Abstract
Chemotherapy for blood-dwelling schistosomes kills the worms and exposes parasite antigen to the circulation. In many people from areas of endemicity, this treatment increases parasite-specific immunoglobulin E (IgE) and other Th2 responses in the months following therapy, responses that have been associated with subsequent resistance to reinfection. Here we investigate much earlier changes in immune reactions after praziquantel therapy in Schistosoma mansoni-infected fishermen working in an area of high transmission in Uganda. The subjects gave blood before treatment and at 1 and 21 days posttreatment. Blood cultures were incubated with schistosome soluble worm antigen (SWA) or soluble egg antigen (SEA). Interleukin-4 (IL-4), IL-5, IL-10, IL-13, gamma interferon, and transforming growth factor β levels were measured in the cultures and in plasma. A marked transient increase in plasma IL-5 levels was observed in 75% of the subjects (n = 48) by 1 day posttreatment. This response was dependent on pretreatment intensity of infection and was accompanied by a transient decrease in eosinophil numbers. One day posttreatment, blood cultures from the 16 subjects with the greatest increase in plasma IL-5 level (>100 pg/ml) displayed reduced IL-5, IL-13, and IL-10 responses to SWA, and in contrast to the rest of the cohort, these high-IL-5 subjects displayed reduced levels of SWA-specific IgE in plasma 21 days posttreatment. Twenty months after treatment, the intensity of reinfection was positively correlated with the increase in plasma IL-5 level seen 1 day posttreatment. These studies describe the heterogeneity in early immune reactions to treatment, identifying subgroups who have different patterns of reaction and who may have different capacities to mount the responses that have been associated with resistance to reinfection.
Schistosomiasis is a condition caused by parasitic helminths of the genus Schistosoma, with S. mansoni, S. haematobium, and S. japonicum accounting for most of the world's estimated 200 million cases of human schistosomiasis. The disease is contracted from waterborne larvae that penetrate the skin and enter the bloodstream, where they develop, pair, and reach sexual maturity. Adult pairs of S. mansoni reside in the mesenteric veins of the intestine, where each female lays up to 300 eggs per day.
In numerous treatment studies carried out in areas of endemicity (4, 6, 7, 11, 12, 16, 23, 45, 46), the intensity of reinfection after chemotherapy has been shown to be highest in children and adolescents and then to decrease during adult life. This suggests that acquired resistance to infection may develop gradually with age. It has, however, been difficult to draw firm conclusions from these age profiles, as in most communities of endemicity adults have less contact with infected water than do children. For this reason, Kabatereine et al. (28) examined the intensity of S. mansoni reinfection in a Ugandan fishing community following treatment with praziquantel (PZQ). Due to occupational contact with water in these communities, adults are more exposed to infectious larvae than are children. The study confirmed that resistance to reinfection increases with increasing age.
The resistance to infection that occurs in areas of endemicity has been correlated with a number of parasite-specific immune responses. For example, in follow-up studies after chemotherapy, reduced reinfection has been associated with higher levels of schistosome-specific immunoglobulin E (IgE) (10, 23, 38, 47), eosinophilia (22), and interleukin-4 (IL-4) and IL-5 (34, 39) and gamma interferon (IFN-γ) (12) production by peripheral blood mononuclear cells. Chemotherapy can promote those responses that have been linked to resistance. For example, PZQ treatment increases the eosinophil count (29) and IL-4 production by peripheral blood mononuclear cells in response to schistosome antigens (20). Studies in Kenya have shown that chemotherapy can increase IgE levels specific for the blood-dwelling adult worm stage of S. mansoni (44) and that the posttreatment and not the pretreatment levels of antiworm IgE are correlated with resistance to reinfection (10).
PZQ is the present drug of choice for treatment of schistosomiasis, acting within the definitive host to kill adult worms and mature eggs (17, 25). Studies in vitro have shown that PZQ initially affects the outer tegument of worms, causing rupture of the membranes with release or exposure of internal antigens (24, 37). Studies of mice have shown that by 17 h after PZQ treatment cells of the host immune system have adhered to and penetrated the worms (3, 8, 35). Thus, in humans, it is to be expected that PZQ therapy would cause disruption of the adult worm tegument with a relatively rapid release of parasite molecules directly into the blood.
In the present investigation we have used PZQ to treat a group of S. mansoni-infected fishermen who live and work in an area of high endemicity. We have collected blood samples before treatment and at 1 day and 21 days posttreatment. This has enabled us to examine the immediate and short-term changes in immune cell function in a group of adults expected to possess some degree of immunity to infection. These early immunological events are likely to relate to the induction of the immune responses that have previously been associated with resistance to reinfection.
MATERIALS AND METHODS
Study cohort selection.
A cohort of adult males was selected in the fishing village of Bugoigo, Lake Albert, Uganda. The cohort comprised 69 males aged 18 to 45 years (mean, 34.5 years), all having resided in Bugoigo for at least 3 years (range, 3 to 43 years; mean, 11.3 years). Of the subjects 31 were Alur, 22 were Mugungu, and the remaining 16 belonged to seven other tribal groups. Selection was made after parasitological examination of three stool samples per individual, with two Kato thick smears per sample (43). All the selected individuals had detectable S. mansoni eggs. The median pretreatment egg count for the selected cohort was 225 eggs per g of feces (range, 3 to 1,340 eggs). Very few eggs of other helminths were found. Twenty months later fecal egg counts were performed again (three stool samples per individual, with two Kato thick smears per sample).
Informed consent was obtained from all whose who participated in this study, in line with the national guidelines of the Ugandan Ministry of Health, whose ethical review committees approved all the protocols used, and the U.S. Department of Health and Human Services. At the completion of this study the whole Bugoigo community was treated with PZQ.
Parasite antigens.
