Abstract
Cephalic allodynia (CA) can be observed in 50-70% of patients with chronic migraine (CM). The aim of this trial was to assess the efficacy of botulinum toxin type A (Botx-A) in the treatment of CA associated with CM. In this placebo-controlled trial, patients were randomized either into Botx-A or 0.9% saline injections and efficacy measures were assessed every 4 weeks for 3 months. Efficacy endpoints were number of migraine episodes associated with CA, changes from baseline in visual analogical scale scores for pain (VAS) and frequency of common analgesics use for migraine. A total of 38 subjects were randomized to saline (n=18) or Botx-A (n=20). There were no significant differences in baseline between active intervention or placebo groups regarding mean age, number of headache episodes [mean 12.1 (9.22) and 17.00 (9.69) respectively; P=0.12], pain severity as measured by the VAS or frequency of analgesic use for headache episodes. Efficacy analysis showed that Botx-A injections led to an important decrease from baseline in the mean migraine episodes associated with CA after 12 weeks (5.20 versus 11.17; P=0.01). Also, VAS scores and frequency of analgesics use for headache were significantly reduced in the Botx-A group. This study suggests that Botx-A injections are superior to saline in the treatment of CA associated with CM, with mild self limited side effects.
Key words: migraine, randomized controlled trial, allodynia, botulinum toxin, adverse event
Introduction
Cutaneous allodynia (CA) is defined as perception of pain when a non-painful stimulus is applied to the normal skin.1,2 Chronic migraine (CM) is diagnosed when the subject presents with more than 15 episodes of headache per month for at least three months, excluding other etiologies.3 During an acute episode of migraine, abnormal sensitivity of cephalic extra-cranial structures may be observed in up to 80% of patients,4 generally referred in the periorbital area.5 The process of abnormal sensitization involving central trigeminovascular neurons is the mechanism believed to promote the underlying allodynia in migraine subjects.6 The prevalence of CM can reach 2-4% of the population worldwide, leading to great occupational disability and reduced quality of life.7 Subjects with CM frequently complain of CA, and previous reports have suggested its association with frequency and duration of migraine attacks.8
The presence of CA, considered as a marker of central sensitization, may reduce the responsiveness to triptans, decreasing the threshold for recurrence of headache episodes.9 However, growing evidence points towards a positive effect of botulinum toxin type A (Botx-A) injections in the prophylactic treatment of CM,10,11 with optimal response in subgroups of patients with associated allodynia and pericranial muscle tenderness.12
The efficacy and safety of Botx-A injections in reducing pain severity during migraine attacks and frequency of headache episodes were extensively assessed in randomized placebo-controlled trials, open trials and case reports.13-16 However, to our knowledge, there is no reference in the literature whether Botx-A injections are effective in cephalic CA associated to chronic migraine. In this way, the objective of this preliminary study was to evaluate the efficacy and safety of Botx-A injections in the treatment of cephalic CA associated with chronic migraine, when compared to placebo.
Materials and Methods
Participants
This randomized placebo-controlled pilot trial was conducted in an outpatient headache clinic in Northeastern, Brazil, from January 2011 to April 2012. Subjects enrolled were men or women aged 18-85 years with previous diagnosis of CM associated with CA, according to the International Classification of Headache Disorders diagnostic criteria (edition revised, 2006). Exclusion criteria included diagnosis of neuromuscular diseases (myasthenia gravis, Eaton-Lambert syndrome and amyotrophic lateral sclerosis) and all conditions that could affect pericranial muscles. Additional exclusion criteria included other primary or secondary headaches, complicated migraine, CM associated with analgesic overuse, severe systemic diseases, other neurologic or psychiatric disorders, fibromyalgia, myofascial syndrome, temporomandibular disorder, regional complex pain syndrome or neuropathic pain. Subjects using opiates, benzodiazepines, muscle relaxants or medications that could interfere with neuromuscular function in the last four weeks prior to baseline assessments, or who have experienced previous injection of any type of botulinum toxin were also excluded. Pregnant females or lactants were excluded and potential childbearing women were required to use a reliable contraception method.
Study protocol
In this pilot study, all eligible subjects who consented to participate fulfilled a headache diary 30 days prior to baseline assessments. The questionnaire included number of headache episodes, type, frequency and dose of oral analgesics used to treat acute episodes, allodynic cephalic points and the visual analogical scale (VAS) for pain assessment. During this period, all prophylactic treatments for CM were withdrawn. The study was approved by the local ethic committee in the Federal University and all participants signed an informed consent prior to any study-related procedure. The trial protocol was registered in the site clinicaltrials.gov under the code NCT01357798.
