Abstract
Transposon mutagenesis of rsbU leads to a biofilm-negative phenotype in Staphylococcus epidermidis. However, the pathway of this regulatory mechanism was unknown. To investigate the role of RsbU in the regulation of the alternative sigma factor σB and biofilm formation, we generated different mutants of the σB operon in S. epidermidis strains 1457 and 8400. The genes rsbU, rsbV, rsbW, and sigB, as well as the regulatory cascade rsbUVW and the entire σB operon, were deleted. Transcriptional analysis of sarA and the σB-dependent gene asp23 revealed the functions of RsbU and RsbV as positive regulators and of RsbW as a negative regulator of σB activity, indicating regulation of σB activity similar to that characterized for Bacillus subtilis and Staphylococcus aureus. Phenotypic characterization of the mutants revealed that the dramatic decrease of biofilm formation in rsbU mutants is mediated via σB, indicating a crucial role for σB in S. epidermidis pathogenesis. However, biofilm formation in mutants defective in σB or its function could be restored in the presence of subinhibitory ethanol concentrations. Transcriptional analysis revealed that icaR is up-regulated in mutants lacking σB function but that icaA transcription is down-regulated in these mutants, indicating a σB-dependent regulatory intermediate negatively regulating IcaR. Supplementation of growth media with ethanol decreased icaR transcription, leading to increased icaA transcription and a biofilm-positive phenotype, indicating that the ethanol-dependent induction of biofilm formation is mediated by IcaR. This icaR-dependent regulation under ethanol induction is mediated in a σB-independent manner, suggesting at least one additional regulatory intermediate in the biofilm formation of S. epidermidis.
Staphylococcus epidermidis, a normal inhabitant of human skin and mucous membranes, is the predominant cause of foreign-body-associated infections (67). In addition, S. epidermidis is being isolated with increasing frequency as the causative pathogen of nosocomial sepsis and other nosocomial infections and now ranks among the five most frequent nosocomial pathogens (67, 72). The pathogenesis of S. epidermidis infections is correlated with the ability to form biofilms on polymeric surfaces (12, 83); cells are more resistant to a variety of antimicrobial substances in such biofilms (39).
Biofilm formation proceeds in two phases (27, 43). Primary attachment of bacterial cells to a polymer surface is a complex process influenced by a variety of factors, including hydrophobic interactions, the presence of host proteins, and specific staphylococcal factors like the capsular polysaccharide adhesin, the autolysin AtlE, and other staphylococcal surface proteins (30, 33, 55, 57, 73, 75). This attachment step is followed by the second phase, leading to the accumulation of bacteria in a multilayered biofilm embedded in an amorphous glycocalyx. Synthesis of the polysaccharide intercellular adhesin (PIA), which mediates cell-to-cell adhesion of the proliferating cells, is essential for S. epidermidis cell accumulation (45-47, 50). PIA is synthesized by the gene products of the icaADBC gene cluster (23, 31). In addition to its function in intercellular adhesion, PIA is essential for hemagglutination of S. epidermidis (19, 48, 66, 69). The significance of PIA as a virulence factor and of biofilm formation as an important process in foreign-body-associated infections was demonstrated in a central venous catheter infection model of the rat and a subcutaneous foreign-body infection model in mice (68, 70, 71). PIA and biofilm expression in S. epidermidis are influenced by a variety of environmental stress conditions (17, 20, 36, 37, 64, 65).
Recently, we demonstrated that the inactivation of rsbU, the first gene of the σB operon in S. epidermidis, dramatically decreased icaADBC transcription, PIA synthesis, and biofilm formation (36, 49). In contrast, Kies et al. (35) demonstrated that the expression of the icaADBC locus in trans from plasmid pCN27 (31) in a sigB mutant in the icaADBC-negative genetic background of S. epidermidis Tü3298 was able to form large cell clusters. These findings lead to the conclusion that the σB-independent biofilm formation of this strain might therefore be explained by a nonfunctional RsbU-mediated regulatory pathway (35). However, the icaR gene, encoding a recently characterized negative regulator of icaADBC transcription (14), is lacking in plasmid pCN27, which could also explain the observed biofilm-positive phenotype in the sigB mutant.
In contrast to S. epidermidis biofilm expression, in which the majority of icaADBC-positive strains display biofilm formation in standard media like Trypticase soy broth (TSB) (11, 22, 83), S. aureus displays a stronger dependence of biofilm formation on distinct growth conditions and requires high sugar concentrations (38). Recently, Valle et al. (74) demonstrated that icaADBC transcription in Staphylococcus aureus is controlled mainly by SarA. Interestingly, the deletion of σB in a sarA deletion mutant was able to partially reverse the effect of the sarA deletion and restored biofilm formation in this S. aureus strain, whereas the deletion of σB in the wild-type strain had no effect on biofilm formation (74). For the mucosal isolate S. aureus MA12, the influence of σB on biofilm formation under high osmolarity was demonstrated (63). However, several other investigated S. aureus strains were biofilm-negative despite the expression of the sigB gene (63), indicating that the influence of σB on the regulation of biofilm formation could be dependent on different genetic S. aureus backgrounds. Additionally, the overexpression of σB from a tetracycline-dependent promoter could induce biofilm formation in S. aureus (2).
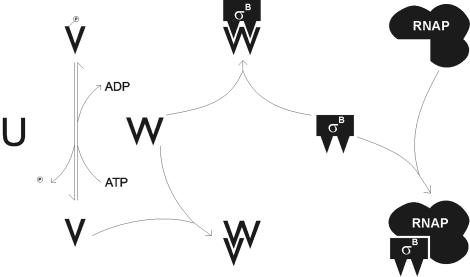
The regulation and function of the alternative sigma factor σB is well characterized for Bacillus subtilis and S. aureus. In B. subtilis, the alternative sigma factor σB is controlled by a complex signal transduction pathway (9, 79). The central module (RsbP, RsbV, and RsbW) is activated by signals of energy stress like carbon, phosphate, or oxygen starvation and directly regulates the activity of σB by forming a σB and RsbW complex (4, 18, 78). An upstream module of σB regulation in B. subtilis (RsbX, RsbR, RsbS, RsbT, and RsbU) is activated by environmental stress such as salt, heat, acid, or ethanol shock (1, 3, 5, 7-9, 76). In S. aureus, a species closely related to S. epidermidis, the σB operon consists of only the four genes rsbU, rsbV, rsbW, and sigB (40, 80), as was observed for S. epidermidis (36). Recently, it was demonstrated that RsbW acts as an anti-sigma factor in S. aureus (52) and that RsbU and RsbV act as positive regulators of σB activity (56), similar to the case for B. subtilis (Fig. 1). In B. subtilis, far more than 100 genes which are in part differentially induced by different stress conditions like heat, oxidative, acid, and ethanol stress, as well as starvation, are characterized within the σB regulon (28, 29, 32, 58-61, 77). In S. aureus, the σB regulon also comprises a wide variety of σB-regulated genes (25), including several virulence factors like clumping factor, fibronectin binding protein A, and coagulase (53, 54), which are positively regulated, as well as alpha- and beta-hemolysin, thermonuclease, enterotoxin B, serine protease SplA, cysteine protease SplB, metalloprotease Aur, staphopain, and leucotoxin D, which are negatively regulated (41, 82). Regulation of virulence factors can be mediated either directly by σB-dependent promoters or indirectly by the influence of σB on additional global regulators like SarA and the agr system (6).
FIG. 1.
Model of the regulatory pathway for the activity of the alternative sigma factor σB of S. aureus, which is homologous to the core regulatory pathway of B. subtilis. In these species, σB is negatively regulated by the anti-sigma factor RsbW, which additionally acts as a specific kinase for the anti-anti-sigma factor RsbV. RsbV activity depends on phosphorylation status, and inactive phosphorylated RsbV could be activated by dephosphorylation by the specific phosphatase RsbU. Transcriptional analysis of the deletion mutants generated in S. epidermidis in this study suggests that regulation of σB activity in this species is homologous to that in S. aureus and B. subtilis.
