Abstract
As a member of 18 glycosyl hydrolase (GH) family, chitotriosidase (Chitinase 1, CHIT1) is a true chitinase mainly expressed in the differentiated and polarized macrophages. CHIT1 is an innate immune mediator that digests the cell walls of chitin-containing eukaryotic pathogens, such as fungi. However, CHIT1 is dysregulated in granulomatous and fibrotic interstitial lung diseases characterized by inflammation and tissue remodeling. These include tuberclosis, sarcoidosis, idiopathic pulmonary fibrosis, scleroderma-associated interstitial lung diseases (SSc-ILD), and chronic obstructive lung diseases (COPD). CHIT1 serum concentration correlates with the progression or the severity of these diseases, suggesting a potential use of CHIT1 as a biomarker or a therapeutic target. Recent studies with genetically modified mice demonstrate that CHIT1 enhances TGF-β1 receptor expression and signaling, suggesting a role in initiating or amplifying the response to organ injury and repair. This additional CHIT1 activity is independent of its enzymatic activity. These studies suggest that CHIT1 serves a bridging function; it is both an innate immune mediator and a regulator of tissue remodeling. This review will focus on recent data linking CHIT1 to the pathogenesis of inflammation, interstitial lung disease, and COPD.
Keywords: Chitotriosidase, sarcoidosis, scleroderma, idiopathic pulmonary fibrosis, inflammation, TGF-beta
INTRODUCTION
Chitin is a nitrogen-containing carbohydrate polymer that is a component of the cell walls of fungi, the exoskeletons of insects and crustaceans. Chitotriosidase (CHIT1) and acidic mammalian chitinase (AMCase) are active enzymes that bind and degrade chitin. They belong to members of 18 glycosyl hydrolase (GH) gene family,1,2 together with various chitinase-like proteins, such as Chi3l1/YKL-40, Ym1, and Ym2 that are not enzymatically active but bind the chitin polysaccharide.3 CHIT1 is the best characterized chitinase from both biologic and clinical perspectives. It is gaining recognition as a key component of the innate host defense mechanism against bacterial and fungal infections.4,5 The general characteristics of CHIT1 are summarized in Table.
Table. Summary of known characteristics of chitotriosidase.
| Other names (Aliases) | Chitinase 1 (CHIT1); CHI3; CHITD |
|---|---|
| Site of expression (tissues and cells) | Mature monocytes derived macrophages; lung macrophages |
| Gaucher cells | |
| Neutrophils | |
| Lung alveolar epithelial cells | |
| Intestinal epithelial cells | |
| Biological activity | Chitolytic enzyme activity: hydrolytic and trans-glycosylation activity |
| Disease association | Infectious diseases |
| - Fungal | |
| - Bacterial | |
| - Malaria | |
| Lysosomal storage diseases | |
| - Gaucher disease | |
| - Niemann Pick disease | |
| - Fabry disease | |
| β-thalassemia | |
| Atherosclerosis | |
| Lung diseases | |
| - Tuberculosis | |
| - Sarcoidosis | |
| - Idiopathic pulmonary fibrosis | |
| - Scleroderma associated interstitial lung disease | |
| - Asthma/atopy associated with fungal infection | |
| Liver disease | |
| - Non-alcoholic steatohepatitis (NASH) | |
| Neurodegenerative diseases | |
| - Alzheimer's disease | |
| - Ischemic cerebrovascular dementia |
CHIT1 is expressed in myeloid cells including, neutrophils, mature monocyte-derived macrophages, lung macrophages, and other specific subsets of tissue macrophages and epithelial cells in the lung and intestine.6,7,8,9,10,11 CHIT1 is predominantly expressed in the human lung, while AMCase is abundantly expressed in the rodent lung, suggesting tissue- and species-specific differences in the expression of chitinases.1 Neutrophils and Macrophages release CHIT1 after toll-like receptor (TLR) stimulation.12 Macrophages also release CHIT1 after stimulation with interferon (IFN)-γ, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF).7 Alternatively, monocytes down-regulated CHIT1 expression after TLR stimulation,12 suggesting regulation of CHIT1 expression depends on the stage of myeloid lineage differentiation with mature tissue macrophages but not blood monocytes being competent to produce this arm of the innate immune response.
