. M. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol. 1991 May;65(5):2357–2361. doi: 10.1128/jvi.65.5.2357-2361.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel T. M., Williams S. A., DeGrado W. F. Metal ion-dependent modulation of the dynamics of a designed protein. Science. 1993 Aug 13;261(5123):879–885. doi: 10.1126/science.8346440. [DOI] [PubMed] [Google Scholar]
- Harbury P. B., Zhang T., Kim P. S., Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993 Nov 26;262(5138):1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- Hecht M. H., Richardson J. S., Richardson D. C., Ogden R. C. De novo design, expression, and characterization of Felix: a four-helix bundle protein of native-like sequence. Science. 1990 Aug 24;249(4971):884–891. doi: 10.1126/science.2392678. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H. Type D (delta) hepatitis. JAMA. 1989 Mar 3;261(9):1321–1325. [PubMed] [Google Scholar]
- Kuo M. Y., Chao M., Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989 May;63(5):1945–1950. doi: 10.1128/jvi.63.5.1945-1950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Chao Y. C., Chang M. F., Lin J. H., Gust I. Functional studies of hepatitis delta antigen and delta virus RNA. Prog Clin Biol Res. 1991;364:283–292. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lau S. Y., Taneja A. K., Hodges R. S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J Biol Chem. 1984 Nov 10;259(21):13253–13261. [PubMed] [Google Scholar]
- Lee C. Z., Lin J. H., Chao M., McKnight K., Lai M. M. RNA-binding activity of hepatitis delta antigen involves two arginine-rich motifs and is required for hepatitis delta virus RNA replication. J Virol. 1993 Apr;67(4):2221–2227. doi: 10.1128/jvi.67.4.2221-2227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Chang M. F., Baker S. C., Govindarajan S., Lai M. M. Characterization of hepatitis delta antigen: specific binding to hepatitis delta virus RNA. J Virol. 1990 Sep;64(9):4051–4058. doi: 10.1128/jvi.64.9.4051-4058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Rizzetto M. The delta agent. Hepatology. 1983 Sep-Oct;3(5):729–737. doi: 10.1002/hep.1840030518. [DOI] [PubMed] [Google Scholar]
- Taylor J. M. Hepatitis delta virus: cis and trans functions required for replication. Cell. 1990 May 4;61(3):371–373. doi: 10.1016/0092-8674(90)90516-h. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. J Biol Chem. 1982 Jun 10;257(11):6010–6015. doi: 10.2210/pdb1mlt/pdb. [DOI] [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982 Jun 10;257(11):6016–6022. [PubMed] [Google Scholar]
- Wang J. G., Cullen J., Lemon S. M. Immunoblot analysis demonstrates that the large and small forms of hepatitis delta virus antigen have different C-terminal amino acid sequences. J Gen Virol. 1992 Jan;73(Pt 1):183–188. doi: 10.1099/0022-1317-73-1-183. [DOI] [PubMed] [Google Scholar]
- Wang J. G., Jansen R. W., Brown E. A., Lemon S. M. Immunogenic domains of hepatitis delta virus antigen: peptide mapping of epitopes recognized by human and woodchuck antibodies. J Virol. 1990 Mar;64(3):1108–1116. doi: 10.1128/jvi.64.3.1108-1116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. G., Lemon S. M. Hepatitis delta virus antigen forms dimers and multimeric complexes in vivo. J Virol. 1993 Jan;67(1):446–454. doi: 10.1128/jvi.67.1.446-454.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Choo Q. L., Wang K. S., Govindarajan S., Redeker A. G., Gerin J. L., Houghton M. A single antigenomic open reading frame of the hepatitis delta virus encodes the epitope(s) of both hepatitis delta antigen polypeptides p24 delta and p27 delta. J Virol. 1988 Feb;62(2):594–599. doi: 10.1128/jvi.62.2.594-599.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. P., Lai M. M. Oligomerization of hepatitis delta antigen is required for both the trans-activating and trans-dominant inhibitory activities of the delta antigen. J Virol. 1992 Nov;66(11):6641–6648. doi: 10.1128/jvi.66.11.6641-6648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. P., Yeh C. T., Ou J. H., Lai M. M. Characterization of nuclear targeting signal of hepatitis delta antigen: nuclear transport as a protein complex. J Virol. 1992 Feb;66(2):914–921. doi: 10.1128/jvi.66.2.914-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]