When these data are taken together, several questions arise with respect to the impact of RsbU on the regulation of the alternative sigma factor σB and the regulation of biofilm formation by RsbU or σB in S. epidermidis. To answer these questions in this study, we deleted each single gene of the σB operon as well as the regulatory cascade rsbUVW and the entire σB operon and investigated the impact of these mutations on biofilm formation and transcription of icaADBC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. S. epidermidis cells were grown in TSB (Becton Dickinson, Cockeysville, Md.) or on Trypticase soy agar (TSB plus 1.5% agar; Becton Dickinson) at 37°C. For phenotypic characterization of the S. epidermidis strains, TSB was supplemented with 4% NaCl or 2, 3, or 4% ethanol. Escherichia coli cells were grown in Luria-Bertani broth or on Luria-Bertani agar at 37°C. Antibiotics were used at the following concentrations: erythromycin, 100 μg/ml; chloramphenicol, 10 μg/ml; ampicillin, 100 μg/ml; and kanamycin, 50 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Reference | Commentsa |
|---|---|---|
| S. epidermidis | ||
| 1457 | 50 | Isolate from infected central venous catheter |
| M15 | 36 | rsbU-Tn917 mutant of S. epidermidis 1457 used as recipient by electroporation |
| 1457rsbU | This study | rsbU::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 1457rsbV | This study | rsbV::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 1457rsbW | This study | rsbW::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 1457sigB | This study | sigB::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 1457rsbUVW | This study | rsbUVW::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 1457rsbUVWsigB | This study | rsbUVWsigB::erm derivate from S. epidermidis 1457 derived by allelic gene replacement |
| 8400 | 50 | Blood culture isolate |
| 8400-M15 | 36 | rsbU-Tn917 mutant of S. epidermidis 8400 derived by phage transduction |
| 8400rsbU | This study | rsbU::erm derivate from S. epidermidis 8400 derived by phage transduction |
| 8400rsbV | This study | rsbV::erm derivate from S. epidermidis 8400 derived by phage transduction |
| 8400rsbW | This study | rsbW::erm derivate from S. epidermidis 8400 derived by phage transduction |
| 8400sigB | This study | sigB::erm derivate from S. epidermidis 8400 derived by phage transduction |
| 8400rsbUVW | This study | rsbUVW::erm derivate from S. epidermidis 8400 derived by phage transduction |
| 8400rsbUVWsigB | This study | rsbUVWsigB::erm derivate from S. epidermidis 8400 derived by phage transduction |
| E. coli | ||
| TOP 10 | Invitrogen | |
| SJrsbU | This study | TOP 10 containing pSJrsbU |
| SJrsbV | This study | TOP 10 containing pSJrsbV |
| SJrsbW | This study | TOP 10 containing pSJrsbW |
| SJsigB | This study | TOP 10 containing pSJsigB |
| SJrsbUVW | This study | TOP 10 containing pSJrsbUVW |
| SJrsbUVWsigB | This study | TOP 10 containing pSJrsbUVWsigB |
| Plasmids | ||
| pCRII TOPO | Invitrogen | E. coli cloning vector for direct cloning of PCR fragments |
| pBT2 | 10 | ts E. coli/Staphylococcus shuttle vector |
| pSJrsbU | This study | ts vector for allelic gene replacement of rsbU in S. epidermidis |
| pSJrsbV | This study | ts vector for allelic gene replacement of rsbV in S. epidermidis |
| pSJrsbW | This study | ts vector for allelic gene replacement of rsbW in S. epidermidis |
| pSJsigB | This study | ts vector for allelic gene replacement of sigB in S. epidermidis |
| pSJrsbUVW | This study | ts vector for allelic gene replacement of rsbUVW in S. epidermidis |
| pSJrsbUVWsigB | This study | ts vector for allelic gene replacement of rsbUVWsigB in S. epidermidis |
ts, temperature sensitive.
Phenotypic characterization.
Biofilm formation of S. epidermidis was measured by a semiquantitative adherence assay with the indicated media in 96-well tissue culture plates with a NunclonΔ surface (Nunc, Roskilde, Denmark) as described previously (13, 44). For the detection of PIA by immunofluorescence assay, S. epidermidis cells were grown in tissue culture dishes (Nunc) for 22 h in the media. Cells were scraped off and diluted in phosphate-buffered saline (PBS) to an optical density at 578 nm of 0.3 to 0.5. The immunofluorescence assay procedure was then performed as described previously with a rabbit antiserum raised against purified PIA (44). For the detection of growth rates, cells were cultured in tissue culture dishes or in 100-ml glass bottles in a horizontal shaker at 200 rpm. Cells were harvested after 2, 4, 6, 8, 10, and 24 h, sonicated for 10 s (3/16-in. tapered Microtip at 50% maximal amplitude) with a Digital Sonifier 250-D (Branson, Danbury, Conn.) to disrupt cell clusters. The optical density of the culture at 600 nm was detected with a DU 530 spectrophotometer (Beckman, Krefeld, Germany). For the assessment of the cell density of stationary-phase cultures, the sonicated cultures were serially diluted and plated on Trypticase soy agar.
Genetic methods.
Chromosomal DNA of S. epidermidis was prepared as described previously (44). Plasmid DNA of E. coli was prepared by using a QIAprep Spin Miniprep kit (QIAGEN, Hilden, Germany). DNA was cleaved with restriction enzymes as suggested by the manufacturer (Pharmacia, Freiburg, Germany), and DNA fragments were separated by electrophoresis in 0.7% agarose gels in Tris-borate buffer. DNA restriction fragments of the expected size were purified from agarose gels with a QIAquick gel extraction kit (QIAGEN). Amplification of DNA fragments was performed by using a DyNazyme DNA polymerase kit (Finnzyme, Espoo, Finland) as described by the manufacturer.
For amplification of fragments used for the allelic gene replacement procedure, the Expand High Fidelity PCR system (Roche, Mannheim, Germany) was used with oligonucleotides shown in Table 2. Amplified fragments were ligated into the pCRII TOPO vector (Invitrogen, Karlsruhe, Germany) and cloned by electroporation into E. coli TOP 10. The fragments flanking the erythromycin resistance cassette (erm) were ligated at their respective positions in the pCRII background, and the entire fragment was subsequently ligated into the temperature-sensitive, chloramphenicol-resistant E. coli/Staphylococcus shuttle vector pBT2 (10). The resulting plasmids were cloned into the restriction-deficient staphylococcal genetic background of S. aureus RN4220 by electroporation. Plasmids isolated from this staphylococcal host could be transformed by electroporation into mutant M15 and were subsequently transduced into the recipient strain, S. epidermidis 1457, by phage transduction with phage 71, kindly provided by V. T. Rosdahl, Statens Seruminstitut Copenhagen, Copenhagen, Denmark, as described previously (44). The cells were grown at nonpermissive temperatures, and clones were screened for double crossover with an erythromycin-resistant but chloramphenicol-sensitive phenotype. The correct chromosomal insertion of the cassette into the respective mutants was demonstrated by PCR with erm-specific primers paired with primers flanking the genetically manipulated site and subsequent sequencing of the resulting fragments (data not shown). Transduction of the respective mutations into the independent biofilm-producing wild-type strain 8400 was performed essentially as described above with S. epidermidis phage 71.
TABLE 2.