In humans, CHIT1 is localized to chromosome 1q31-q32 and encompasses 12 exons, spanning ~20 kb.2 Loss of CHIT1 function is produced by a 24-bp duplication allele rs3831317.13,14,15,16 Homozygous individuals completely lack chitinase activity. At a population level, about 35% of humans are heterozygous, and 6% are homozygous for this mutant allele in areas with low parasitic burden suggesting heterozygote advantage.17 In malaria endemic areas, however, there is a low prevalence individuals without chitinase activity, a finding consistent with chitinase providing innate protection from malaria.13 Further, case reports of individuals bearing genetic variants of CHIT1 have demonstrated increased susceptibility to chitin-containing pathogens, including Wuchereria bancrofti (filariasis), Plasmodium falciparum (malaria), Cryptococcus neoformans, and Candida albicans.9,16,18,19 These population and individual level data suggest that CHIT1 contributes to innate resistance from infections with chitin containing eukaryotes.20
CHIT1 has pleiotropic effects in immune responses to pathogens without chitin and in non-infectious inflammation.21 The CHIT1 24-bp duplication allele was a risk factor for Gram-negative bacteremia in children with acute myeloid leukemia (AML). CHIT1 expression is increased neonates suffering from bacterial infection.19 A CHIT1 A442G polymorphism was associated with increased childhood atopy.22 These phenotypes of CHIT1 polymorphisms hint at a variety biological effects important in the pathogenesis of specific human diseases that are still largely undetermined.
CHIT1 expression is altered in a number of non-infectious inflammatory diseases. The circulating level of CHIT1 is a biomarker of lysosomal storage diseases such as Gaucher's disease and are being used to monitor progression or the efficacy of treatment.23,24,25,26 Elevated serum CHIT1 levels were also reported in β-thalassemia, multiple sclerosis, atherosclerosis (both coronary and cerebrovascular), and Alzheimer's disease.27 The levels of CHIT1 in the circulation were significantly dysregulated in granulomatous or fibrotic lung disease as well as chronic obstructive pulmonary diseases (COPD).28,29 On the other hand, elevated CHIT1 soon after dust exposure reduced the risk of subsequent lung injury.30 These biomarker studies, however, are unable to determine if CHIT1 has a mechanistic role in any of these diseases.
Recent studies in genetically modified mouse models have provided insight to novel mechanisms on CHIT1 action. Bleomycin-induced pulmonary fibrosis was significantly reduced in CHIT1 null mutant mice and significantly enhanced in lungs from CHIT1 overexpressing transgenic animals. Further CHIT1 interacts with TGF-β1 to augment fibroblast TGF-β receptor 1 and 2 expression and TGF-β-induced signaling.11 As an effector molecule of macrophage differentiation and activation, the role of CHIT1 is getting more attention as a modifier of tissue fibrosis or airway remodeling in addition to its role in innate immune response.11,31 Since ILD and COPD are representative chronic lung diseases resulting from abnormal injury and tissue repair responses, the role and effects of CHIT1 in the development of these diseases are of great interest to understand the pathogenic mechanism of these diseases. In this perspective, studies directed to test the implication of CHIT1 as a biomarker and therapeutic target in the ILD and COPD are comprehensively reviewed and potential underlying mechanism(s) of CHIT1 in the pathogenesis of these diseases are also discussed.
Tuberculosis
Pulmonary tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis, a slow growing bacteria without chitin. Over one third of the world's population has latent tuberculosis infection leading to 1.5 million deaths per year. The gold standard of TB diagnosis is demonstration of mycobacteria in tissues or body fluids.32 Necrotizing granuloma characterizes the pathology of active infection. Activated macrophages are involved in granuloma development as are other innate cells, such as natural killer T cells. The cellular immune response, however, is required to control the infection. Antigen-specific T lymphocytes participate with macrophages in forming granulomas which produce cytokines, chemokines, and other soluble immune mediators.33,34 While there is some variability, most studies have demonstrated CHIT1 levels are increased in the serum of TB patients, correlate with disease severity and decline with treatment.35,36 These studies suggest that pulmonary granuloma lead to release of CHIT1 into the serum and that resolution of the inflammation diminish the release. It appears, then, that CHIT1 can be used as a marker of tuberculosis activity, severity, and response to treatment.
