Abstract
Prostatic stromal sarcoma (PSS) is a rare tumor that normally occurs in adult age. Its management relies mainly on surgery. We report the first case of PSS occurring in an adolescent. There was evidence of a good response to chemotherapy including ifosfamide, doxorubicin, vincristine and actinomycin-D, although the final outcome was dismal. A review of the English literature revealed 14 additional patients with PSS treated with chemotherapy: tumor shrinkage was reported in 4 of the 6 evaluable patients. Patients with PSS may benefit from the use of chemotherapy in combination with early aggressive local treatment.
Key words: stromal sarcoma, specialized prostatic stroma, chemotherapy, prostatic neoplasm
Introduction
Prostatic stromal sarcoma (PSS) is a rare spindle cell lesion that arises from mesenchymal pluripotent stem cells in the prostatic stroma. It accounts for 0.1-0.2% of all prostatic cancers and typically affects adults.1,2 It can be distinguished from the more indolent lesions called stromal tumors of uncertain malignant potential (STUMP) on the basis of a marked increase in cellularity and the presence of mitotic figures, necrosis and cellular atypia. Immunohistochemistry is positive for progesterone receptor and CD34, helping to differentiate it from other tumors of muscular origin.2
Prostatic stromal sarcoma is an aggressive disease capable of metastasizing to the lung, liver, and bone.3-8 Complete resection has been recommended by several authors but little information is available on any associated treatments.2,9 Chemotherapy has rarely been used, so its role in the treatment of PSS is still not clear.
To the best of our knowledge, no cases of PSS in adolescence have been published so far. This report describes a case occurring in a young patient, with a review of the literature and comments on the possible utility of chemotherapy in the treatment of this rare disease.
Case Report
A 14-year-old boy with a medical history characterized only by an operation at one year of age for pulmonary cysts was referred to his local hospital for dysuria, increased urinary frequency followed by urinary retention. He underwent urgent cystoscopy with removal of clots from the bladder and a biopsy of prostatic areas that looked abnormal. An inflammatory myofibroblastic tumor was initially diagnosed. A computed tomography (CT) scan showed a heterogeneous lesion occupying the prostate and extending to the seminal vesicles. The patient was transferred to our hospital where a new transurethral biopsy was performed. Microscopic examination showed a mesenchymal lesion characterized by elongated cells with slender eosinophilic cytoplasm and oval angulated nuclei with finely dispersed cromatin. A focal storiform pattern could be seen. Some areas were more densely cellular with evidence of a rich vascular network and interspersed glandular structures corresponding to prostatic glands entrapped in the proliferation. In other areas the cellularity decreased with evidence of a loose myxoid stroma containing small fascicles of cells haphazardly arranged. Moreover scattered atypical cells, with remarkable pleomorphism were evident. Mitotic rate was very high (in some fields more than 10 mitoses/10HPF). Foci of necrosis were also found. No features of phyllodes tumor were seen. The lesion was extensively sampled and no evidence of round cell morphology was seen. Immunohistochemically, the tumor cells were focally reactive for desmin, smooth muscle actin, caldesmon, CD34, and progesterone receptor (40%), while no Myf4, CD117, S100, EMA, or CK7 immunoreactivity was apparent. Reverse-transcriptase polymerase chain reaction (RT-PCR) for MyoD and Myogenin was negative (Figure 1). These morphological and immunohistochemical features led to a final diagnosis of PSS.
Figure 1.
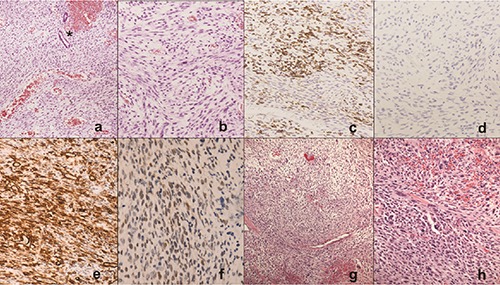
Prostatic stromal sarcoma: histological features. a) The tumor contained elongated-spindle cells with a rich vascular network and two prostatic glands (*) embedded in the proliferation. b) At higher power, cytologic atypia and mitoses were seen. c) Desmin immunostaining showed some positive cells. d) Myogenin was negative. Strong and diffuse immunostaining for CD34 (e) and nuclear staining for progesterone receptor f) in more than 40% of cells. g) Scattered cells with hypercromic nuclei and occasional multinucleated cells. h) Higher power highlights the cytologic detail. In the center a multinucleated cell simulating a rhabdomyoblast.
