Abstract
We report the case of a young woman diagnosed with metastatic urachal carcinoma. A multimodal approach was used for the management of this patient. Due to disease progression despite surgery and two different chemotherapy regimens (neoadjuvant capecitabine + irinotecan + oxaliplatin and docetaxel + cisplatin after surgery), treatment with sunitinib was eventually started. Treatment with sunitinib resulted in stable disease and improvement of symptoms. Sunitinib was discontinued due to the occurrence of metrorrhagia, and restarted one week later. Disease eventually progressed and the patient died 18 months after the onset of symptoms. This is the first report on the use of sunitinib for the management of urachal carcinoma and provides initial evidence supporting the use of targeted therapy in this setting.
Key words: sunitinib, targeted therapies, urachal adenocarcinoma
Introduction
The urachal ligament, an embryologic remnant, connects the dome of the bladder to the umbilicus via the ligamentum commune; it is the main excretory organ of the fetus and it is still present in all newborns, then gradually degenerates into a single fibrous band.1 In approximately 30% of the general population, the urachal remnant may persist with tubular or cystic structures consisting of mucosa, connective tissue and smooth muscle. Tumors of the urachal ligament are extremely rare, accounting for 0.2% of all bladder cancers.2 These tumors are usually diagnosed at an advanced stage due to the extravescical growth and the lack of symptoms during early disease. Symptoms often occur only after the disease has progressed, the most common clinical presentation being gross hematuria and,3 at more advanced stages, abdominal pain and/or development of abdominal mass. Adenocarcinoma is the most common histological type and accounts for over 90% of all cases, the enteric type being the most frequent subtype.4 Two different staging systems have been described for urachal cancers, i.e. the Sheldon system and the Mayo system (Table 1): these systems predict cancer-specific mortality equally well.5 The diagnostic approach should include computed tomography (CT) or magnetic resonance imaging (MRI) evaluation of the abdomen and pelvis; a cystoscopy is crucial for precisely localizing and performing biopsies of the tumor, since most lesions are located in the dome and anterior wall of the bladder. Since the embryological origin of the urachus is the same as the colon, and most urachal carcinomas are adenocarcinomas, an elevation in tumor markers associated with gastrointestinal tumors, including carcinoembryonic antigen (CEA), CA 125 and CA 19.9, is common.6,7 The primary therapeutic approach is surgical resection with partial or radical cystectomy and en bloc resection of the urachal ligament with the umbilicus and bladder.8 At present, no guidelines or standard of care for the management of this tumor in local and/or advanced disease exist, mainly due to the infrequency of this cancer. Available information on the treatment of this cancer is mainly derived from case reports, and therefore we believe that it is of great importance to make the experiences on the management of patients with urachal ligament carcinoma available to clinicians facing this rare malignancy. We report a case of metastatic urachal carcinoma treated with multimodal approach (surgery, chemotherapy and targeted therapy). Considering the lack of guidelines and clinical experience in this disease a discussion in a multidisciplinary team was made in order to select a treatment oriented on the patient and disease.
Table 1.
Urachal cancer staging systems.
| Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|
| Sheldon staging system | Urachal cancer confined to urachal mucosa | Urachal cancer with invasion confined to urachus itself | Local urachal cancer extension to: A) bladder; B) abdominal wall; C) peritoneum | Metastatic urachal cancer to: A) lymph nodes; B) distant sites |
| Mayo staging system | Tumor confined to the urachus and/or bladder | Tumor extending beyond the muscular layer of the urachus and/or the bladder | Tumors infiltrating the regional lymph nodes | Tumor infiltrating nonregional lymph nodes or other distant sites |
Case Report
Presentation of case and initial assessment
On November 2009 a 33-year old woman with no significant previous medical history was referred to her gynecologist due to complaints of pelvic pain. A right ovarian cyst was diagnosed upon examination. However, due to persistent pain, a CT scan was performed that revealed a right pelvic mass. On 12 November 2009 the patient’s gynecologist performed laparoscopic surgery during which a sub peritoneal lesion likely to start from the bladder was found. The mass was removed but ruptured during surgery, with intraoperative spillage of mucinous material. A cystoscopy was performed postoperatively, which showed a reddish lesion of the dome of the bladder. The intraoperative histological diagnosis was mucinous adenocarcinoma. This was then confirmed by the final histological examination.