Antigen extracts from S. mansoni eggs (soluble egg antigen [SEA]) and from adult worms (soluble worm antigen [SWA]) have been described previously (14). Both SWA and SEA were filtered through sterile 0.22-μm-pore-size filters, and endotoxin content was measured using the Limulus amebocyte lysate kit (QCL-1000; Bio-Whittaker, Inc., Walkersville, Md.). The levels of endotoxin in the antigens used in these studies were as follows: 10.7 ng of endotoxin/mg of SEA and 25 ng of endotoxin/mg of SWA. Purified endotoxin at the concentrations found when using 10 μg of these SEA or SWA preparations (<0.3 ng/ml)/ml does not stimulate detectable production of any of the cytokines that we have measured by enzyme-linked immunosorbent assay (ELISA) in whole-blood culture.
Blood collection and PZQ treatment.
Blood samples taken by venipuncture in heparinized syringes (10 U of heparin sodium [Sigma, Poole, United Kingdom]/ml) were collected from the subjects before they received a single dose of 40 mg of PZQ/kg of body weight. They donated further blood samples at 24 h and 21 days posttreatment. At each time point, blood eosinophil counts were carried out using single-use counting chambers (Fast Read; ISL Immune System Ltd., Paignton, United Kingdom) after dilution of blood 10 times in eosin counting fluid (0.1% eosin Y, 0.3% sodium citrate, and 19% acetone in water).
Whole-blood cultures.
Heparinized blood was diluted 1:6 in RPMI 1640 medium (Sigma) with penicillin (50 U/ml), streptomycin (50 μg/ml), and l-glutamine (2 mM) added (Sigma) and was dispensed into duplicate 48-well flat-bottomed plates (Costar, Acton, Mass.), and parasite antigens were added to a final concentration of 10 μg/ml. The plates were placed in sealed boxes after the addition of a “gas generating kit” (Oxoid Ltd., Basingstoke, United Kingdom). The cultures were incubated at 37°C and harvested 96 h later. The supernatants were immediately frozen at −20°C.
Cytokine assays.
IL-4, IL-5, IL-10, IL-13, and IFN-γ were measured using capture ELISAs and anticytokine antibodies as described previously (27). In assays for transforming growth factor β (TGF-β), samples were acid treated to activate latent cytokine before they were added to antibody-coated ELISA plates. Thus, for plasma samples, 5 μl of plasma was added to 10 μl of 1.25 M acetic acid-5 M urea, incubated for 10 min, and then neutralized with 5 μl of 2.7 M NaOH-1 M HEPES before dilution. For measurement of TGF-β in blood culture supernatants, samples were incubated with an equal volume of 1 N HCl for 10 min and then neutralized with the same volume of 1.2 M NaOH-0.5 M HEPES. All supernatants (from all individuals and all cultures) or, alternatively, all plasma samples were assayed for each cytokine simultaneously.
Antibodies used in cytokine assays.
The paired monoclonal antibodies (capture antibody and biotinylated detection antibody) used for the cytokine assays were as follows: IL-4 (PharMingen 8D4-8 and MP4-25D2), IL-5 (PharMingen TRFK5 and JES1-5A10), IL-13 (PharMingen JES10-5A2 and B69-2), IFN-γ (PharMingen N1B42 and 4S.B3), and IL-10 (JES3-9D7 and JES3-12G8). In the TGF-β assay, antibody clone 9016 (R&D Systems) was used for capture and a polyclonal chicken antibody (R&D Systems) was used for detection.
Determination of schistosome-specific antibodies and CAA in plasma.
SEA- and SWA-specific IgE and IgG isotypes were quantified in the plasma of the subjects by ELISA as described previously (10). S. mansoni circulating cathodic antigen (CAA) levels were measured by ELISA as described previously (36).
Statistical analysis.
For each donor, all cytokine determinations were carried out in duplicate and antibody determinations were carried out in triplicate. Unless otherwise stated, Wilcoxon's signed rank test was used for pairwise comparison of variables and correlations between variables were determined by calculating Spearman's rank correlation coefficients. Statistical significance was considered at P < 0.05.
RESULTS
Transient increase in levels of IL-5 in plasma 1 day after treatment.
Sixty-nine Ugandan fishermen, all of whom were infected with S. mansoni, consented to be bled before they had received PZQ. Of these, 64 donated blood 1 day posttreatment and 51 donated blood again at 21 days posttreatment. The levels of IL-4, IL-5, IL-13, IFN-γ, IL-10, and TGF-β were measured in plasma from each time point, and the results for the 51 donors who gave blood at all three time points are listed in Table 1. The 1-day posttreatment time point was chosen because this was the earliest feasible posttreatment time point under the field conditions. The 21-day posttreatment time point was chosen to allow time for immunological changes requiring cell proliferation (eosinophilia, lymphocyte proliferation, and antibody responses) to occur.
TABLE 1.
Plasma cytokine concentrations before and after treatment
| Cytokine | Medial concna (25th-75th percentile range) (pg/ml)
|
||
|---|---|---|---|
| Pretreatment | 1 day posttreatment | 21 days posttreatment | |
| IL-4 | 6.3 (3.3-12.2) | 7.5 (4.7-15.2) | 8.0 (4.1-15.8) |
| IL-5 | 12.6 (5.9-18.5) | 33.2*** (17.2-102.6) | 8.5* (4.2-14.9) |
| IL-13 | 74.0 (0-460.0) | 60.8 (17.9-257.0) | 67.9 (16.5-257.7) |
| IFN-γ | 146 (83-293) | 183 (103-293) | 175 (120-294) |
| IL-10 | 32.5 (12.0-65.3) | 31.30 (16.4-54.4) | 27.85 (10.5-55.3) |
| TGF-β | 3,870 (1,976-7,412) | 5,474 (2,790-10,566) | 6,033* (3,477-11,898) |
Significance symbols: *, P = 0.01-0.05; **, P = 0.001-0.01; ***, P < 0.001. No correction has been made for multiple testing. At all time points, n = 51.
In the cohort as a whole, the only measured plasma cytokines to show a statistically significant change following treatment were IL-5 and TGF-β (Table 1). The most striking of these was IL-5, the median level of which increased from 12.6 pg/ml before treatment to 33.2 pg/ml 1 day posttreatment, returning to below pretreatment levels by 21 days. A range of plasma IL-5 increases was seen in the participants. Of the 64 individuals who gave blood at the first two time points, 16 showed no significant increase, while the 16 most responsive increased by between 100 and 1,180 pg/ml.