Sarcoidosis
Sarcoidosis is a multisystem granulomatous disease of unknown origin. While there has been an intense search for an infectious etiology of this disease, none has been reproducibly found. The pathology is characterized by non-necrotizing ("non-caseating") granulomas that result from accumulation of activated proliferating mononuclear phagocytes and T lymphocytes primarily affecting the lungs.37 Sarcoidosis presents many different clinical forms, and its onset may be acute, subacute, or chronic; it may be asymptomatic and remit spontaneously.38,39 Spontaneous remission is more common in patients with acute sarcoidosis (about 50% of all cases). Chronic sarcoidosis has insidious onset and slow progression with wide variations between individuals and almost invariable lung involvement. In progressive advanced forms with fibrotic lung involvement, there may be severe hypoxemia and pulmonary hypertension.40 In recent years, the unpredictable clinical course of sarcoidosis has prompted several investigators to look for reliable indicators of disease outcome. Biomarkers proposed for clinical evaluation of sarcoidosis severity have included radiological features, bronchoalveolar lavage (BAL) cell profile, CD4/CD8 ratio, neopterin, lysozyme, angiotensin-converting enzyme (ACE), and cytokines/chemokines and their receptors, such as soluble interleukin-2 receptor (sIL-2R).41,42,43,44,45,46,47
The idea of evaluating CHIT1 from serum and BAL as a biomarker of sarcoidosis disease progression arose from evidence of direct involvement of activated macrophages in the pathogenesis of sarcoidosis and granuloma formation.48,49 CHIT1 concentrations are elevated in sarcoid patients when compared to controls, and levels correlate with disease severity and progression.50,51,52 The lowest concentrations of CHIT1 were found in untreated patients in remission, while the highest enzyme concentrations were found in symptomatic patients with persistent disease on steroids and with functional deterioration in the last year. In this symptomatic group, CHIT1 decreased significantly after the increasing of steroid dose or the introduction of a new immunosuppressant therapy (P<0.01).52
Mechanistically, granuloma formation requires activation of both T cells and macrophages.53 A pivotal step in the generation of specific T cell responses in sarcoidosis is the activation of T cells by antigen-presenting cells, including alveolar macrophages. Several groups independently found that alveolar macrophages from patients with sarcoidosis disclosed an increased antigen-presenting capacity compared to controls.54,55,56 Nevertheless, immunopathogenesis of sarcoidosis remains poorly understood. Challenges to understanding disease mechanisms in sarcoidosis include: (1) the fact that a causative agent is unknown; (2) the lack of a universally accepted animal model, and (3) the various manifestations of the disease, as well as the marked heterogeneity of the disease between different individuals and ethnic groups.57 Despite of these limitations, an immunological response triggered by an as-yet unknown environmental antigen(s) in a genetically susceptible individual is a generally accepted concept as a potential mechanism of sarcoidosis.58
Serum CHIT1 levels correlate with sIL-2R concentration,59 a known marker of T-cell activation and sarcoidosis severity.42,60 Sarcoid macrophages secrete high quantities of both CHIT1 and chemokine (C-C motif) ligand 18 (CCL18).61 CCL18 is expressed by a broad range of monocytes/macrophages and dentritic cells, and enhanced CCL18 production has been demonstrated in various malignancies and inflammatory diseases.62 The relative changes in CHIT1 and CCL18 during the course of disease closely mimicked each other. Therapy with steroids resulted in clear reductions, in plasma CHIT1 and CCL18 and relapse of disease activity was preceded by increases in these parameters. The coordinated regulation of CCL18 and CHIT1 is consistent with the hypothesis that they are components of the same immune pathway in this granulomatous lung disease. The establishment of an appropriate animal model of sarcoidosis together with CHIT1-specific targeting will be instrumental to test this possibility. If this is the case, CHIT1 could be a therapeutic target for the intervention of inflammation and/or tissue remodeling associated with sarcoidosis.