Further assessment at a referral center
The patient was referred to our hospital, Istituto Nazionale Tumori (National Tumors Institute), Milan, Italy, a referral center for the treatment of oncological disease in Italy, and a histological review was performed by our genitourinary pathology expert. The immunohistochemical analysis was positive for CDX-2 and CK20 and negative for CK 7, suggesting a diagnosis of mucinous adenocarcinoma originating from the urachal ligament.3,9 We then performed a whole body CT scan that showed two metastases in the right lung, one at the lower lobe and one in the middle lobe, with a diameter of 15.6 and 8.5 mm, respectively, and one lesion anterior to the bladder wall and the dome that extended through the bladder wall, protruding into the lumen. Serum CEA was 15.39 ng/mL (normal <5), CA 19.9 was 70.1 U/mL (normal <37), CA 125 negative, CA 15.3 negative.
Management
We discussed the case of the patient with the urological surgeon and, given the extension of the disease, we decided to treat the patient with systemic chemotherapy rather than performing surgery.
The histological type, the strong mucinous component and the phenotypic similarities with a cancer of gastroenteric origin, rather than urothelial, prompted us to use the association of three drugs: irinotecan 180 mg/m2 (300 mg tot.) on day 1, oxaliplatin 85 mg/m2 (145 mg tot.) on day 2 and capecitabine 2000 mg/m2/day (days 2-6); cycles were repeated every 2 weeks. This association was undertaken based on the clinical experience of our group in gastrointestinal tumors.10
We started this chemotherapy regimen on 16 Dec 2009 and continued for 6 cycles, until 03 March 2010, with evidence of radiologically stable disease on CT scans after 3 and 6 cycles, and biochemical response (CA 19.9: 15.2 U/mL, CEA 1.59 ng/mL).
Treatment was well tolerated, except for nausea (G2) and neutropenia (G2). Since the second cycle, granulocyte colony-stimulating factor (G-CSF) for secondary prophylaxis was administered on days 8-13 of each cycle.
In light of the young age of the patient and the stabilization of the disease we decided to reconsider the surgical approach, after a pre-operative abdomen MRI was performed (Figure 1). On 06 April 2010 umbilical resection with the bladder dome and the urachal remnant was performed (Figure 2). No postoperative complications arose.
Figure 1.
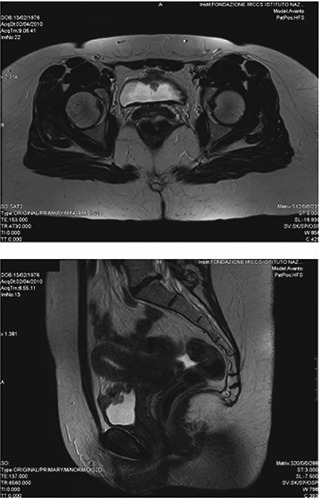
Abdominal magnetic resonance imaging (pre-surgery).
Figure 2.
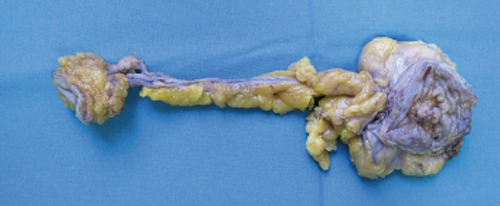
Macroscopic tumor sample.
The final histology report confirmed the diagnosis of urachal adenocarcinoma with mucinous components (CDX-2 positive, CK 20 positive, CK 7 negative, CK 34 beta E12 negative, beta-catenin positive); surgery was radical, with negative surgical margins. Follow up was then activated (Figure 3).
Figure 3.
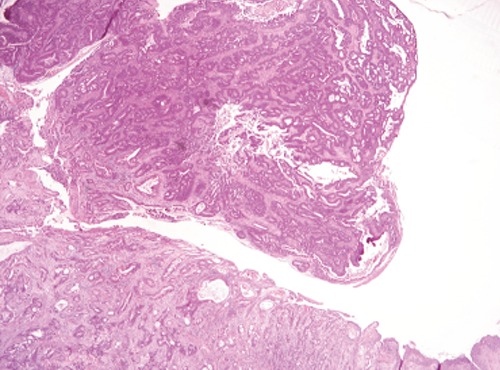
Histopathological preparation (Haematoxylin and Eosin). Urachal mucinous adenocarcinoma.
The CT scan performed after two months showed the presence of two right lung nodules.