After baseline assessments, all subjects enrolled went through a 12-week randomized, double-blind placebo-controlled follow up period, with 3 monthly pre-scheduled visits to the center. All procedures related to the study were performed during the visits. If an extra visit was necessary or elicited by the patient, the reasons and procedures performed were also recorded.
Randomization process and study intervention
Fifty-eight subjects who fulfilled the inclusion/exclusion criteria were invited to participate in the trial. Thirty-eight patients who signed the informed consent were assigned into one of two intervention groups, according to a simple random allocation sequence generated by computer. Patients and investigators who administered the study interventions and assessed the outcomes were masked to group assignments. One designated investigator who had access to the randomization list provided the study medication to the principal investigator according to the allocation list generated. Patients and investigators remained blinded to study interventions until the last study assessment, according to protocol. If necessary, for safety or ethical reasons, allocation code could be broken by the principal investigator before the end of the study.
Botx-A 100 U (Prosigne®, Cristalia, Lanzhou Institute, Beijing, China) was conserved in a freezer between –20°C and –5°C before utilization. During the first intervention visit (day 0), patients randomized in a double-blinded way confirmed allodynic points according to their register in the headache diary and received intramuscular injections of Botx-A or saline solution. Doses applied in each muscle injected during trial visits are specified in Table 1. Injections were administered with a syringe and 0.5 inch needle containing Botx-A diluted in 3 mL of sterile 0.9% saline solution or placebo in each point, according to the doses and sites previously determined. After injections, subjects were oriented to return for subsequent visits with 4-weeks interval, up to 12-weeks, total follow-up period (end of study). During each visit, before intramuscular injections, data on each patient were collected by a blinded investigator and registered in a standardized form (headache diary information in the previous 30 days and VAS score).
Table 1.
Allodynic sites and total injected in each point.
| Dose | Points | |
|---|---|---|
| Right frontal | 3U | 2-4 |
| Left frontal | 3U | 2-4 |
| Right temporal | 4U | 2-4 |
| Left temporal | 4U | 2-4 |
| Right occiptal | 5U | 2-4 |
| Left occiptal | 5U | 2-4 |
Study endpoints and safety assessments
The primary efficacy endpoint was mean change from baseline in frequency of headache episodes with allodynia to end-of-study assessments, in week 12. Secondary efficacy endpoints were mean change from baseline to end-of-study in a 12-week observation period of the following outcomes: intensity of headache pain, measured through the VAS, and frequency of analgesics use for headache (Figure 1).
Figure 1.
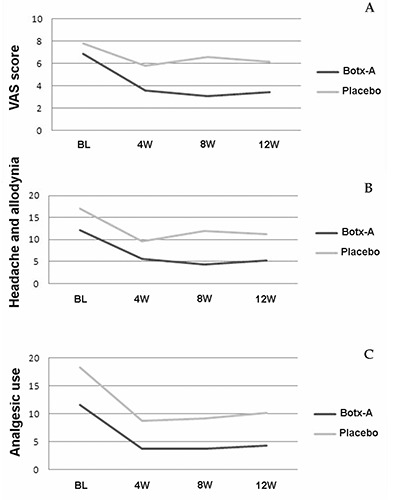
Mean change from baseline to end-of-study in visual analogical scale scores (A), frequency of headache episodes associated with allodynia (B) and frequency of analgesic use for headache (C).
All adverse events were registered during the scheduled visits. Data regarding headache episodes such as onset and resolution date, severity (mild, moderate or severe), duration, frequency, relation with study intervention (not related, possible or probable), treatment (if required) and outcomes were assessed by a blinded investigator. Events that required hospitalization were classified as severe. Safety measures were also assessed by complete physical and neurological examination. Complementary laboratory tests were performed if necessary.
Statistical analysis
Analysis were performed at SPSS version 16.0 with all randomized patients, according to intention-to-treat protocol. Continuous variables were reported as mean ± standard deviation (SD) and comparison in several time-points was performed using the Wilcoxon rank-sum test. Categorical variables were reported as frequency (%) and were compared with the chi-square statistics or Fisher’s exact test. The significance level was established at 0.05.
Results
Demographic characteristics of the studied population
Fifty-eight subjects were considered eligible according to study protocol. However, twenty subjects were not randomized and considered in the analysis because did not sign the informed consent, due to personal reasons. Thirty-eight eligible subjects were randomized to Botx-A (n=20) or 0.9% saline solution (n=18), being 29 women and 9 men. Baseline characteristics of the studied population were summarized in Table 2. There were no significant differences between active intervention or placebo groups in the studied population regarding mean age, number of headache episodes, pain severity as measured by the VAS or frequency of analgesic use for headache episodes.
Table 2.