PCR primers
| Primer | Restriction site | Sequencea |
|---|---|---|
| Primers used for allelic gene replacement | ||
| ermR2 | SacI | 5′-CTC GAG CTC TGA CGG TGA CAT CTC TCT ATT G-3′ |
| ermL1 | NheI | 5′-CTC GCT AGC GAA AAG TAC CAT AAA CGG TCG-3′ |
| vrsbUR1 | BamHI | 5′-CTC GGA TCC AGC GAA AAT ACC AAC CCA CG-3′ |
| vrsbUL1 | SacI | 5′-CTC GAG CTC GAA ATG CGC CTC CTT ACT TC-3′ |
| hrsbUR1 | NheI | 5′-CTC GCT AGC GAT GGT GTT ACA GAG GCA CG-3′ |
| hrsbUL1 | KpnI | 5′-CTC GGT ACC AGC TGG CAA CCG CAT TTC-3′ |
| hrsbWR1 | NheI | 5′-CTC GCT AGC GTT ATT TCA GAC CAA GGT G-3′ |
| hrsbWL1 | KpnI | 5′-CTC GGT ACC TTA TCA TTC TGT TGT CCC AT-3′ |
| vsigBR1 | BamHI | 5′-CTC GGA TCC CCA ATG AGA CAA GAA GGC AC-3′ |
| vsigBL1 | SacI | 5′-CTC GAG CTC CTT GAG CTT GGC TAT CTT CG-3′ |
| hsigBR1 | NheI | 5′-CTC GCT AGC CGA AAG AAG CTC AGG TGG AC-3′ |
| hsigBL1 | KpnI | 5′-CTC GGT ACC GAT GCT GAA TAA ACT GAT GCG-3′ |
| Primers used for Northern blot hybridization probes | ||
| erm.for | None | 5′-AAT TGG AAC AGG TAA AGG GC-3′ |
| erm.rev | None | 5′-AAC ATC TTG GGT ATG GCG G-3′ |
| sigB.for | None | 5′-AGA TTT AGT TCA AGT TGG TA-3′ |
| sigB.rev | None | 5′-TTA TCA TCT TGT TGT CCC AT-3′ |
| rsbU.for | None | 5′-GAA GTG GAA GTA AGG AGG CG-3′ |
| rsbU.rev | None | 5′-TCG ATG TGT TAC CAG AAG TCG-3′ |
| sarA.for | None | 5′-ATA GGG AGG TTT CAT TAA TGG C-3′ |
| sarA.rev | None | 5′-TTT GCT TCT GTG ATA CGG TTG-3′ |
| asp23.for | None | 5′-AAA ATC AAA AAG CAC TTG AGC G-3′ |
| asp23.rev | None | 5′-AAA AAA TTG CAG GTAT TGC AG-3′ |
| icaA.for | None | 5′-GAA TCC AAA ATT AGG CGC AG-3′ |
| icaA.rev | None | 5′-AAC ATC CAG CAT AGA GCA CG-3′ |
| icaR.for | None | 5′-TCC GAA AAG GGG TAC GAT G-3′ |
| icaR.rev | None | 5′-CCT CTT TAT CCA AAG CGA TG-3′ |
| Primers used for quantitative RT-PCR | ||
| asp23.real1 | None | 5′-TCC AAC TTC TAC AGA TAC GCC-3′ |
| asp23.real2 | None | 5′-AAA ATT GCA GGT ATT GCA GC-3′ |
| icaA.real1 | None | 5′-TGT ATC AAG CGA AGT CAA TCT C-3′ |
| icaA.real2 | None | 5′-GGC ACT AAC ATC CAG CAT AG-3′ |
| icaR.real1 | None | 5′-TGA AGA TGG TGT TTG ATT TGT G-3′ |
| icaR.real2 | None | 5′-CCA TTG ACG GAC TTT ACC AG-3′ |
| rsbU.real1 | None | 5′-AGC GTT TGA GGA AAT TGG TGT G-3′ |
| rsbU.real2 | None | 5′-CCT CTA CAT CTC GTG CCT CTG-3′ |
| gyrB.real1 | None | 5′-CTG ACA ATG GCC GTG GTA TTC-3′ |
| gyrB.real2 | None | 5′-GAA GAT CCA ACA CCG TGA AGA C-3′ |
Restriction sites are underlined.
Nucleotide sequence analysis was performed with an ABI Prism 310 sequencer by capillary electrophoresis with an ABI PrismdGTP BigDye Terminator Ready Reaction kit (PE Applied Biosystems, Foster City, Calif.). Nucleotide sequences were subsequently analyzed with HUSAR software (DKFZ, Heidelberg, Germany) or Vector NTI suite II software (InforMax, Frederick, Md.).
RNA preparation.
For RNA extraction, cells were cultivated under biofilm conditions in 9.5-cm-diameter cell culture dishes with a NunclonΔ surface (Nunc). RNA was prepared with an RNeasy bacteria kit (QIAGEN) by using a modified protocol. Cells were harvested on ice with a cell scraper and by centrifugation for 5 min at 4°C. The resulting pellets were overlaid with 2 ml of sterile PBS, and cells were resuspended by sonication (10 s with a 3/16-in. tapered Microtip at 70% maximal amplitude; Branson sonifier). A 1.5-ml portion of the cell suspension was mixed with 3 ml of RNA protect solution (QIAGEN) and incubated for 5 min at ambient temperature. Cells were harvested by centrifugation for 10 min at ambient temperature. To remove PIA, cells were resuspended in 10 ml of sterile PBS and treated twice by sonication (3 cycles of 30 s with a 3/16-in. tapered Microtip at 70% maximal amplitude) with a PBS wash step between treatments. After sonication, cells were harvested by centrifugation for 5 min at ambient temperature and the resulting cell pellets were resuspended in 180 μl of Tris-EDTA buffer. Twenty microliters of a lystostaphin solution (1,500 U/ml; Sigma, Deisenhofen, Germany) was added, and the reaction mixture was incubated at 37°C for 10 min. The subsequent extraction of RNA was performed according to the instructions of the manufacturer. Extracted RNA was quantified with a GeneQuant photometer (Pharmacia).
Northern blot analysis.
For electrophoresis, 5 μg of total RNA was resuspended in RNA loading buffer and denatured at 70°C for 10 min. The RNA was separated on 1% agarose-formaldehyde gels and blotted on Zeta Probe nylon membranes (Bio-Rad, Munich, Germany) by using capillary transfer with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). After 20 h, the RNA was fixed by baking at 80°C for 2 h. After prehybridization at 52°C for 30 min in DIG easy Hyb (Roche), the membranes were incubated at 52°C overnight with specific digoxigenin (DIG)-labeled probes, generated by PCR labeling (PCR DIG probe synthesis kit; Roche) with primers shown in Table 2. Membranes were washed at 52°C twice in 2× SSC-0.1% sodium dodecyl sulfate (SDS), twice in 0.1× SSC-0.1% SDS, and finally in washing buffer (Roche). The membranes were blocked in blocking solution, incubated with anti-digoxigenin-AP Fab fragments, and equilibrated in detection buffer as directed by the manufacturer (Roche). CDP-Star was added as a substrate for the chemiluminescence detection on Lumi Film (Roche). By boiling in 2× SSC-0.1% SDS twice for 6 min, the membranes could be stripped and reprobed up to three times. All transcriptional analyses were performed at least three times with independent RNA preparations.
Real-time RT-PCR analysis.
For the reverse transcription (RT)-PCR analysis, 4 μg of RNA was treated with 4 U of RNase-free DNase (Promega, Mannheim, Germany) in a 20-μl total volume for 45 min at 37°C as suggested by the manufacturer. The reaction was diluted 1:10, and 5 μl of DNase-treated RNA solution was used for first-strand cDNA synthesis with the iScript cDNA synthesis kit (Bio-Rad) in a total volume of 20 μl. The cDNA reaction was diluted 1:4, and 3 μl was used as a template in real-time PCR analysis with an iQ SYBR Green Supermix (Bio-Rad) with oligonucleotides shown in Table 2 in an iCycler iQ thermal cycler under following conditions: (i) 95°C for 3 min; (ii) 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and (iii) 4°C for 30 min. All RT-PCR analyses were performed in triplicate for at least two independent experiments. Relative transcriptional levels within distinct experiments were determined by using the 2−ΔΔCT method (42).
RESULTS
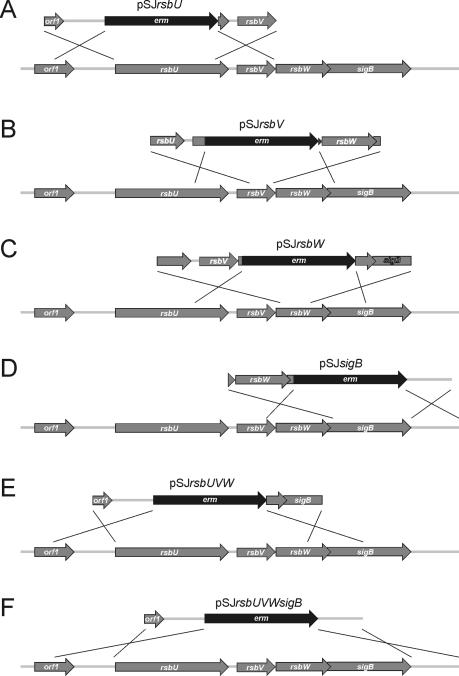
Construction of deletion mutants.