A CT scan and pelvic magnetic resonance imaging (MRI) revealed a 6x5x6.5 cm prostatic mass infiltrating the bladder, seminal vesicles and rectal wall (Figure 2) and extending to a 3.5 cm paravesical lymph node. MRI scan also documented a 1 cm bone T1-hypointense and T2-hyperintense area with intense contrastenhancement located in the left acetabulum that corresponded to a rarefaction area in CT scan. This lesion was also documented by PET (positron emission tomography). The radiological metastatic nature of this lesion was not histologically confirmed by a biopsy.
Figure 2.
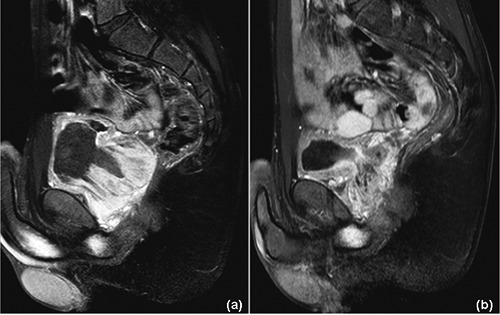
Prostatic stromal sarcoma: magnetic resonance imaging at diagnosis (a), and after 3 cycles of chemotherapy (b) (T1-fat-suppressed sequences with contrast enhancement). a) Non-homogeneous, vascularized mass (6×6×6.5 cm) originating from the prostate and extending into the bladder and seminal vesicles with signs of rectal wall infiltration. b) Significant shrinkage is evident (2×2×6.5 cm), with an estimated volume reduction of approximately 85%.
Complete tumor resection was considered impossible without important functional sequelae such as fecal/urinary incontinence or impotence so we decided to administer chemotherapy in an effort to reduce the tumor volume and allow a more conservative surgery. The patient’s young age was also considered to make this decision. After 3 cycles of chemotherapy according to the IVADo regimen (ifosfamide 3 gr/m2/day on days 1 and 2; vincristine 1.5 mg/m2/day on days 1,8, and 15; actinomycin 1.5 mg/m2/day on day 1; and doxorubicin 40 mg/m2/day on days 1 and 2). MRI showed a reduction of approximately 85% of the initial volume (Figure 2) and the paravesical lymph node was no longer visible. The bone lesion persisted unmodified after the initial 3 cycles (but its enhancement was reduced on MRI after the 9th chemotherapy cycle). Given this good response, the patient continued with the same treatment plus radiotherapy (50.4 Gy). After 9 cycles of chemotherapy, a prostate biopsy demonstrated persistent disease.
Radical cystoprostatectomy with ileal neobladder construction was planned, but at the time of surgery the tumor was found more extensive than it had appeared from a preoperative MRI. The bladder, perineal muscle and rectum infiltration made only partial tumor resection possible. Few weeks after surgery the patient clinical condition worsened and local tumor progression was evident on ultrasound. Only palliative treatments were administered and the patient died 15 months after diagnosis.
Discussion
PSS is a highly aggressive tumor arising from the specialized stroma of the prostatic parenchyma. PSS have been reported in adults aged in 25-86 years (median 55 years).2,9 To our knowledge, our patient - 14 years old at diagnosis - is the youngest case to be reported to date. More frequent soft tissue sarcomas of adolescents were discarded in our case. Rhabdomyosarcoma was excluded by the lack of rhabdomyoblastic differentiation and negative staining for MyoD and myogenin, which was also confirmed by RT-PCR. An inflammatory myofibroblastic tumor was excluded by the absence of a predominant inflammatory infiltrate and the frankly malignant cytologic features observed in some areas: moreover ALK-1 staining was negative, and hormonal receptors and CD34 were positive.