The increase in plasma IL-5 level was associated with high intensity of infection.
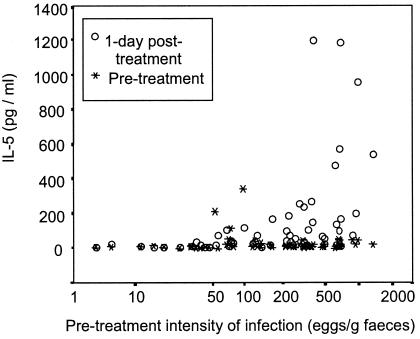
Before treatment, plasma IL-5 levels were not dependent on the intensity of infection (defined by fecal egg count). One day posttreatment, however, there was a strong correlation between the new plasma IL-5 levels and the pretreatment intensity of infection (r = 0.664, P < 0.001, n = 64). Figure 1 shows that those individuals with <50 eggs per g of feces (before treatment) did not show an increase in plasma IL-5 by 1 day posttreatment. Above this threshold there was a relationship between the intensity of infection and the magnitude of the IL-5 increase, and the 16 subjects who experienced the largest increases in IL-5 (>100 pg/ml) all had fecal egg counts exceeding 150 eggs per g. There were, however, donors with egg counts exceeding 150 per g who displayed little or no increase in plasma IL-5 levels.
FIG. 1.
Pretreatment intensity of infection and changes in plasma IL-5 concentration. The intensity of infection before treatment, defined as number of S. mansoni eggs per gram of feces, is plotted against the plasma IL-5 concentration in blood taken before treatment and at 1 day posttreatment (n = 64).
The schistosome secretory product CAA was measured in plasma before treatment (mean, 13.9 ng/ml) and was not detectable 21 days posttreatment, demonstrating effective cure in the cohort. The pretreatment level of CAA (regarded as a measure of worm burden) was correlated with the increased plasma IL-5 concentration at 1 day posttreatment (r = 0.597, P = 0.001).
The transient increase in IL-5 was not dependent on age, on how long subjects had been resident in the village, or on their tribal group. No relationship was observed at any time point between the concentrations of IL-4, IL-13, IFN-γ, or TGF-β in plasma and the pretreatment intensity of infection. In the cohort as a whole, plasma IL-10 levels did not change after treatment; however, the IL-10 concentration at 1 day posttreatment did show a significant positive correlation with pretreatment intensity (r = 0.365, P = 0.004).
Decreased IL-5, IL-13, and IL-10 responses to SWA in blood cultures at 1 day posttreatment.
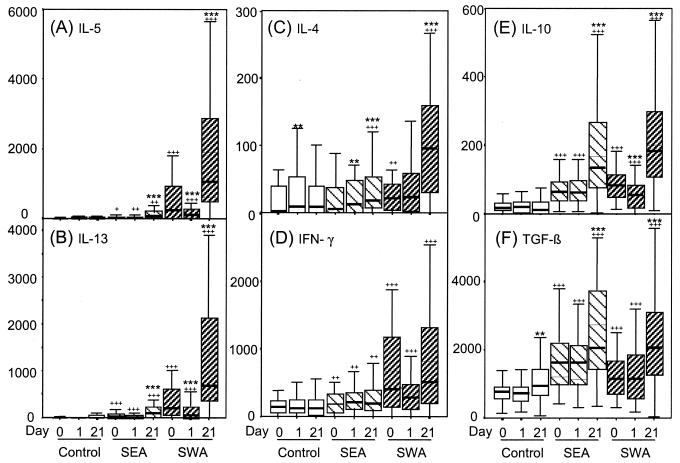
Figure 2 shows cytokine production by whole-blood cultures stimulated with SEA and SWA. At all three time points SWA was the most potent stimulus of both IL-5 and IL-13 production (Fig. 2A and B), with day 21 cultures producing considerably more than pretreatment cultures did. Notably, day 1 cultures produced significantly less IL-5 and IL-13 in response to SWA than pretreatment equivalents did. At all three time points production of IL-5 and that of IL-13 were highly correlated. This was particularly true of the responses to SWA, where coefficients for the correlations between IL-13 and IL-5 levels were 0.903, 0.709, and 0.918 at the three time points, respectively (P < 0.001 in each case).
FIG. 2.
Cytokine production in whole-blood cultures. Blood taken before treatment (day 0) or at 1 or 21 days after treatment was diluted with 5 volumes of medium only (control) or medium containing SEA or SWA to give final antigen concentrations of 10 μg/ml. After 96 h of culture supernatants were harvested for analysis. The broad lines indicate median values (n = 51). The boxes represent the 25th to 75th percentile ranges, and the error bars show the ranges of data excluding outlying values and extremes. For antigen specificity, for a given stimulus, results significantly different (Wilcoxon signed rank test) from the equivalent “medium only” result are indicated by +++ (P < 0.001), ++ (P = 0.001 to 0.01), and + (0.01 to 0.05). For change in response following treatment, for a given time point results significantly different from the equivalent pretreatment result are indicated by *** (P < 0.001), ** (P = 0.001 to 0.01), and * (P = 0.01 to 0.05).
In pretreatment cultures, production of IL-4 was also stimulated by SWA but not by SEA (Fig. 2C). Levels of IL-4 induced by SWA did not change in day 1 cultures, but IL-4 responses to both schistosome antigen preparations, particularly SWA, increased between pretreatment and day 21.
Figure 2D shows that at all three time points SWA was the most potent stimulus for IFN-γ production. This response followed the same trend as IL-5 and IL-13 in that many subjects showed a decrease in IFN-γ production in response to SWA between pretreatment and day 1 posttreatment. Overall, however, neither the difference between pretreatment and day 1 posttreatment (P = 0.161) nor the difference between day 1 and day 21 posttreatment (P = 0.521) achieved statistical significance. SEA induced IFN-γ production to a lesser extent, and the levels were not changed following chemotherapy.