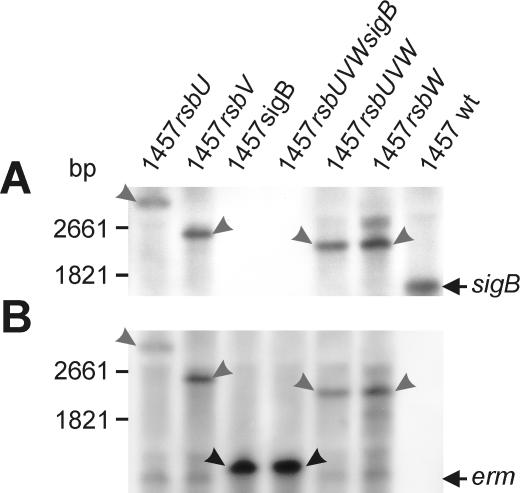
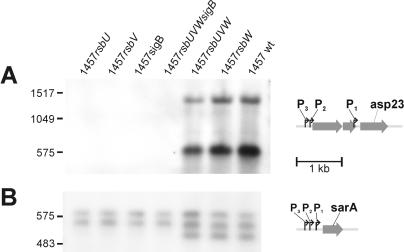
To investigate the relevance of the genes preceding sigB in the σB operon in S. epidermidis to the activity of the alternative sigma factor σB, we constructed mutants with deletions of the single genes rsbU, rsbV, rsbW, and sigB, as well as the regulatory cascade rsbUVW and the entire σB operon, by homologous recombination leading to mutants 1457rsbU, 1457rsbV, 1457rsbW, 1457sigB, 1457rsbUVW, and 1457rsbUVWsigB (Fig. 2). For all mutants, the erythromycin resistance cassette (erm) was inserted in a positive orientation with respect to the σB operon to allow transcription of downstream genes and to avoid gene silencing by induction of antisense RNA transcription. For all mutants, the correct insertion without induction of additional mutations was confirmed by PCR analysis and subsequent sequencing of the chromosomal regions overlapping the manipulated chromosomal regions of the σB operon (data not shown). By transcriptional analysis with a sigB-specific probe (Fig. 3A), an ∼1.7-kb transcript presumably enclosing the internal σB-dependent genes rsbV, rsbW, and sigB could be detected for wild-type S. epidermidis 1457. The largest amount of sigB transcript was observed in the mid-exponential growth phase (7 h under biofilm growth conditions) (data not shown), and all subsequent transcriptional analyses were performed with RNA extracted at this time point. The lack of detection by Northern analysis of the predicted 2.8-kb transcript from the putative σA-dependent promoter preceding rsbU revealed that this promoter has only low activity. However, low transcriptional activity of rsbU was detectable by RT-PCR with RNA prepared from wild-type S. epidermidis 1457 and mutants 1457rsbV, 1457rsbW, and 1457sigB, in contrast to mutant 1457rsbU (data not shown). For mutants 1457sigB and 1457rsbUVWsigB, no sigB-containing transcript was observed, whereas for mutants 1457rsbU, 1457rsbV, 1457rsbW, and 1457rsbUVW, transcripts of 3.45, 2.60, 2.30, and 2.30 kb, respectively, were detected (Fig. 3A). Transcriptional analysis with an erm-specific probe revealed fragments for mutants 1457rsbU, 1457rsbV, and 1457rsbUVW that were identical to the fragments observed with the sigB probe. Mutants 1457sigB and 1457rsbUVWsigB displayed identical fragments of approximately 1.4 kb, whereas for wild-type S. epidermidis 1457 no transcript could be detected. For all mutants, an additional weak transcript of approximately 1.3 kb, comprising only the erm gene, was observed, indicating a weak terminator downstream of the erm gene which is, however, not sufficient to prevent transcription of downstream genes (16). These data revealed the correct insertion of the erm cassette with the expected transcriptional behavior for all mutants. These mutants were further analyzed and were used as hosts for the transduction of the mutations into different genetic backgrounds.
FIG. 2.
Physical map of the sigB operon of S. epidermidis (accession no. AF274004) and construction of deletion mutants. Arrows depict open reading frames and indicate their orientations and sizes. All deleted genes were replaced with the erythromycin resistance gene (erm) as indicated. The erm gene and chromosomal regions flanking the respective deletions were amplified by PCR and cloned into plasmid pBT2, yielding the integration vectors pSJrsbU (A), pSJrsbV (B), pSJrsbW (C), pSJsigB (D), pSJrsbUVW (E), and pSJrsbUVWsigB (F). The crosses indicate the sites of homologous recombination.
FIG. 3.
Influence of deletions on transcription of the sigB gene in S. epidermidis 1457 and its mutants. (A) Northern blot analysis with a sigB-specific probe. S. epidermidis 1457 displayed a 1.5-kb transcript. The σA-dependent transcript of the entire σB operon could not be detected under the conditions used. Mutants with a remaining sigB gene displayed transcripts of increased sizes corresponding to the erm insertion. In sigB-negative mutants, no transcripts were detected. (B) Northern blot analysis with an erm-specific probe. For all mutants, a weak transcript comprising only the erm gene was detected, indicating a weak terminator following the erm gene. In mutants with a remaining sigB gene, additional transcripts with sizes identical to those of transcripts for the sigB-specific hybridization (A) were observed. In mutants with an inactivated sigB gene, the major transcripts detected were only slightly larger than the erm transcript, indicating a strong terminator preceding the sigB gene closely downstream. The genetic maps for the mutants are shown in Fig. 2. wt, wild type.
Regulation of σB in S. epidermidis.
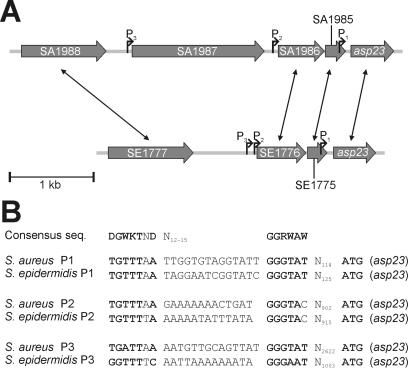
The relevance of the genes rsbU, rsbV, and rsbW to the regulation of the activity of the alternative sigma factor σB was investigated by transcriptional analysis of σB-dependent genes in S. epidermidis 1457 and their respective mutants. Besides the internal σB-dependent promoter of the σB operon (36), the only putative σB-dependent promoter in S. epidermidis published to date is the first of three promoters (P1) of the sarA gene locus (21). As an additional control, we decided to use the asp23 homologue of S. epidermidis ATCC 12228 (81). In S. aureus, the Asp23 protein was identified as a 23-kDa protein, and this protein was clearly missing in σB deletion mutants of S. aureus COL and Newman (41). Further studies revealed that asp23 is transcribed by three independent σB-dependent promoters and that this gene is a good marker for σB activity in S. aureus (24, 26, 41, 52). For the upstream 5,000-bp chromosomal region of the asp23 homologue of S. epidermidis, a consensus sequence search for σB-dependent promoters was performed and three putative σB-dependent promoters (P1 to P3) could be detected (Fig. 4). The promoters P1 and P2 are conserved between S. aureus and S. epidermidis with respect to the −35 and −10 sequences, as well as their approximate distance to asp23, indicating similar functions in both species. In contrast, the putative promoter P3 in S. epidermidis was not conserved compared to that in S. aureus. Interestingly, the SA1987 gene, which is preceded by the P3 promoter in S. aureus, is missing in this chromosomal region of S. epidermidis, whereas the genes flanking SA1987 in S. aureus are conserved in S. epidermidis (Fig. 4).
FIG. 4.