The most challenging differential diagnosis is represented by leiomyosarcoma, being their cytologic features and architectural pattern very similar. Immunostains play a crucial role in the final diagnosis. In our case, the positive staining for progesterone receptors and CD34 supported the diagnosis of PSS. Although most of PSS are negative for SMA and may express only very focally desmin, these are expressed in the prostatic stromal proliferations of uncertain potential, thus, in our opinion, their focal positivity in our case do not contrast with the diagnosis of prostatic stromal sarcoma being both part of a the same morphologic spectrum from benign to malignant.10
Our patient was considered to have a metastatic disease: indeed a prostatic mass extending to bladder, seminal vesicles, rectal wall and paravesical lymph node was radiologically documented. A bone lesion located in the left acetabulum was considered of metastatic origin because of its enhancement reduction after 9th course. Nevertheless, in the absence of a biopsy, this could not be determined with certainty.
The treatment of PSS relies mainly on tumor resection via various surgical approaches, which range from enucleation to pelvic exenteration, depending on the tumor’s extent.2,9,11 Unfortunately, survival is unsatisfactory even after complete tumor resection. In the English literature, we found reports on 20 PSS patients treated with radical surgery at diagnosis (associated with radiotherapy in 4 cases); only 10 were disease-free at the latest reported follow-up.1,2,3,5,6,8,10-20
Chemotherapy has rarely been used against PSS. We initially opted to administer chemotherapy to our patient in the hope of enabling a more conservative local treatment and preserving bladder and erectile function. A marked tumor shrinkage was seen after the first 3 courses and a further volume reduction was recorded after the seventh cycle.
In the English literature, we could find only 14 previously-reported patients who had been given chemotherapy. The characteristics of these patients, along with the information on our adolescent case, are shown in Table 1,2,4-7,11,15,17,19,21-24 amounting to a total of 15 cases. Overall, neo-adjuvant chemotherapy was administered to 10 patients, either as part of their front-line treatment (6 patients) or after they relapsed (4 patients). Chemotherapy was also used in the adjuvant setting in 4 patients and as palliative treatment in 1 patient. Overall, tumor shrinkage was reported in 5 of the 7 evaluable patients. Ifosfamide, cisplatin, and doxorubicin were the most commonly used drugs.
Conclusions
Despite our patient’s dismal outcome, evidence in the literature and from our experience indicates that PSS may be chemosensitive, so chemotherapy could be considered early in the treatment of PSS, i.e. after the initial 3-4 courses of chemotherapy, in combination with aggressive local treatment.
Acknowledgments: this paper is part of the SPeLeS Project (Specializzandi in Pediatria e Letteratura Scientifica), an academic scheme organized by the Pediatrics Department in Padova to improve resident pediatricians’ expertise in analyzing teaching cases and writing scientific reports.
Table 1.
Treatment, response to chemotherapy and outcome in 15 patients with prostatic stromal sarcoma (14 from the English literature and the case we described).