IL-10 production was stimulated by both SEA and SWA at all three time points. The amounts produced in response to the two antigens were highly correlated (r = 0.735, 0.814, and 0.664 at the three times, respectively; P < 0.001 for each). In common with the IL-5 and IL-13 responses, day 1 cultures produced significantly less IL-10 in response to SWA than did pretreatment cultures, but this was not observed when SEA was used as the stimulus. Relative to pretreatment, the IL-10 responses to both SEA and SWA were stronger in day 21 cultures.
SEA was a more potent stimulus for TGF-β production than was SWA in pretreatment and day 1 cultures (Fig. 2F). Levels were substantially higher in day 21 cultures, but at that time there was no significant difference between the responses to SEA and those to SWA. As with IL-10, the SEA responses were strongly correlated with the SWA responses at all three time points (r = 0.735, 0.814, and 0.664, respectively; P < 0.001 in each case).
Reduction of SWA-induced IL-5, IL-13, and IL-10 levels on day 1 in vitro is linked with increase in plasma IL-5 level.
Whole-blood cultures established 1 day posttreatment produced significantly less IL-5, IL-13, and IL-10 in response to SWA than pretreatment cultures did (Fig. 2). This was not the case with responses to SEA, which did not differ significantly between the two time points. The decreases in IL-5 and IL-13 levels were highly correlated (Spearman rank correlation, r = 0.788, P < 0.001), as were the decreases in IL-5 and IL-10 (r = 0.703, P < 0.001) and those of IL-13 and IL-10 (r = 0.608, P < 0.001). These rank correlations demonstrate that reductions in the production of the three cytokines were generally occurring in cultures from the same donors.
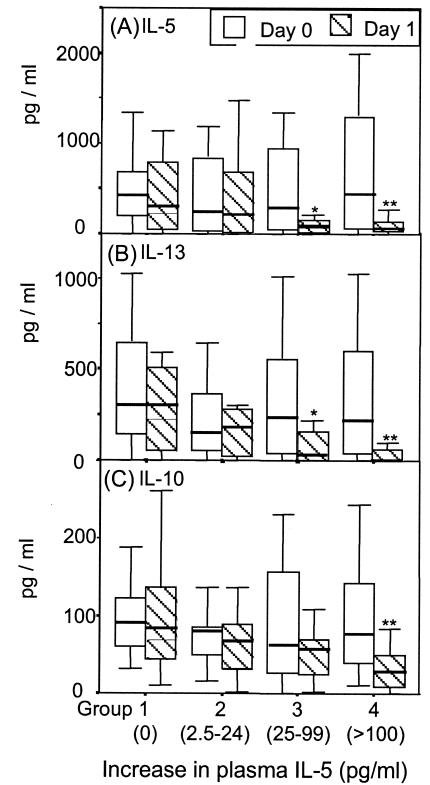
Analysis revealed that there was a relationship between the increase in plasma IL-5 level and the in vitro decrease in IL-5, IL-13, and IL-10 responses to SWA seen 1 day after treatment. This relationship is most clearly seen if the donors are arbitrarily divided into four equal groups (n = 16 for each group) based on their increase in plasma IL-5 level during the first day after treatment (Fig. 3). Group 1 showed no increase in plasma IL-5 level, group 2 showed an increase of up to 24 pg/ml, group 3 showed an increase of between 25 and 100 pg/ml, and group 4 showed an increase greater than 100 pg/ml.
FIG. 3.
Increase in plasma IL-5 level on day 1 posttreatment and the accompanying in vitro decrease in SWA-induced cytokines. Subjects were divided into four equal groups based on the increase in their plasma IL-5 levels between pretreatment and 1 day posttreatment (n = 16 per group). The IL-5 (A), IL-13 (B), and IL-10 (C) responses to SWA of the four groups in cultures established before treatment (open boxes) and at 1 day posttreatment (hatched boxes) are shown as box plots as defined in the legend to Fig. 2. Groups where there was a significant difference (Wilcoxon signed rank test) between pretreatment and 1 day posttreatment are indicated by ** (P = 0.001 to 0.01) and * (P = 0.01 to 0.05).
Those individuals with no change in plasma IL-5 level between pretreatment and 1 day posttreatment (group 1) demonstrated no change in in vitro production of IL-5 between the two time points (Fig. 3A). The individuals with the greatest increase in plasma IL-5 level in the 24 h after treatment (group 4) showed the greatest decrease in in vitro IL-5 production in response to SWA during the same period. There was a similar relationship between plasma IL-5 level and the in vitro IL-13 and IL-10 responses to SWA (Fig. 3B and C).
It should be noted that across the cohort there was no increase in plasma IL-13 or plasma IL-10 level at 1 day posttreatment (Table 1).
The increase in plasma IL-5 level is associated with a transient decrease in circulating eosinophil numbers.
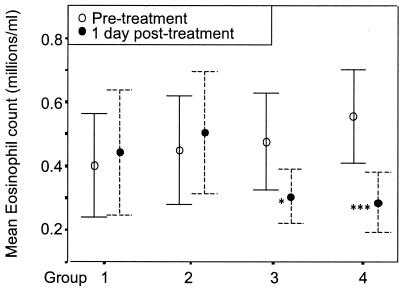
Median eosinophil count before treatment was 0.37 × 106/ml (25th to 75th percentile range = 0.23 × 106 to 0.66 × 106, n = 69). This dropped to 0.34 × 106 the day after treatment (P = 0.004, n = 64) and then rose to 0.78 × 106 by 21 days posttreatment (P < 0.001, n = 51). The elevated plasma IL-5 level at 1 day posttreatment was not correlated with the increase in eosinophil count 20 days later (r = 0.154). Plasma IL-5 level at 1 day posttreatment was, however, correlated with the drop in eosinophil numbers at day 1 (r = 0.507, P < 0.001, n = 63). There was an even stronger correlation (r = 0.559, P < 0.001, n = 63) between the drop in eosinophil numbers and the boost in plasma IL-5 level (day 1 values minus pretreatment values).
Figure 4 shows that the group with no increase in plasma IL-5 level (group 1) experienced no overall change in the number of circulating eosinophils while those with the largest increase (group 4) typically showed a pronounced drop in eosinophil number.
FIG. 4.