Homology of the asp23 promoter regions of S. aureus and S. epidermidis. (A) Physical maps of the asp23 promoter regions of S. aureus N315 (accession no. AP003136) and S. epidermidis ATCC 12228 (accession no. AE016750) are displayed. Homologous genes are indicated by double-headed arrows. In S. epidermidis, no open reading frame homologous to the S. aureus SA1987 gene could be detected in the asp23 promoter region. SA1987 is a homologue to the S. aureus opuD gene. In S. epidermidis, only one opuD homologue (SE0259) could be detected in a distinct chromosomal region (accession no. AE016744). (B) Alignment of σB promoters within the asp23 promoter regions of S. aureus and S. epidermidis with the consensus sequence of σB-dependent promoters in B. subtilis (59). Bases fitting the consensus sequence are displayed in boldface type. The P1 and P3 promoters of both species represent perfect matches, whereas for the respective P2 promoters one identical mismatch with respect to the consensus sequence was observed.
No σB-dependent transcript could be observed by Northern blot analysis with asp23- and sarA-specific probes in mutants 1457rsbV, 1457sigB, and 1457rsbUVWsigB in the mid-exponential growth phase, whereas the σA-dependent sarC (P2) and sarB (P3) designated transcripts of the sarA gene (21) were present (Fig. 5). For wild-type S. epidermidis 1457 and mutants 1457rsbW and 1457rsbUVW, the P1-dependent sarA transcript (designated sarA) and two transcripts of asp23 could be detected. The transcripts observed with the asp23-specific probe were approximately 1.5 and 0.6 kb in size, corresponding to the expected transcript sizes for the putative P1 and P2 promoters. However, the 1.5-kb transcript could represent a double band of transcripts dependent on the closely located putative P2 and P3 promoters in S. epidermidis (Fig. 3). Quantitative analysis of transcription by RT-PCR revealed an at-least-1,000-fold down-regulation of asp23 transcription in σB-negative mutants compared to the wild type (data not shown). By overexposure of the film under some conditions, very weak asp23- and σB-dependent sarA transcripts could be detected in 1457rsbU, indicating very low σB activity remaining in this mutant (data not shown). Transcriptional analysis of mutant 1457rsbW compared to wild-type S. epidermidis 1457 at various time points (4, 7, 10, 14, 17, 20, and 24 h) revealed that the asp23 transcripts and the σB-dependent sarA transcript in 1457rsbW were transcribed continuously, whereas in the wild type these genes were up-regulated at the early time points (4 to 14 h) and down-regulated at the late time points (17 to 24 h). The transcriptional levels of these genes in 1457rsbW were lower than those in the wild type in the mid-exponential growth phase but higher than those of the wild type in the stationary phase (data not shown), indicating a lack of growth phase-dependent regulation of σB activity and functional σB overexpression during the post-exponential phase and the stationary phase in mutants with inactivation of rsbW.
FIG. 5.
Influence of deletions on transcription of σB-dependent genes asp23 and sarA. Shown are Northern blot analyses with asp23-specific (A) and sarA-specific (B) probes, as well as maps of the respective genes with published or putative promoter sites. In mutants defective in sigB or its function, a lack of σB-dependent transcripts of asp23 and sarA transcripts was observed. wt, wild type.
Biofilm formation in deletion mutants of σB operon genes.
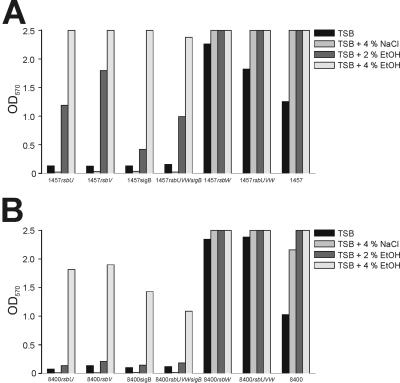
The impact of the different regulators of σB and the influence of σB activity on biofilm formation in S. epidermidis were investigated under different environmental conditions. In S. aureus, the influence of σB on biofilm formation in different genetic backgrounds seems to be extremely variable (63, 74). Therefore, we transduced all mutations generated into the independent icaADBC-positive genetic background of the clinical S. epidermidis isolate 8400. Biofilm assays were performed with TSB and with TSB supplemented with 4% NaCl, 2% ethanol, or 4% ethanol (Fig. 6). Mutants 1457rsbU and 8400rsbU displayed a phenotype similar to that observed for Tn917 mutants M15 and 8400-M15 with the Tn917 insertion site at position 19 of rsbU (36). In these mutants, biofilm formation was dramatically decreased or abolished in TSB and in TSB supplemented with NaCl, whereas supplementation of TSB with ethanol was able to restore biofilm formation in these mutants. Phenotypes similar to those detected in rsbU mutants were observed in mutants 1457rsbV, 1457sigB, 1457rsbUVWsigB, 8400rsbV, 8400sigB, and 8400rsbUVWsigB (Fig. 6). To exclude the possibility that a delay of growth in dysfunctional σB mutants could be responsible for the observed phenotypes, we investigated the growth rates of these strains. Over the time in question, all strains displayed almost identical growth curves in all phases of growth and reached very similar cell densities in the stationary phase (data not shown).
FIG. 6.
Biofilm formation of S. epidermidis 1457 (A) and S. epidermidis 8400 (B) as well as their respective deletion mutants in TSB and in TSB supplemented with 4% NaCl, 2% ethanol (EtOH), or 4% EtOH under different environmental conditions. OD570, optical density at 570 nm.
In contrast to the former mutants, 1457rsbW, 1457rsbUVW, 8400rsbW, and 8400rsbUVW displayed increased biofilm formation compared to their respective wild-type strains (Fig. 6). Biofilm formation in these mutants could be induced by NaCl and ethanol, as was observed for the wild-type strains. However, this increase in biofilm formation could not be quantified because the respective optical density values were outside the detection range of the spectrophotometer used (Fig. 6). The lack of PIA synthesis in the biofilm-negative mutants and the presence of PIA under conditions in which biofilm was expressed was demonstrated by an immunofluorescence assay with PIA-specific antibodies (data not shown).
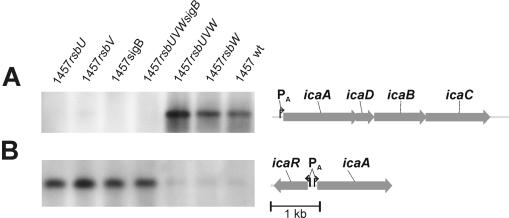
Transcriptional analysis of icaR and icaA under biofilm conditions.
RNA was isolated from S. epidermidis 1457 and its respective mutants in the mid-exponential growth phase under biofilm-forming conditions in TSB. Transcriptional analysis revealed that for mutants 1457rsbV, 1457sigB, and 1457rsbUVWsigB no icaA transcript could be observed (Fig. 7A) but that for mutant 1457rsbU a faint transcript could be observed in some of several experiments (not in the experiment displayed). In mutants 1457rsbW and 1457rsbUVW, transcription of icaA was similar to that observed for wild-type S. epidermidis 1457 (Fig. 7A). In contrast to the transcriptional icaA profiles of the different mutants, icaR encoding a negative regulator of icaADBC transcription was up-regulated in mutants 1457rsbU, 1457rsbV, 1457sigB, and 1457rsbUVWsigB. In mutants 1457rsbW and 1457rsbUVW, as well as the wild type, icaR transcription was repressed (Fig. 7B). Quantitative analysis of transcription by RT-PCR revealed approximately 25- to 30-fold up- or down-regulation of icaR and icaA transcription in σB-negative mutants compared to that in the wild type under these conditions (data not shown).
FIG. 7.
Influence of deletions on transcription of icaR and icaADBC. Shown are Northern blot analyses with icaA-specific (A) and icaR-specific (B) probes, as well as maps of the respective genes with published or putative promoter sites. In mutants defective in sigB or its function, icaADBC transcription was strongly repressed, whereas icaR, encoding a negative regulator (14), was up-regulated. wt, wild type.
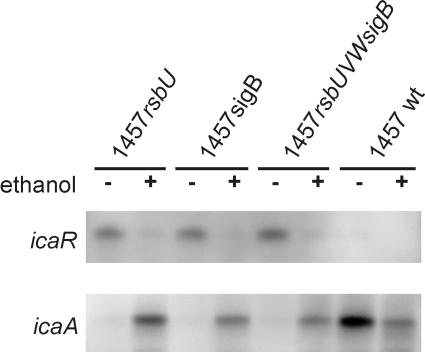
Transcriptional analysis with ethanol stimulation for σB-inactive mutants.