| Ref. (year) | Age | Tumor extension | Treatment | Drugs (cycles) | Rsponse to CT | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| First-line | Second-line | |||||||
| 1 | 2 (1998) | 25 | Prostate | RCP, adjuvant CTh | n.r. | n.r. | NED 12 mo after RP | |
| 2 | 2 (1998) | 59 | Prostate | RCP, RT, adjuvant CTh | n.r. | n.r. | LR, AWD 72 mo after Dx | |
| 3 | 15 (2001) | 36 | Prostate extending to neck of bladder | RP | Neoadjuvant CTh + RT (70 Gy/35 f) | DOXO + IFO (6) | PR | LR, DOD 20 mo after Dx |
| 4 | 6 (2002) | 35 | Prostate, pelvic walls | RP+RT (60 Gy) | Neoadjuvant CTh + lobectomy | DOXO + IFO (5) | PR | DR (lung), NED 5 mo after lobectomy |
| 5 | 7 (2003) | 78 | Prostate extending to bladder, abdominal walls | RP+ adjuvant CTh | VCR + MMCO + DOX + CDDP (6) | n.r. | DR (liver), DOD 6 mo after CTh | |
| 6 | 17 (2006) | 19 | Prostate | RP | Neoadjuvant CTh+ RT (49.2 Gy) | VP-16 + IFO +CDDP (4) | PR | LR, NED 48 mo after CTh and RT |
| 7 | 11 (2006) | 75 | Prostate extending to seminal vesicles | Enucleation, RT, adjuvant CTh | n.r. | n.e. | NED 13 mo after Dx | |
| 8 | 19 (2007) | 52 | Prostate | RCP | Neoadjuvant CTh + metastasectomy | CDDP + PIRA + IFO (3) | n.r. | LR, AWD 12 mo after metas-tasectomy |
| 9 | 22 (2010) | 31 | Prostate | Neoadjuvant C Th + RP | Cstectomy | IFO + DOXO (2) | SD | LR, DOD 3 mo after cystectomy |
| 10 | 5 (2010) | 34 | Prostate | RP+RT (60 Gy) | Lung metastasectomy + palliative RT and CT | IFO + DOXO h | PR | LR, DR (lung), PD, DOD 25 mo after Dx |
| 11 | 4 (2010) | 30 | Prostate extending to bladder, anterior bowel walls, lungs | Neoadjuvant CTh + RT (still ongoing) | n.r. | n.e. | n.r. | |
| 12 | 23 (2011) | 26 | Prostate, lymph nodes | Neoadjuvant CTh | n.r. | PD | DR (mediastinal lymph node), DOD 7 mo after Dx | |
| 13 | 21 (2012) | 63 | Prostate extending to bladder | Neoadjuvant CTh + total pelvic exenteration | CDDP + CPT-11 | n.r. | NED at 16 mo after RCP | |
| 14 | 24 (2012) | 66 | Prostate extending to bladder | Subtotal P + neoadjuvant CTh + RT (50 Gy) + RCP | IFO + DOXO (2) | n.e. | n.r. | |
| 15 | Present case | 14 | Prostate extending to bladder, seminal vesicles, lymph nodes, bone | Neoadjuvant CTh + RT (50 Gy) + RCP | IFO + VCR + ACT +DOXO (9) | PR | PD, DOD 15 mo after Dx | |
ACT, actinomycin; AWD, alive with disease; CDDP, cisplatin; CPT-11, irinotecan; CTh, chemotherapy; DOD, dead of disease; DOXO, doxorubicin; DR, distant recurrence; Dx, diagnosis; IFO, ifosfamide; Gy, Gray; LR, local recurrence; MMC, mitomycin C; mo, months; n.e., not evaluable; NED, no evidence of disease; n.r., not reported; P, prostatectomy; PD, progression of disease; PIRA; pirarubicin; PR, partial response; RCP, radical cystoprostatectomy; RP, radical prostatectomy; RT, radiotherapy; SD, stable disease; VCR, vincristine; VP-16, etoposide.