Increase in plasma IL-5 level and the accompanying decrease in eosinophil counts. Subjects were divided into four equal groups (n = 16 per group) based on the increase in their plasma IL-5 levels between pretreatment and 1 day posttreatment. Group 1 increase, 0 pg/ml; group 2, 2.5 to 24 pg/ml; group 3, 25 to 100 pg/ml; group 4, >100 pg/ml. The mean eosinophil counts ± 95% confidence limits at pretreatment and 1 day posttreatment are shown for the four groups. Groups where there was a significant difference (paired t test) between pretreatment and 1 day posttreatment are indicated by *** (P = 0.001 to 0.01) and * (P = 0.01 to 0.05).
The transient rise in plasma IL-5 level was associated with reduced IgE in response to SWA at 21 days.
No significant changes in antibody levels were detected between pretreatment values and 1 day posttreatment (results not shown).
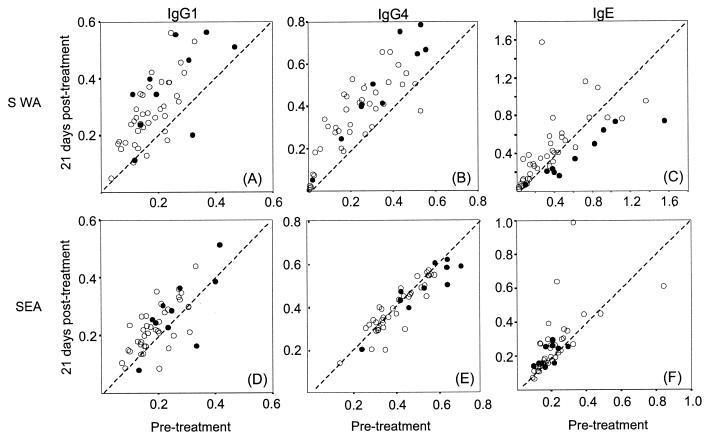
All donors had detectable SWA-specific IgG1 before treatment (n = 47), and over 70% showed an increase by 21 days (Fig. 5A). In a similar manner, more than 90% of individuals expressed IgG4 in response to SWA, and of these, over 80% had increased by 21 days (Fig. 5B). No relationship was found between either of these responses and the peak in plasma IL-5 level at 1 day posttreatment, and the responses of group 4 individuals were typical of the cohort as a whole (Fig. 5A and B, filled circles).
FIG. 5.
Antibody responses before and after treatment and the influence of the peak in plasma IL-5 level. Shown are scatter graphs of the pretreatment versus 21-day-posttreatment levels of IgG1, IgG4, and IgE directed against SWA and SEA. Each circle represents an individual volunteer (n = 51), and those in group 4 (first day posttreatment increase in plasma IL-5, >100 pg/ml) are indicated as filled circles. Levels were determined by isotype-specific ELISA, and the values represent means of triplicate optical density measurements.
Figure 5C shows changes in levels of IgE to SWA. More than 90% of the donors' plasma contained detectable SWA-specific IgE, but by 21 days posttreatment one-third had increased levels, one-third remained unchanged, and one-third had decreased levels. The changes in levels of SWA-specific IgE between pretreatment and 21 days posttreatment were negatively correlated with the elevated plasma IL-5 concentrations observed at 1 day posttreatment (r = −0.546, P < 0.001). In fact, of the donors in group 4 (plasma IL-5 increase of >100 pg/ml) all but one experienced a reduction in SWA-specific IgE.
The changes in levels of antibodies to SEA following treatment were much less marked. Levels of IgG4 to SEA both before and 3 weeks after chemotherapy were correlated with pretreatment intensity of infection (r = 0.646 and 0.633, respectively). The group 4 individuals, all having high intensities of infection, had higher SEA-specific IgG4 levels than did the rest of the cohort before treatment (P = 0.033), and this was not changed by chemotherapy (Fig. 5E).
The increase in plasma IL-5 level is linked with increased reinfection 20 months later.
Fecal samples were obtained from 45 of the original cohort members 20 months after treatment. Sixty-seven percent (n = 30) had been reinfected (7 to 2,677 eggs/g of feces). In those subjects who were reinfected, the intensity of reinfection was correlated with the increase in plasma IL-5 level observed at 1 day posttreatment (r = 0.439, P = 0.019).
DISCUSSION
Treatment of schistosomiasis induces marked changes in antibody (21, 44) and cytokine (20, 34) responses during the months following treatment, increasing IgE levels and other Th2 responses that have been associated with resistance to reinfection (10, 23, 34, 38, 39, 47). Little is known about events soon after disruption of the parasites in the bloodstream. However, these early events are likely to be complex, involving not only the release of inflammatory parasite antigens but also compensatory immune regulators such as prostaglandins, IL-10, and TGF-β.
In this study we have measured plasma cytokine levels in infected men 1 day after PZQ treatment, to determine whether changes would be apparent in the circulation at this time. We observed a marked transient increase in plasma IL-5 levels, most likely representing an in vivo release of IL-5 in response to the antigens discharged from damaged worms. Indeed, there was a strong relationship between the intensity of the original schistosome infection and the magnitude of the IL-5 release. This showed that the release was firmly linked to treatment for schistosomiasis rather than any other infection or condition and was not an effect of the drug per se.
Interestingly, there was no correlation between the levels of IL-5 seen in plasma 1 day posttreatment and the capacity of pretreatment blood cultures to produce IL-5 in response to worm antigen (SWA). It is possible that the SWA used to stimulate cultures did not contain the worm components responsible for the in vivo IL-5 response. However, it is also possible that the in vivo peak of IL-5 release was not produced by circulating blood cells. For example, human mast cells readily release a variety of mediators, including IL-5, in response to IgE receptor cross-linking (19, 26). In infected individuals the intestinal mucosa is rich in mast cells. Given the peri-intestinal location of adult worms and eggs, activation of mucosal mast cells may contribute to transient antigen-dependent IL-5 production after treatment. This is supported by the observation that, in mice infected with S. japonicum, there is rapid activation of mast cells following PZQ treatment (33).