Baseline demographic characteristics of the studied population.
| BotxA (n=20), mean (±SD) | Placebo (n=18), mean (±SD) | P | |
|---|---|---|---|
| Age (years) | 44.8 (12.6) | 45.8 (14.2) | 0.81 |
| Visual analogical score | 6.90 (1.37) | 7.78 (1.59) | 0.08 |
| Headache episodes with allodynia | 12.1 (9.22) | 17.00 (9.69) | 0.12 |
| Frequency of analgesic use for headache | 11.65 (13.86) | 18.33 (12.97) | 0.13 |
Efficacy measures
All primary and secondary study endpoints were achieved (Table 3), with a significant improvement in the group treated with Botx-A when compared to placebo (P<0.05). Differences between groups were observed after the first intervention visit, favoring the Botx-A group, being more accentuated in the 8-week assessments for frequency of headache episodes associated with allodynia and VAS scores (Tables 2 and 3). When considering frequency of analgesic use for headache episodes, differences between groups were observed after the first intervention visit, being stable during all protocol assessments, also favoring the Botx-A group (Table 4).
Table 3.
Mean changes in primary and secondary endpoints from baseline to week 12.
| BotxA (n=20), mean (±SD) | Placebo (n=18), mean (±SD) | P | |
|---|---|---|---|
| Visual analogical score | |||
| Baseline | 6.90 (1.37) | 7.78 (1.59) | 0.08 |
| 4 weeks | 3.60 (2.16) | 5.83 (1.94) | <0.01 |
| 8 weeks | 3.05 (2.62) | 6.55 (2.23) | <0.01 |
| 12 weeks | 3.45 (2.39) | 6.17 (2.50) | <0.01 |
| Headache episodes with allodynia | |||
| Baseline | 12.1 | 17.00 (9.69) | 0.12 |
| 4 weeks | 5.65 (4.89) | 9.61 (5.24) | 0.02 |
| 8 weeks | 4.30 (3.99) | 11.94 (6.83) | P<0.01 |
| 12 weeks | 5.20 (6.76) | 11.17 (6.69) | 0.01 |
| Frequency of analgesic use for headache | |||
| Baseline | 11.65 (13.86) | 18.33 (12.97) | 0.13 |
| 4 weeks | 3.75 (4.65) | 8.72 (4.93) | P<0.01 |
| 8 weeks | 3.70 (5.02) | 9.17 (6.77) | P<0.01 |
| 12 weeks | 4.25 (6.95) | 10.22 (6.11) | P<0.01 |
Table 4.
Adverse events reported in the studied population.
| Botx-A (n=20) | Placebo (n=18) | |
|---|---|---|
| Adverse events (total) | 9 (45.0%) | 7 (38.8%) |
| Pain in injected points | 6 (30.0%) | 2 (11.1%) |
| Burning sensation in injected points | 4 (20.0%) | 2 (11.1%) |
| Headache after injection | 2 (10.0%) | 1 (5.5%) |
Safety assessments
A total of 16 subjects reported adverse events during the study protocol, the most common being pain in the site of injections, which were resolved without sequelae (Table 4). There were no severe adverse events reported in this group.
Discussion
This pilot study suggests that Botx-A injections are safety and efficacious in the treatment of cephalic CA associated with CM, leading to significant improvement in the frequency of headache episodes, use of analgesics for headache or pain severity after 12 weeks of treatment. The differences between groups were more accentuated after 8 weeks of Botx-A injections.
Patients with migraine may experience CA, reflecting the central sensitization of trigeminal pathways.17 CA can be present in up to 60% of subjects with episodic migraine and might be associated with long disease duration and phono- or photo-phobia.18 In addition, the presence of CA during migraine attacks may be a risk factor for the development of chronic disease.19 Although the most accepted mechanisms of CA during migraine attacks are the hiperexcitability of trigeminovascular pathways with central involvement of neurons in the trigeminal caudal nucleus and thalamus leading to sensitization of peripheral neurons,19,20 skin areas outside the trigeminal territory also may be affected.21
Several trials assessed the efficacy and safety of Botx-A in the treatment of chronic daily headache.13-15 Studies that compared classic drugs used in the prophylactic treatment of CM, such as amytriptiline, suggested that Botx-A injections were equally efficacious, with less side effects.22
Chronic pain improves after Botx-A injections, suggesting that one single pathophysiologic mechanism might be responsible for this result.23,24 As has been pointed out by some authors, Botx-A mediate releasing of several neurotransmitters and neuromodulators involved in central and peripheral sensitization, such as substance P, calcitonin-gene related peptide and glutamate.25,26 Modulation of these substances may explain improvement in CA associated with CM in our study. Nevertheless, Botx-A injections are not associated with chronic tension type headache improvement.27-29
In this way, it is possible that multiple mechanisms may interact to improve CA associated to CM. As far as we know, there is no placebo-controlled trial where the primary endpoint was CA associated with CM.