References
- 1.Chang YS, Chuang CK, Ng KF, Liao SK. Prostatic stromal sarcoma in a young adult: a case report. Arch Androl 2005;51:419-24. [DOI] [PubMed] [Google Scholar]
- 2.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol 1998;22:148-62. [DOI] [PubMed] [Google Scholar]
- 3.Löpez-Beltran A, Gaeta JF, Huben R, Croghan GA. Malignant phyllodes tumor of prostate. Urology 1990;35:164-7. [DOI] [PubMed] [Google Scholar]
- 4.Stoll LM, Johnson MW, Rosenthal DL. High-grade prostatic sarcoma seen in a catheterized urine specimen: case report and differential diagnosis. Diagn Cytopathol 2010;39:762-6. [DOI] [PubMed] [Google Scholar]
- 5.Colombo P, Ceresoli GL, Boiocchi L, et al. Prostatic stromal tumor with fatal outcome in a young man: histopathological and immunohistochemical case presentation. Rare Tumors 2010;2:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam KC, Yeo W. Chemotherapy induced complete remission in malignant phyllodes tumor of the prostate metastasizing to the lung. J Urol. 2002;168:1104-5. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal V, Sharma D, Wadhwa N. Case report: malignant phyllodes tumor of prostate. Int Urol Nephrol 2003;35:37-9. [DOI] [PubMed] [Google Scholar]
- 8.Yum M, Miller JC, Agrawal BL. Leiomyosarcoma arising in atypical fibromuscular hyperplasia (phyllodes tumor) of the prostate with distant metastasis. Cancer 1991;68:910-5. [DOI] [PubMed] [Google Scholar]
- 9.Bostwick DG, Hossain D, Qian J, et al. Phyllodes tumor of the prostate: long-term followup study of 23 cases. J Urol 2004;172:894-9. [DOI] [PubMed] [Google Scholar]
- 10.Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol 2006;30:694-704. [DOI] [PubMed] [Google Scholar]
- 11.Froehner M, Bartholdt E, Meye A, et al. Adult prostate sarcoma diagnosed from tissue spontaneously excreted through the urethra. Urol Oncol 2004;22:119-20. [DOI] [PubMed] [Google Scholar]
- 12.Young JF, Jensen PE, Wiley CA. Malignant phyllodes tumor of the prostate. A case report with immunohistochemical and ultrastructural studies. Arch Pathol Lab Med 1992;116:296-9. [PubMed] [Google Scholar]
- 13.Olson EM, Trambert MA, Mattrey RF. Cystosarcoma phyllodes of the prostate: MRI findings. Abdom Imaging 1994;19:180-1. [DOI] [PubMed] [Google Scholar]
- 14.Probert JL, O’Rourke JS, Farrow R, Cox P. Stromal sarcoma of the prostate. Eur J Surg Oncol 2000;26:100-1. [DOI] [PubMed] [Google Scholar]
- 15.De Raeve H, Jeuris W, Wyndaele JJ, Van Marck E. Cystosarcoma phyllodes of the prostate with rhabdomyoblastic differentiation. Pathol Res Pract 2001;197:657-62. [DOI] [PubMed] [Google Scholar]
- 16.Osaki M, Osaki M, Takahashi C, et al. Prostatic stromal sarcoma: case report and review of the literature. Pathol Int 2003;53:407-11. [DOI] [PubMed] [Google Scholar]
- 17.Sakura M, Tsukamoto T, Yonese J, et al. Successful therapy of a malignant phyllodes tumor of the prostate after postoperative local failure. Urology 2006;67:845 e11-13. [DOI] [PubMed] [Google Scholar]
- 18.Huang YC, Wang JY, Lin PY, et al. Synchronous prostate stromal sarcoma and gastrointestinal stromal tumor of rectum: case report and review of the literature. Urology 2006;68:672 e11-13. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa T, Goto A, Tomita K, et al. Recurrent prostatic stromal sarcoma with massive high-grade prostatic intraepithelial neoplasia. J Clin Pathol. 2007;60:330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraggetta F, Pepe P, Giunta ML, Aragona F. Primary high grade sarcoma of the specialised prostatic stroma: a case report with clinico-pathological considerations. Pathologica 2008;100:482-4. [PubMed] [Google Scholar]
- 21.Yamazaki H, Ohyama T, Tsuboi T, et al. Prostatic stromal sarcoma with neuroectodermal differentiation. Diagn Pathol 2012;7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JY, Cho YM, Ro JY. Prostatic stromal sarcoma with rhabdoid features. Ann Diagn Pathol 2010;14:453-6. [DOI] [PubMed] [Google Scholar]
- 23.Tamada T, Sone T, Miyaji Y, et al. MRI appearance of prostatic stromal sarcoma in a young adult. Korean J Radiol 2011;12:519-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese AC, Ball MW, Efron JE, et al. Favorable response to neoadjuvant chemotherapy and radiation in a patient with prostatic stromal sarcoma. J Clin Oncol 2012;30:e353-5. [DOI] [PubMed] [Google Scholar]