Nutman and colleagues (18, 31) reported a similar peak in plasma IL-5 levels in the first few days after patients were treated for lymphatic filariasis with diethylcarbamazine. They also observed a decrease in circulating eosinophils immediately after treatment followed by an increase at least 4 days afterwards. IL-5 is a potent growth factor for eosinophils (40), and the authors linked the timing of the peak in IL-5 level to the timing of the subsequent eosinophilia. In the present study, the rise in plasma IL-5 level in the 24 h after PZQ treatment was not correlated with the elevated eosinophil counts 20 days later. It was, however, strongly correlated with the drop in blood eosinophil count observed 1 day after treatment. Thus, when intensity of infection was high, chemotherapy resulted in an IL-5 release in responsive individuals who also experienced a drop in eosinophil numbers. The sudden release of antigen in these subjects may have resulted not only in the binding of eosinophils to damaged parasites but also in their adhesion to the activated vascular endothelium. IL-5 can induce eosinophils to express β2 integrins and to adhere to vascular endothelial cells (2, 42), and thus the high concentration of IL-5 in plasma soon after treatment may have contributed to the adhesion and removal of these cells from the circulation.
Events in vivo shortly after treatment also influenced cytokine production by peripheral blood cells, resulting in reduced IL-5, IL-13, and IL-10 production in response to SWA. Interestingly, the extent of these decreases was correlated with the magnitude of the accompanying increase in plasma IL-5 level. There are two likely explanations for these observations. The first is that, as part of the inflammatory response following release of worm antigen, the leukocytes capable of producing these cytokines may have been temporarily removed from the circulation. Again, the extent of this response would have depended on the amount of antigen released and on how responsive an individual was to that release. The second possibility is that the cells producing IL-5, IL-13, and IL-10 may have been suppressed or depleted in vivo before they could be tested with antigen in vitro.
Pretreatment blood cultures produced IL-4, IL-5, IL-13, IFN-γ, IL-10, and TGF-β in response to SWA but were much less responsive to SEA. Adult S. mansoni worms can live in the human bloodstream for many years (15), and many antigens will be released only when the worm dies. Schistosome eggs, however, are repeatedly deposited in the host's tissues, where, although short-lived, they secrete soluble antigens. It is thought that this chronic exposure results in a hyporesponsiveness to egg antigens mediated by IL-10 and perhaps TGF-β (5, 9, 30).
Three weeks after treatment there were dramatic increases in the production of Th-2-type cytokines IL-5 and IL-13, in response to SWA but much less so to SEA. This supports our previous results from a study carried out in the neighboring Ugandan village of Booma (27a). There, when 187 infected people gave blood samples before and 7 weeks after PZQ treatment, IL-4, IL-5, and IL-13 responses to SWA but not to SEA were increased posttreatment. Worm antigens may be exposed only rarely in infected people, and chemotherapy is likely to release a pulse of this material into the bloodstream. This may be the stimulus for the marked increase in worm responsiveness weeks later.
In this study no relationships were found between any of the early responses and changes in cytokine production measured at 21 days. This may reflect the difficulty in studying the dynamic changes in the immune responses of human volunteers when it is not possible to include multiple time points. Nonetheless, it was possible to establish a link between the early peak in plasma IL-5 level and subsequent changes in IgE levels. That is, there was an inverse relationship between the increase in plasma IL-5 level and the levels of SWA-specific IgE 20 days later. In fact, in the subjects with the most pronounced peak in plasma IL-5 level, the levels of IgE against SWA actually decreased in the weeks following PZQ treatment. It is possible that a strong IL-5 response marked a group in whom the dynamics of the posttreatment boost in SWA-specific IgE were different from those of others. This may be interesting in the light of previous findings in Kenya (10) which indicated that people with the highest posttreatment levels of worm-specific IgE were more resistant to reinfection over the next 30 months and those with low levels were less resistant.
In the present study, the majority of those examined were shown to have been reinfected 20 months after treatment. In this relatively small group, no relationship was found between the posttreatment IgE levels and the intensity of reinfection nearly 2 years later. However, the intensity of this infection was positively correlated with the increase in plasma IL-5 levels that had been observed 1 day after treatment. Intriguingly, it may be that those subjects with a strong early systemic response to treatment (release of IL-5 and loss of circulating eosinophils) suffered more subsequent reinfection. These observations must be viewed with a certain degree of caution, however, as detailed water-contact studies were not performed during the reinfection period. Indeed, it is not clear why 33% of the subjects remained uninfected given that fishing in highly infectious water was virtually the only occupation open to members of the cohort. Further systematic reinfection studies will be needed to confirm or disprove our observations.
This study is the first to describe early perturbations in immune function following treatment for schistosomiasis in individuals living in an area of endemicity. We have shown that these early reactions are heterogeneous but that there are clear, strong relationships between schistosome infection and a number of important posttreatment immune responses. The heterogeneity in both acute responses and susceptibility to reinfection must be in some part due to differences in genetic background. Dessein and colleagues have demonstrated the importance of a particular gene locus (SM1) in dictating individual susceptibility by influencing T-cell differentiation and cytokine pathways (1, 32, 41). The observations on acute responses in the present study will direct future reinfection studies to further define different phenotypes in an attempt to better understand the mechanisms of acquired resistance to the condition.
Acknowledgments
We gratefully acknowledge Timothy Kamau for performing all of the phlebotomy and thank Maureen Laidlaw and Karen Plant for their skillful technical assistance.
This work was supported by the British Medical Research Council, the Wellcome Trust, and The Commission of the European Community, Science and Technology for Development Programme (INCO-DC contract IC18 CT97-0237 and INCO-DEV contract ICA4-CT-1999-10003).