On 17 June 2010 the patient underwent precision resection of the 3 right lung nodules (for 2 of them, the CT scan was positive for metastasis from urachal carcinoma; the third, supposed by the surgeon to be malignant, resulted benign fibrotic parenchymal tissue). Surgery was radical and then follow up was started. A subsequent CT scan (24 August 2010) was negative for metastatic disease, although some small lesions, equivocal but suggestive of peritoneal involvement, were detected. Given both the absence of symptoms and the negativity of serum markers we decided, in agreement with the patient, to proceed only with clinical and radiological follow up. A CT scan performed after three months showed peritoneal disease in close contact with the uterus and bladder wall. Serum markers were elevated (CEA: 174 ng/mL, CA 19.9: 321 U/mL).
The patient was symptomatic for abdominal pain, therefore transdermal therapy with fentanyl (50 mcg/h) was started. Since the disease was progressing, we considered starting a second-line chemotherapy. Among the few chemotherapeutic regimens tested in this rare tumor,8 we chose a platinum/taxane combination. On 01 December 2010, we started chemotherapy with cisplatin (75 mg/m2: 130 mg tot.) and docetaxel 75 mg/m2 (130 mg tot.) day 1, q 21 for three cycles (until 18 January 2011). Primary prophylaxis with a single subcutaneous injection of pegfilgrastim was also performed. Treatment was well tolerated, with only nausea (G2) as adverse event; however, a CT scan performed after the end of the third cycle showed evidence of abdominal disease progression. Within few weeks since the end of chemotherapy, the patient reported moderate asthenia. Physical examination revealed paleness and a palpable, hard central pelvic mass (maximum diameter 5 cm). Hematological tests showed acute anemia (Hb 7.3 g/dL); the patient received transfusion of 2 units of red blood cells (05 February 2011) with return to satisfactory Hb values (Hb 10.5 g/dL on 08 February 2011).
The disease was progressing despite chemotherapy and surgery on both primary and metastatic sites. Nevertheless, the patient was in relatively good clinical conditions: vital signs were within the normal range and organ functions, as assessed by blood laboratory tests, were good. Given the young age of the patient, who was also a mother of two kids, we decided to try another treatment and to initiate off label therapy with the multikinase inhibitor sunitinib. On 23 February 2011 we started therapy with sunitinib at the dose of 25 mg continuously, with close clinical and hematological monitoring; after two weeks since the start of treatment the patient reported feeling better, with improvement of pain, no adverse events and good hematological profile. After about 50 days, analgesic treatment was discontinued because of pain disappearance; we performed a CT scan that showed stable disease, with evidence of necrotic evolution of the abdominal mass, which was also smaller and softer upon physical examination. The patient received sunitinib until May 2011, when she developed metrorrhagia. For this reason, sunitinib therapy was discontinued for seven days, with resolution of bleeding but also a prompt recurrence of abdominal pain. Treatment with sunitinib was started again, but metrorrhagia persisted. A radiation oncology consult was obtained, and hemostatic radiotherapy (total dose: 15 Gy in 3 fraction) was administered, with control of bleeding.
The patient continued sunitinib until 10 July 2011, when symptoms of intestinal obstruction appeared; treatment was finally stopped and the patient started only supportive care at home. She died on 29 July 2011.
Discussion
Urachal carcinomas are rare, and there is paucity of data on the best chemotherapy regimen for the treatment of this disease. Since the commonest histologic subtype of urachal carcinoma is adenocarcinoma with enteric features,3 chemotherapy regimens used to treat gastrointestinal tumors, i.e. 5-fluorouracil and taxanes, are commonly used in the management of this malignancy.8 Other drugs that have been used in this setting include cisplatin, methotrexate, vinblastine and gemcitabine.8 Although surgery is generally recommended for urachal carcinoma, due to the extension of disease we did not perform curative surgery initially, and we decided to administer systemic chemotherapy with capecitabine, irinotecan and oxaliplatin, a regimen used for the treatment of metastatic colon cancer.11 The patient exhibited a marker response to this regimen, which also resulted in radiological stabilization of disease. Umbilical resection with removal of the bladder dome and urachal remnant was then performed, followed by resection of lung metastases. However, few months after surgery we observed recurrence of disease, with elevation of serum markers and peritoneal spread that did not respond to chemotherapy with cisplatin and docetaxel. Peritoneal dissemination of urachal adenocarcinoma is a rarely encountered aggressive facet of this disease, observed typically with the mucinous subtype.12 