To evaluate the regulatory influence of ethanol induction on biofilm formation in mutants with a deletion of sigB, rsbU, or the complete σB operon, we performed transcriptional analysis of the icaR, icaA, and asp23 genes under biofilm formation conditions. RNA was isolated from S. epidermidis 1457 and its respective mutants in the mid-exponential growth phase under biofilm-forming conditions in TSB and in TSB supplemented with an optimal stimulating concentration of 3% ethanol. In the mutants defective in sigB or its function, 1457rsbU, 1457sigB, and 1457rsbUVWsigB, supplementation of the growth medium with ethanol led to the repression of icaR transcription and a subsequent up-regulation of icaA transcription (Fig. 8). Despite the increased biofilm formation in wild-type S. epidermidis 1457, the icaR and icaA transcripts were slightly repressed by ethanol supplementation. In all investigated mutants with a lack of sigB function, no asp23 transcript could be detected under these conditions (data not shown).
FIG. 8.
Influence of ethanol on the transcription of icaR and icaADBC. Shown are Northern blot analyses with icaR- and icaA-specific probes. RNA was extracted in the mid-exponential growth phase in TSB supplemented with 3% ethanol. In mutants defective in sigB or its function, up-regulated icaR mRNA was repressed by the addition of ethanol, leading to increased transcription of icaADBC. wt, wild type.
DISCUSSION
Biofilm formation of S. epidermidis is regarded as the major virulence factor in this staphylococcal species and is therefore the subject of intense research. The biphasic mode of biofilm formation with a primary attachment step followed by the accumulation of cells as a multilayered biofilm is well characterized (27, 43). However, the regulation of the expression of PIA is poorly understood. Some contradictory results regarding the impact of the alternative sigma factor σB or its regulator RsbU in the regulation of PIA synthesis and biofilm formation in S. epidermidis exist. These data were based on phenotypic characterization of S. epidermidis biofilm formation under different environmental conditions (62), the biofilm-negative phenotype of an rsbU transposon mutant in an icaADBC-positive S. epidermidis strain (36), and the biofilm-positive phenotype of a sigB mutant in an icaADBC-negative genetic background after complementation in trans with icaADBC (35). In the present study, we evaluated the role of the alternative sigma factor σB and its regulators in the biofilm formation of S. epidermidis by using allelic gene replacements.
We were able to delete all single genes of the σB operon as well as the regulatory cascade rsbUVW and the entire operon. The correct insertion of the erm cassette used was determined by sequencing the σB operons of the generated mutants. Transcriptional analysis with sigB- and erm-specific probes revealed that genes downstream of the introduced resistance cassette were transcribed from the σA-dependent erm promoter, which displays activity stronger than that of the putative σA-dependent promoter upstream of the σB operon. The double bands with fragments of approximately 1.3 and 1.4 kb observed in transcriptional analyses of mutants 1457sigB and 1457rsbUVWsigB (Fig. 3B) suggest the existence of a strong transcription terminator approximately 150 to 200 bp downstream of the stop codon of the sigB gene.
To evaluate the activity of the alternative sigma factor σB, we used the σB-dependent transcript of the sarA locus of S. epidermidis (21), which is a good marker when Northern blot analysis after separation of RNA by gel electrophoresis is used as a detection method. However, due to the two additional σA-dependent promoters preceding sarA, this gene locus is not useful for alternative detection methods like Northern analysis of slot blots or RT-PCR. Therefore, we performed a promoter search with the consensus sequence of σB-dependent promoters upstream of the asp23 gene of S. epidermidis. This gene is a homologue to the alkaline shock protein 23 of S. aureus, which is solely transcribed from three σB-dependent promoters and was used in several studies as a marker gene for σB activity in S. aureus (24, 26, 41, 52, 81). Three putative σB-dependent promoters could be detected upstream of asp23 in S. epidermidis. The P1 and P2 promoters displayed conserved −10 and −35 regions in both species and were located at similar distances upstream of the start codon of the respective asp23 genes. Even the mismatch of the last base of the −10 region of promoter P2 was found to be conserved in S. epidermidis and S. aureus when compared to the consensus sequence. These data indicate similar σB-dependent transcription of asp23 from promoters P1 and P2 in both species. Interestingly, the P3 promoters were not conserved in their −10 and −35 region sequences. Additionally, in S. aureus, the P3 promoter precedes an open reading frame (SA1987) homologous to the S. aureus gene opuD, which could not be detected in this chromosomal region of S. epidermidis.
The lack of the σB-dependent transcript designated sarA (21) and the asp23 transcripts in mutant 1457sigB compared to the presence of these transcripts in the wild-type S. epidermidis 1457 confirmed the expected σB-dependent transcription of these genes, indicating the usefulness of both genes as indicators of σB activity. Additionally, the σA-dependent transcripts of the sarA locus designated sarB and sarC (21) could act as a control for comparability between different RNA preparations within Northern hybridization experiments. The more-than-1,000-fold decrease in asp23 transcription in S. epidermidis 1457sigB compared to the ∼30-fold decrease in indirectly σB regulated genes detected by quantitative RT-PCR suggests an almost exclusively σB-dependent transcription of this gene locus. Therefore, asp23 seems to be an excellent marker for σB activity in S. epidermidis when quantitative real time RT-PCR analysis is used.
To evaluate the regulatory roles of the genes rsbU, rsbV, and rsbW on the activity of σB in S. epidermidis, we performed transcriptional analysis of asp23 and sarA in wild-type S. epidermidis 1457 and all of its respective mutants. The dramatic decrease in and lack of σB-dependent transcripts in mutants 1457rsbU and 1457rsbV, respectively, indicate that these genes encode positive regulators of σB activity. The phenotypic observation of increased biofilm formation in mutant 1457rsbW in contrast to the decreased biofilm formation in mutants with dysfunctional σB indicates that this gene encodes a negative regulator of σB. However, an increase in σB activity compared to that in the wild type could be detected only in the post-exponential growth phase, indicating that for the regulation of σB activity during growth, the autoregulation from the internal σB-dependent promoter of the σB operon is superordinate compared to RsbW regulation. The low σB activity in mutant 1457rsbU indicates that RsbU is necessary for full σB activation in different growth phases and under different environmental conditions. However, the basal σB activity in this mutant demonstrates that σB could be activated at a low level in an RsbU-independent manner. In view of these data, it can be suggested that the activity of σB in S. epidermidis is regulated in a manner similar to that described for B. subtilis and S. aureus (Fig. 1). The high homology between the σB operons of gram-positive bacteria (51) also suggests the prediction of similar gene functions in these species (Fig. 1).
Biofilm formation for mutants 1457rsbU and 8400rsbU displayed a phenotype similar to that already observed for mutants M15 and 8400-M15 (36), with dramatic decreases of biofilm formation in TSB and in TSB supplemented with NaCl compared to those for the respective wild types and a strong biofilm-positive phenotype in TSB supplemented with ethanol, indicating that the observed phenotype of mutants M15 and 8400-M15 (36) is mediated by Tn917 insertion in rsbU and not by polar effects of the transposon. Mutants 1457sigB and 8400sigB displayed phenotypes almost identical to those of mutants 1457rsbU and 8400rsbU. In these mutants, no differences in transcriptional levels of rsbU could be detected, while σB activity was abolished, indicating that the RsbU-dependent regulation of biofilm formation is mediated via the alternative sigma factor σB. This idea is supported further by the phenotypic properties of mutants 1457rsbV and 8400rsbV, in which the genes for the second positive regulator of σB activity were inactivated. This finding suggests that the observation of Kies et al. (35) of a biofilm-positive phenotype in a sigB deletion mutant, S. epidermidis TüΔsigB, containing the plasmid pCN27 carrying the icaADBC locus seems to be caused by the overexpression of the icaADBC genes. This overexpression despite a sigB-negative genotype could be explained by the lack of the icaR gene (31), encoding the negative regulator of icaADBC transcription IcaR (14, 15), in pCN27. However, the possibility that an additional, yet-unknown regulatory intermediate between σB and the icaADBC operon is missing in an icaADBC-negative genetic background cannot be completely excluded. The phenotypic characterization of mutants 1457rsbW, 1457rsbUVW, 8400rsbW, and 8400rsbUVW revealed an increase in biofilm formation compared to that of their respective wild-type strains. Apparently, the lack of regulation of σB activity during the growth cycle, resulting in constitutive sigB transcription with lower σB activity during the exponential growth phase and higher activity during the post-exponential phase, leads to overexpression of PIA and biofilm formation.