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abel, L., F. Demenais, A. Prata, A. E. Souza, and A. Dessein. 1991. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am. J. Hum. Genet. 48:959-970. [PMC free article] [PubMed] [Google Scholar]
- 2.Bochner, B. S., and R. P. Schleimer. 1994. The role of adhesion molecules in human eosinophil and basophil recruitment. J. Allergy Clin. Immunol. 94:427-438. [DOI] [PubMed] [Google Scholar]
- 3.Brindley, P. J., and A. Sher. 1987. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. J. Immunol. 139:215-220. [PubMed] [Google Scholar]
- 4.Butterworth, A. E., M. Capron, J. S. Cordingley, P. R. Dalton, D. W. Dunne, H. C. Kariuki, G. Kimani, D. Koech, M. Mugambi, J. H. Ouma, M. A. Prentice, B. A. Richardson, T. K. Siongok, R. F. Sturrock, and D. W. Taylor. 1985. Immunity after treatment of human schistosomiasis mansoni. II. Identification of resistant individuals, and analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 79:393-408. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, P. J., I. Espinel, W. Paredes, R. H. Guderian, and T. B. Nutman. 1998. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J. Infect. Dis. 178:1133-1138. [DOI] [PubMed] [Google Scholar]
- 6.Demeure, C. E., P. Rihet, L. Abel, M. Ouattara, A. Bourgois, and A. J. Dessein. 1993. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J. Infect. Dis. 168:1000-1008. [DOI] [PubMed] [Google Scholar]
- 7.Dessein, A. J., P. Couissinier, C. Demeure, P. Rihet, S. Kohlstaedt, D. Carneiro-Carvalho, M. Ouattara, V. Goudot-Crozel, H. Dessein, A. Bourgois, L. Abel, E. M. Carvallo, and A. Prata. 1992. Environmental, genetic and immunological factors in human resistance to Schistosoma mansoni. Immunol. Investig. 21:423-453. [DOI] [PubMed] [Google Scholar]
- 8.Doenhoff, M. J., A. A. Sabah, C. Fletcher, G. Webbe, and J. Bain. 1987. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Trans. R. Soc. Trop. Med. Hyg. 81:947-951. [DOI] [PubMed] [Google Scholar]
- 9.Doetze, A., J. Satoguina, G. Burchard, T. Rau, C. Loliger, B. Fleischer, and A. Hoerauf. 2000. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 12:623-630. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, D. W., A. E. Butterworth, A. J. C. Fulford, H. C. Kariuki, J. G. Langley, J. H. Ouma, A. Capron, R. J. Pierce, and R. F. Sturrock. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483-1494. [DOI] [PubMed] [Google Scholar]
- 11.Dunne, D. W., and A. Montford. 1998. Resistance to infection in human and animal models, p. 133-212. In A. A. F. Mahmoud (ed.), Schistosomiasis. Imperial College Press, London, United Kingdom.
- 12.El Ridi, R., C. B. Shoemaker, F. Farouk, N. H. El Sherif, and A. Afifi. 2001. Human T- and B-cell responses to Schistosoma mansoni recombinant glyceraldehyde 3-phosphate dehydrogenase correlate with resistance to reinfection with S. mansoni or Schistosoma haematobium after chemotherapy. Infect. Immun. 69:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etard, J. F., M. Audibert, and A. Dabo. 1995. Age-acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. Am. J. Trop. Med. Hyg. 52:549-558. [DOI] [PubMed] [Google Scholar]
- 14.Fallon, P. G., P. Smith, and D. W. Dunne. 1998. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. Eur. J. Immunol. 28:1408-1416. [DOI] [PubMed] [Google Scholar]
- 15.Fulford, A. J., A. E. Butterworth, J. H. Ouma, and R. F. Sturrock. 1995. A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitology 110:307-316. [DOI] [PubMed] [Google Scholar]
- 16.Fulford, A. J. C., M. Webster, J. H. Ouma, G. Kimani, and D. W. Dunne. 1998. Puberty and age-changes in susceptibility to schistosome infection. Parasitol. Today 14:23-26. [DOI] [PubMed] [Google Scholar]
- 17.Giboda, M., and J. M. Smith. 1994. Schistosoma mansoni eggs as a target for praziquantel: efficacy of oral application in mice. J. Trop. Med. Hyg. 97:98-102. [PubMed] [Google Scholar]
- 18.Gopinath, R., L. E. Hanna, V. Kumaraswami, V. Perumal, V. Kavitha, V. Vijayasekaran, and T. B. Nutman. 2000. Perturbations in eosinophil homeostasis following treatment of lymphatic filariasis. Infect. Immun. 68:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon, J. R., P. R. Burd, and S. J. Galli. 1990. Mast cells as a source of multifunctional cytokines. Immunol. Today 11:458-464. [DOI] [PubMed] [Google Scholar]
- 20.Grogan, J. L., P. G. Kremsner, A. M. Deelder, and M. Yazdanbakhsh. 1996. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobium infection. Eur. J. Immunol. 26:1365-1370. [DOI] [PubMed] [Google Scholar]
- 21.Grogan, J. L., P. G. Kremsner, G. J. van Dam, W. Metzger, B. Mordmuller, A. M. Deelder, and M. Yazdanbakhsh. 1996. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J. Infect. Dis. 173:1242-1247. [DOI] [PubMed] [Google Scholar]
- 22.Hagan, P., U. J. Blumenthal, M. Chaudri, B. M. Greenwood, R. J. Hayes, J. Hodgson, C. Kelly, M. Knight, A. J. G. Simpson, S. R. Smithers, and H. A. Wilkins. 1987. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 81:938-946. [DOI] [PubMed] [Google Scholar]
- 23.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 24.Harnett, W., and J. R. Kusel. 1986. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology 9:401-405. [DOI] [PubMed] [Google Scholar]
- 25.Harnett, W. 1988. The antihelminthic action of praziquantel. Parasitol. Today 4:144-146. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe, J. S., M. C. Glaum, D. G. Raible, T. J. Post, E. Dimitry, D. Govindarao, Y. Wang, and E. S. Schulman. 1995. Human lung mast cell IL-5 gene and protein expression: temporal analysis of upregulation following IgE-mediated activation. Am. J. Respir. Cell Mol. Biol. 13:665-675. [DOI] [PubMed] [Google Scholar]