Furthermore, the possibility that peritoneal spread was due to tumor rupture during the first surgery cannot be excluded. Given the young age of the patient and the relatively good clinical conditions despite disease progression, we initiated off-label treatment with sunitinib. We chose sunitinib because of its great efficacy in other advanced malignancies and good safety profile.13,14 We started with a reduced dose, to test tolerability. To the best of our knowledge, this is the first report on the use of sunitinib for the treatment of urachal carcinoma. Few, scattered reports are available on the use of targeted therapies for this malignancy.8 Sunitinib is a multikinase inhibitor indicated for the treatment of advanced malignant gastrointestinal stromal tumors, renal cell metastatic carcinoma and progressive, well-differentiated pancreatic neuroendocrine tumors. Sunitinib inhibits the vascular endothelial growth factor (VEGF) receptor, platelet-derived growth factor (PDGF) receptor, and c-Kit in addition to other kinases.15 In the present case, treatment with sunitinib resulted in partial necrosis of the tumor that was paralleled by an improvement of symptoms. Unfortunately, treatment had to be stopped due to the occurrence of metrorrhagia. Bleeding is an established adverse effect of sunitinib,16 but whether metrorrhagia was due to treatment or to the disease itself cannot be conclusively determined, since eventually the tumor infiltrated the uterus. Although sunitinib was started again after seven days, the temporary suspension of treatment may have limited the efficacy of the drug. However, published data suggest that the median survival of patients with urachal carcinoma ranges from 12 to 24 months.5,17 In the present case, treatment with sunitinib noticeably delayed the progression of disease, leading to an overall survival of 18 months since the onset of symptoms and to rapid symptomatic benefit.
Conclusions
The case discussed here indicates that targeted therapy with sunitinib might have a role in the management of patients with advanced urachal carcinoma. However, this is the first report on the use of sunitinib in this setting, and the indications and clinical benefits of this strategy remain to be determined. Multi-institutional collaborations will be important to explore the impact of targeted treatments in the management of urachal carcinoma.
Acknowledgments
Editorial assistance for the preparation of this manuscript was provided by Luca Giacomelli, PhD, and was supported by internal funds.
References
- 1.Begg RC. The urachus: its anatomy, histology and development. J Anatom 1930;64: 170-8. [PMC free article] [PubMed] [Google Scholar]
- 2.Bruins HM, Visser O, Ploeg M, et al. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol 2012;188:1102-7. [DOI] [PubMed] [Google Scholar]
- 3.Gopalan A, Sharp DS, Fine SW, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009;33:659-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984;131:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer 2006;107:712-20. [DOI] [PubMed] [Google Scholar]
- 6.Guarnaccia S, Pais V, Grous J, Spirito N. Adenocarcinoma of the urachus associated with elevated levels of CA 125. J Urol 1991;145:140-1. [DOI] [PubMed] [Google Scholar]
- 7.Kikuno N, Urakami S, Shigeno K, et al. Urachal carcinoma associated with increased carbohydrate antigen 19-9 and carcinoembryonic antigen. J Urol 2001;166:604. [PubMed] [Google Scholar]
- 8.Siefker-Radtke A. Urachal adenocarcinoma: a clinician’s guide for treatment. Semin Oncol 2012;39:619-24. [DOI] [PubMed] [Google Scholar]
- 9.Lee W. Urachal adenocarcinoma metastatic to the ovaries resembling primary ovarian mucinous carcinoma: a case report with the immunohistochemical study. Int J Clin Exp Pathol 2010;4:118-23. [PMC free article] [PubMed] [Google Scholar]
- 10.Bajetta E, Verzoni E, Ferrario E, et al. Feasibility study of biweekly capecitabine, oxaliplatin, and irinotecan in patients with untreated advanced gastric cancer. Tumori 2009;95:43-7. [DOI] [PubMed] [Google Scholar]
- 11.Zarate R, Rodriguez J, Bandres E, et al. Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): a dose-finding study and pharmacogenomic analysis. Br J Cancer 2010;102:987-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugarbaker PH, Verghese M, Yan TD, Brun E. Management of mucinous urachal neoplasm presenting as pseudomyxoma peritonei. Tumori 2008;94:732-6. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Engl J Med 2007;356:115-24. [DOI] [PubMed] [Google Scholar]
- 14.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. New Engl J Med 2011;364:501-13. [DOI] [PubMed] [Google Scholar]
- 15.Mena AC, Pulido EG, Guillen-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs 2010;21:S3-11. [DOI] [PubMed] [Google Scholar]
- 16.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol 2009;10:967-74. [DOI] [PubMed] [Google Scholar]
- 17.Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: the M. D. Anderson Cancer Center experience. J Urol 2003;169:1295-8. [DOI] [PubMed] [Google Scholar]