Conclusions
This randomized controlled trial suggests that Botx-A injections are superior to saline solution in the treatment of CA associated with CM. However, the conclusions in this pilot trial must be interpreted carefully, as the small sample size and characteristics of the studied population may limit the generalization of the results. In this way, further larger multicentric randomized controlled trials are necessary to reassure the results achieved in this study.
Acknowledgements
This work was funded by Brazilian National Institutes of Science (CITECS/INNT/CNPq), CAPES, and UFBA.
References
- 1.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998;79:964-82. [DOI] [PubMed] [Google Scholar]
- 2.Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache 1987;27:45-50. [DOI] [PubMed] [Google Scholar]
- 3.Levin M. Chronic daily headache and the revised international headache society classification. Curr Pain Headache Rep 2004;8:59-65. [DOI] [PubMed] [Google Scholar]
- 4.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry 1960;23:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goadsby PJ. Migraine, allodynia, sensitisation and all of that .. Eur Neurol 2005;53:10-6. [DOI] [PubMed] [Google Scholar]
- 6.Burnstein R. Replacement of damaged neural cells. J R Soc Med 2000;93:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep 2011;15:70-8. [DOI] [PubMed] [Google Scholar]
- 8.Mathew NT. Pathophysiology of chronic migraine and mode of action of preventive medications. Headache 2011;51:84-92. [DOI] [PubMed] [Google Scholar]
- 9.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004;55:19-26. [DOI] [PubMed] [Google Scholar]
- 10.Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804-14. [DOI] [PubMed] [Google Scholar]
- 11.Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010;50:921-936. [DOI] [PubMed] [Google Scholar]
- 12.Meadors L. Predictors of response to botulinum toxin type A (BoNTA) in chronic daily headache. Headache 2008;48:194-200. [DOI] [PubMed] [Google Scholar]
- 13.Mathew NT, Frishberg BM, Gawel M, et al. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:293-307. [DOI] [PubMed] [Google Scholar]
- 14.Silberstein SD, Stark SR, Lucas SM, et al. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 2005;80:1126-37. [DOI] [PubMed] [Google Scholar]
- 15.Freitag FG, Diamond S, Diamond M, Urban G. Botulinum Toxin Type A in the treatment of chronic migraine without medication overuse. Headache 2008;48:201-9. [DOI] [PubMed] [Google Scholar]
- 16.Dodick DW, Mauskop A, Elkind AH, et al. Botulinum toxin type a for the prophylaxis of chronic daily headache: subgroup analysis of patients not receiving other prophylactic medications: a randomized double-blind, placebo-controlled study. Headache 2005;45:315-24. [DOI] [PubMed] [Google Scholar]
- 17.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guven H, Cilliler AE, Comoglu SS. Cutaneous allodynia in patients with episodic migraine. Neurol Sci 2013;34:1397-402 [DOI] [PubMed] [Google Scholar]
- 19.Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain 2008;9:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache 2006;46:S182-91. [DOI] [PubMed] [Google Scholar]
- 21.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol 2000;47:614-24. [PubMed] [Google Scholar]
- 22.Magalhaes E, Menezes C, Cardeal M, Melo A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg 2010;112:463-6. [DOI] [PubMed] [Google Scholar]
- 23.Gobel H, Heinze A, Reichel G, et al. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain 2006;125:82-8. [DOI] [PubMed] [Google Scholar]
- 24.Jabbari B, Machado D. Treatment of refractory pain with botulinum toxins: an evidence-based review. Pain Med 2011;12:1594-606. [DOI] [PubMed] [Google Scholar]
- 25.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005;26:785-93. [DOI] [PubMed] [Google Scholar]
- 26.Durham PL, Cady R, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: implications for migraine therapy. Headache 2004;44:35-42. [DOI] [PubMed] [Google Scholar]
- 27.Padberg M, de Bruijn SF, de Haan RJ, Tavy DL. Treatment of chronic tension-type headache with botulinum toxin: a double-blind, placebo-controlled clinical trial. Cephalalgia 2004;24:675-80. [DOI] [PubMed] [Google Scholar]
- 28.Silberstein SD, Gobel H, Jensen R, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicentre, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia 2006;26:790-800. [DOI] [PubMed] [Google Scholar]
- 29.Jeffrey L, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headache in adults: a meta-analysis. JAMA 2012;307:1736-45. [DOI] [PubMed] [Google Scholar]