- 27.Joseph, S., F. M. Jones, G. Kimani, J. K. Mwatha, T. Kamau, F. Kazibwe, J. Kemijumbi, N. B. Kabatereine, M. Booth, H. C. Kariuki, J. H. Ouma, B. J. Vennervald, and D. W. Dunne. 2004. Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect. Immun. 72:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Joseph, S., F. M. Jones, K. Walter, A. J. Fulford, G. Kimani, J. K. Mwatha, T. Kamau, H. C. Kariuki, F. Kazibwe, E. Tukahebwa, N. B. Kabatereine, J. H. Ouma, B. J. Vennervald and D. W. Dunne. Increases in human Th2 cytokine responses to Schistosoma mansoni worm and worm tegument antigens are specifically induced by treatment with Praziquantel. J. Infect. Dis., in press. [DOI] [PubMed]
- 28.Kabatereine, N. B., B. J. Vennervald, J. H. Ouma, J. Kemijumbi, A. E. Butterworth, D. W. Dunne, and A. J. Fulford. 1999. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology 118:101-105. [DOI] [PubMed] [Google Scholar]
- 29.Kimani, G., C. N. Chung, G. Gachihi, T. Kamau, B. Mungai, and G. M. Mkoji. 1992. Immune responses after treatment for Schistosoma mansoni infections. Scand. J. Immunol. Suppl. 11:29-33. [DOI] [PubMed] [Google Scholar]
- 30.King, C. L., A. Medhat, I. Malhotra, M. Nafeh, A. Helmy, J. Khaudary, S. Ibrahim, M. El-Sherbiny, S. Zaky, R. J. Stupi, K. Brustoski, M. Shehata, and M. T. Shata. 1996. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J. Immunol. 156:4715-4721. [PubMed] [Google Scholar]
- 31.Limaye, A. P., E. A. Ottesen, V. Kumaraswami, J. S. Abrams, J. Regunathan, V. Vijayasekaran, K. Jayaraman, and T. B. Nutman. 1993. Kinetics of serum and cellular interleukin-5 in posttreatment eosinophilia of patients with lymphatic filariasis. J. Infect. Dis. 167:1396-1400. [DOI] [PubMed] [Google Scholar]
- 32.Marquet, S., L. Abel, D. Hillaire, H. Dessein, J. Kalil, J. Weissenbach, and A. J. Dessein. 1996. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 14:181-184. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto, J., and H. Matsuda. 2002. Mast-cell-dependent histamine release after praziquantel treatment of Schistosoma japonicum infection: implications for chemotherapy-related adverse effects. Parasitol. Res. 88:888-893. [DOI] [PubMed] [Google Scholar]
- 34.Medhat, A., M. Shehata, K. Bucci, S. Mohamed, A. D. Dief, S. Badary, H. Galal, M. Nafeh, and C. L. King. 1998. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobium adult worm antigens correlates with lack of reinfection after treatment. J. Infect. Dis. 178:512-519. [DOI] [PubMed] [Google Scholar]
- 35.Mehlhorn, H., B. Becker, P. Andrews, H. Thomas, and J. K. Frenkel. 1981. In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung 31:544-554. [PubMed] [Google Scholar]
- 36.Naus, C. W., G. Kimani, J. H. Ouma, A. J. Fulford, M. Webster, G. J. van Dam, A. M. Deelder, A. E. Butterworth, and D. W. Dunne. 1999. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect. Immun. 67:3444-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redman, C. A., A. Robertson, P. G. Fallon, J. R. Modha, M. Kusel, M. J. Doenhoff, and R. J. Martin. 1996. Praziquantel: an urgent and exciting challenge. Parasitol. Today 12:14-20. [DOI] [PubMed] [Google Scholar]
- 38.Rihet, P., C. E. Demeure, A. Bourgois, A. Prata, and A. J. Dessein. 1991. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 21:2679-2686. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, M., A. E. Butterworth, G. Kimani, T. Kamau, A. J. C. Fulford, D. W. Dunne, J. H. Ouma, and R. F. Sturrock. 1993. Immunity after treatment of human schistosomiasis: association between responses and resistance to reinfection. Infect. Immun. 61:4984-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roboz, G. J., and S. Rafii. 1999. Interleukin-5 and the regulation of eosinophil production. Curr. Opin. Hematol. 6:164-168. [DOI] [PubMed] [Google Scholar]
- 41.Rodrigues, V., Jr., K. Piper, P. Couissinier-Paris, O. Bacelar, H. Dessein, and A. J. Dessein. 1999. Genetic control of schistosome infections by the SM1 locus of the 5q31-q33 region is linked to differentiation of type 2 helper T lymphocytes. Infect. Immun. 67:4689-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedgwick, J. B., S. F. Quan, W. J. Calhoun, and W. W. Busse. 1995. Effect of interleukin-5 and granulocyte-macrophage colony stimulating factor on in vitro eosinophil function: comparison with airway eosinophils. J. Allergy Clin. Immunol. 96:375-385. [DOI] [PubMed] [Google Scholar]
- 43.Sturrock, R. F., A. E. Butterworth, and V. Houba. 1976. Schistosoma mansoni in the baboon (Papo anubis): parasitological responses of Kenya baboons to different exposures of a local parasite strain. Parasitology 73:239-252. [DOI] [PubMed] [Google Scholar]
- 44.Webster, M., P. G. Fallon, A. J. Fulford, A. E. Butterworth, J. H. Ouma, G. Kimani, and D. W. Dunne. 1997. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 19:333-335. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins, H. A., U. J. Blumenthal, P. Hagan, R. J. Hayes, and S. Tulloch. 1987. Resistance to reinfection after treatment of urinary schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 81:29-35. [DOI] [PubMed] [Google Scholar]
- 46.Wu, Z., S. Zhang, B. Pan, L. Hu, R. Wei, Z. Gao, J. Li, and U. Brinkman. 1994. Reinfection with Schistosoma japonicum after treatment with praziquantel in Poyang Lake region, China. Southeast Asian J. Trop. Med. Public Health 25:163-169. [PubMed] [Google Scholar]
- 47.Zhang, Z. S., H. W. Wu, S. C. Chen, L. S. Hu, Z. W. Xie, Y. X. Qiu, C. Su, J. P. Cao, Y. P. Wu, S. J. Zhang, and G. L. Wu. 1997. Association between IgE antibody against soluble egg antigen and resistance to reinfection with Schistosoma japonicum. Trans. R. Soc. Trop. Med. Hyg. 91:606-608. [DOI] [PubMed] [Google Scholar]