Transcriptional analysis of the icaADBC locus revealed that the positive regulation of biofilm formation in S. epidermidis by σB is based on transcriptional activation of icaADBC, as has already been demonstrated for the rsbU mutant M15 (49). However, the lack of a σB-dependent promoter upstream of the icaADBC transcription start site (31, 36) necessitates a σB-dependent regulatory intermediate which controls icaADBC transcription. Recently, it was demonstrated that IcaR acts as a negative regulator of icaADBC transcription in S. epidermidis (14, 15). In S. aureus, IcaR was characterized as a DNA binding protein which interacts with the promoter region of icaADBC (34). Transcriptional analysis of the icaR gene revealed that the positive σB regulation of biofilm formation is mediated by negative transcriptional control of IcaR, the negative regulator of icaADBC transcription (14), which is up-regulated in mutants which are defective in sigB or its function. This regulatory pathway could explain the biofilm-positive phenotype of mutants with a lack of σB function in media supplemented with ethanol. In these mutants, icaR transcription is repressed by ethanol by a yet-unknown mechanism, as was already demonstrated by Conlon et al. for the clinical isolate S. epidermidis CSF41498 (15). These data indicate that icaR transcription is negatively controlled by two divergent regulatory pathways. Hence, for the σB-dependent control of biofilm formation, a negative regulatory intermediate of icaR transcription, which is itself under positive control of σB, must be predicted. Despite the increased biofilm formation in wild-type S. epidermidis 1457, a decrease in icaADBC transcription under the influence of ethanol was detected. Recently, a dissociation of icaADBC transcription and PIA synthesis was characterized in S. epidermidis 1457 with glucose limitation (17). A glucose-dependent posttranscriptional regulator of PIA synthesis was predicted, and this regulator might also be responsible for the observed dissociation between icaADBC transcription and biofilm formation under ethanol induction.
The data obtained in this study together with the current knowledge about the regulation of PIA synthesis in S. epidermidis enables us to draw up a model of transcriptional and posttranscriptional regulation (Fig. 9). This model reveals a complex regulation of PIA synthesis, which is regulated by at least three different regulatory pathways. Two of these pathways act through transcriptional regulation of the negative regulator IcaR of icaADBC transcription, and the third pathway is a glucose-dependent proteinaceous factor of PIA synthesis (17). However, in this provisional model several gaps of yet-uncharacterized regulatory intermediates remain in this complex regulatory system. The σB-dependent negative regulator and the ethanol-mediated negative regulation of icaR transcription, as well as the nature of the glucose-dependent posttranscriptional activator of PIA synthesis, are still unknown. The regulatory role of SarA in S. epidermidis biofilm formation could not be conclusively assessed. The σB-dependent sarA transcript was still absent in mutants with dysfunctional σB despite a biofilm-positive phenotype under ethanol induction, indicating the lack of influence of SarA on the IcaR-dependent regulation of PIA synthesis in S. epidermidis. In the σB-dependent regulatory pathway of PIA synthesis, the possibility of an influence of SarA could not be excluded. However, the sarB and sarC transcripts were not affected in the mutants with inactivation of σB function, indicating a still-present activity of SarA. Interestingly, regulation of PIA synthesis in S. aureus seems to be different, as was already predicted by phenotypic characterization (38). In S. aureus, regulation of biofilm formation is mainly dependent on SarA, whereas σB-dependent regulation plays only a minor role (74). The differential role of the alternative sigma factor σB in these species indicates that investigation of the transcriptional profile during biofilm formation in S. aureus is not suitable for drawing conclusions about S. epidermidis or, presumably, about other staphylococcal species.
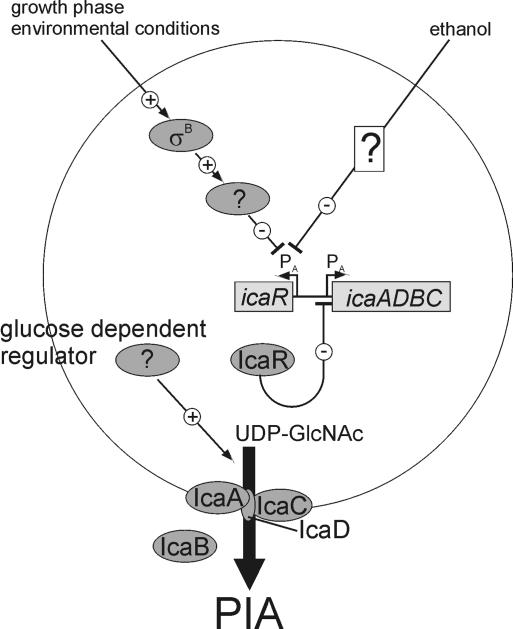
FIG. 9.
Model of the regulation of PIA synthesis in S. epidermidis. Transcription of icaADBC is regulated by the activity of the negative regulator IcaR (14). Transcription of icaR is negatively regulated by ethanol (15) and independently by a σB-dependent negative regulator, which is yet unknown. Additionally, PIA synthesis is regulated posttranscriptionally by a yet-unknown glucose-dependent regulator (17).
PIA synthesis resulting in biofilm formation is the major pathogenetic factor of S. epidermidis in foreign-body-associated infections. Therefore, the regulatory system of biofilm formation could be an important target in the prevention and therapy of foreign-body-related infections due to S. epidermidis, requiring further studies.
Acknowledgments
We thank Rainer Laufs for his continuous support. The kind gift of plasmid pBT2 by Reinhold Brückner is gratefully acknowledged.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the Werner Otto Stiftung, and the Forschungsförderungsfonds Medizin des Universitätsklinikums Hamburg-Eppendorf, Germany, given to J.K.-M.K. and D.M. S.J. was supported by a fellowship of the Werner Otto Stiftung, Hamburg, Germany.
Editor: V. J. DiRita
REFERENCES
- 1.Akbar, S., C. M. Kang, T. A. Gaidenko, and C. W. Price. 1997. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol. Microbiol. 24:567-578. [DOI] [PubMed] [Google Scholar]
- 2.Bateman, B. T., N. P. Donegan, T. M. Jarry, M. Palma, and A. L. Cheung. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 69:7851-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls sigma B expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis sigma B is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 90:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, A. K., and W. G. Haldenwang. 1993. The sigma B-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the sigma B transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, S. A., A. R. Redfield, and C. W. Price. 1993. Transcription factor sigma B of Bacillus subtilis controls a large stationary-phase regulon. J. Bacteriol. 175:3957-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boylan, S. A., A. Rutherford, S. M. Thomas, and C. W. Price. 1992. Activation of Bacillus subtilis transcription factor sigma B by a regulatory pathway responsive to stationary-phase signals. J. Bacteriol. 174:3695-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Cho, S. H., K. Naber, J. Hacker, and W. Ziebuhr. 2002. Detection of the icaADBC gene cluster and biofilm formation in Staphylococcus epidermidis isolates from catheter-related urinary tract infections. Int. J. Antimicrob. Agents 19:570-575. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, G. D., L. Baldassari, and W. A. Simpson. 1994. Colonisation of medical devices by coagulase-negative staphylococci, p. 45-78. In F. A. Waldvogel and A. L. Bisno (ed.), Infection associated with indwelling medical devices. American Society for Microbiology, Washington, D.C.
- 13.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2002. Regulation of icaR gene expression in Staphylococcus epidermidis. FEMS Microbiol. Lett. 216:171-177. [DOI] [PubMed] [Google Scholar]
- 16.Dobinsky, S., K. Bartscht, and D. Mack. 2002. Influence of Tn917 insertion on transcription of the icaADBC operon in six biofilm-negative transposon mutants of Staphylococcus epidermidis. Plasmid 47:10-17. [DOI] [PubMed] [Google Scholar]
- 17.Dobinsky, S., K. Kiel, H. Rohde, K. Bartscht, J. K. M. Knobloch, M. A. Horstkotte, and D. Mack. 2003. Glucose-related dissociation between icaADBC transcription and biofilm expression by Staphylococcus epidermidis: evidence for an additional factor required for polysaccharide intercellular adhesin synthesis. J. Bacteriol. 185:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-sigma factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fey, P. D., J. S. Ulphani, F. Götz, C. Heilmann, D. Mack, and M. E. Rupp. 1999. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J. Infect. Dis. 179:1561-1564. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick, F., H. Humphreys, E. Smyth, C. A. Kennedy, and J. P. O'Gara. 2002. Environmental regulation of biofilm formation in intensive care unit isolates of Staphylococcus epidermidis. J. Hosp. Infect. 52:212-218. [DOI] [PubMed] [Google Scholar]
- 21.Fluckiger, U., C. Wolz, and A. L. Cheung. 1998. Characterization of a sar homolog of Staphylococcus epidermidis. Infect. Immun. 66:2871-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galdbart, J. O., J. Allignet, H. S. Tung, C. Ryden, and N. El Solh. 2000. Screening for Staphylococcus epidermidis markers discriminating between skin-flora strains and those responsible for infections of joint prostheses. J. Infect. Dis. 182:351-355. [DOI] [PubMed] [Google Scholar]
- 23.Gerke, C., A. Kraft, R. Süßmuth, O. Schweitzer, and F. Götz. 1998. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 273:18586-18593. [DOI] [PubMed] [Google Scholar]
- 24.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 1999. Regulation of σB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 25.Gertz, S., S. Engelmann, R. Schmid, A. K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Götz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 28.Hecker, M., W. Schumann, and U. Völker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 29.Hecker, M., and U. Völker. 1998. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the σB regulon. Mol. Microbiol. 29:1129-1136. [DOI] [PubMed] [Google Scholar]
- 30.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 31.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 32.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 34.Jefferson, K. K., S. E. Cramton, F. Götz, and G. B. Pier. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 48:889-899. [DOI] [PubMed] [Google Scholar]
- 35.Kies, S., M. Otto, C. Vuong, and F. Götz. 2001. Identification of the sigB operon in Staphylococcus epidermidis: construction and characterization of a sigB deletion mutant. Infect. Immun. 69:7933-7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knobloch, J. K. M., K. Bartscht, A. Sabottke, H. Rohde, H. H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, P. M. Kaulfers, and D. Mack. 2002. Alcoholic ingredients in skin disinfectants increase biofilm expression of Staphylococcus epidermidis. J. Antimicrob. Chemother. 49:683-687. [DOI] [PubMed] [Google Scholar]
- 38.Knobloch, J. K. M., M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. (Berlin) 191:101-106. [DOI] [PubMed] [Google Scholar]
- 39.Knobloch, J. K. M., H. von Osten, M. A. Horstkotte, H. Rohde, and D. Mack. 2002. Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med. Microbiol. Immunol. (Berlin) 191:107-114. [DOI] [PubMed] [Google Scholar]
- 40.Kullik, I., and P. Giachino. 1997. The alternative sigma factor σB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151-159. [DOI] [PubMed] [Google Scholar]
- 41.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 43.Mack, D. 1999. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect. 43(Suppl.):S113-S125. [DOI] [PubMed] [Google Scholar]
- 44.Mack, D., K. Bartscht, C. Fischer, H. Rohde, C. de Grahl, S. Dobinsky, M. A. Horstkotte, K. Kiel, and J. K. M. Knobloch. 2001. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. Methods Enzymol. 336:215-239. [DOI] [PubMed] [Google Scholar]
- 45.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack, D., M. Haeder, N. Siemssen, and R. Laufs. 1996. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J. Infect. Dis. 174:881-884. [DOI] [PubMed] [Google Scholar]
- 47.Mack, D., M. Nedelmann, A. Krokotsch, A. Schwarzkopf, J. Heesemann, and R. Laufs. 1994. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62:3244-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mack, D., J. Riedewald, H. Rohde, T. Magnus, H. H. Feucht, H. A. Elsner, R. Laufs, and M. E. Rupp. 1999. Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect. Immun. 67:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mack, D., H. Rohde, S. Dobinsky, J. Riedewald, M. Nedelmann, J. K. M. Knobloch, H. A. Elsner, and H. H. Feucht. 2000. Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidis polysaccharide intercellular adhesin and biofilm formation. Infect. Immun. 68:3799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittenhuber, G. 2002. A phylogenomic study of the general stress response sigma factor σB of Bacillus subtilis and its regulatory proteins. J. Mol. Microbiol. Biotechnol. 4:427-452. [PubMed] [Google Scholar]
- 52.Miyazaki, E., J. M. Chen, C. Ko, and W. R. Bishai. 1999. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J. Bacteriol. 181:2846-2851.10217777 [Google Scholar]
- 53.Nair, S. P., M. Bischoff, M. M. Senn, and B. Berger-Bächi. 2003. The sigma B regulon influences internalization of Staphylococcus aureus by osteoblasts. Infect. Immun. 71:4167-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholas, R. O., T. Li, D. McDevitt, A. Marra, S. Sucoloski, P. L. Demarsh, and D. R. Gentry. 1999. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect. Immun. 67:3667-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilsson, M., L. Frykberg, J. I. Flock, L. Pei, M. Lindberg, and B. Guss. 1998. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect. Immun. 66:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palma, M., and A. L. Cheung. 2001. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascual, A., A. Fleer, N. A. Westerdaal, and J. Verhoef. 1986. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur. J. Clin. Microbiol. 5:518-522. [DOI] [PubMed] [Google Scholar]
- 58.Petersohn, A., H. Antelmann, U. Gerth, and M. Hecker. 1999. Identification and transcriptional analysis of new members of the σB regulon in Bacillus subtilis. Microbiology 145:869-880. [DOI] [PubMed] [Google Scholar]
- 59.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Völker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 62.Rachid, S., S. Cho, K. Ohlsen, J. Hacker, and W. Ziebuhr. 2000. Induction of Staphylococcus epidermidis biofilm formation by environmental factors: the possible involvement of the alternative transcription factor sigB. Adv. Exp. Med. Biol. 485:159-166. [DOI] [PubMed] [Google Scholar]
- 63.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rohde, H., J. K. M. Knobloch, M. A. Horstkotte, and D. Mack. 2001. Correlation of biofilm expression types of Staphylococcus epidermidis with polysaccharide intercellular adhesin synthesis: evidence for involvement of icaADBC genotype-independent factors. Med. Microbiol. Immunol. (Berlin) 190:105-112. [DOI] [PubMed] [Google Scholar]
- 66.Rupp, M. E., and G. L. Archer. 1992. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect. Immun. 60:4322-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rupp, M. E., and G. L. Archer. 1994. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin. Infect. Dis. 19:231-243. [DOI] [PubMed] [Google Scholar]
- 68.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Götz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 69.Rupp, M. E., N. Sloot, H. G. Meyer, J. Han, and S. Gatermann. 1995. Characterization of the hemagglutinin of Staphylococcus epidermidis. J. Infect. Dis. 172:1509-1518. [DOI] [PubMed] [Google Scholar]
- 70.Rupp, M. E., J. S. Ulphani, P. D. Fey, K. Bartscht, and D. Mack. 1999. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect. Immun. 67:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rupp, M. E., J. S. Ulphani, P. D. Fey, and D. Mack. 1999. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model. Infect. Immun. 67:2656-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thylefors, J. D., S. Harbarth, and D. Pittet. 1998. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect. Control Hosp. Epidemiol. 19:581-589. [DOI] [PubMed] [Google Scholar]
- 73.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 74.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 75.Veenstra, G. J., F. F. Cremers, H. van Dijk, and A. Fleer. 1996. Ultrastructural organization and regulation of a biomaterial adhesin of Staphylococcus epidermidis. J. Bacteriol. 178:537-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Völker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Völker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 77.Völker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Völker, U., A. Völker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-sigma B antagonist, RsbV, by stress- or starvation-induced phosphatase activities. J. Bacteriol. 178:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]
- 82.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]
- 83.Ziebuhr, W., C. Heilmann, F. Götz, P. Meyer, K. Wilms, E. Straube, and J. Hacker. 1997. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 65:890-896. [DOI] [PMC free article] [PubMed] [Google Scholar]