Idiopathic Pulmonary Fibrosis and Scleroderma-Associated Interstitial Lung Diseases (SSc-ILDs)
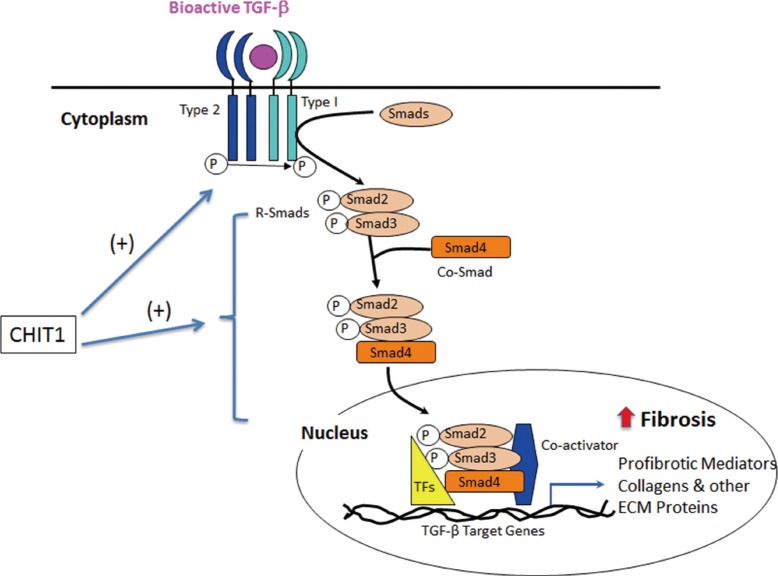
ILD is a heterogeneous group of lung disorders with varying clinical courses and pathogenetic mechanisms. Other than sarcoidosis, the most common ILDs include idiopathic pulmonary fibrosis (IPF). IPF is rapidly progressive and typically unresponsive to therapy.63,64 The etiology and pathogenesis of IPF are unknown, although IPF is thought to stem from epithelial lung injury.64,65 The unpredictable clinical course of IPF has motivated research to identify the potential prognostic biomarkers for the disease.66 A study showed that CHIT1 activity was elevated in the BAL of IPF patients compare to controls, but not in serum.51 This result suggests that CHIT1 expression in specific macrophage subsets might be responsible for remodeling and tissue damage seen in the lung from IPF patients. As such, it is conceivable that CHIT1 could be involved in fibrogenesis of other ILD. In systemic sclerosis (SSc), ILD represents the leading cause of death and the incidence of ILD is up to two-third of SSc patients.67 Because of the extremely variable incidence and outcome of ILD in connective tissue disease, there are huge unmet medical needs to develop biomarker(s) for diagnosis, prognosis, patient subtyping, response to treatment, or as surrogate endpoints in clinical trials. A study by Lee et al.11 demonstrated that, in 2 different SSc patient cohorts, CHIT1 bioactivity and protein levels were significantly increased in the circulation and lung of patients compared to demographically matched controls. Compared to SSc patients without lung involvement, patients with ILD had higher levels of circulating CHIT1 activity, and CHIT1 activity was correlated with disease severity. Using animal models of fibrosis and transgenic mice, they further demonstrated that CHIT1 enhances bleomycin- or IL-13-stimulated pulmonary fibrosis. They further identified that CHIT1 enhances TGF-β1 receptor expression and its signaling in fibroblasts, suggesting CHIT1 is a modifier of pathologic tissue fibrosis31 in that TGF-β1 play a significant role. The potential CHIT1 regulation of TGF-β1 pathway associated with tissue phenotypes is schematically illustrated in Figure. These studies, for the first time, identifies CHIT1 as a biomarker of subset of SSc patients with pulmonary fibrosis and also raises the possibility that CHIT1 and/or the interaction between CHIT1 and TGF-β1 are potential therapeutic targets of this disease. In this regard, identification of specific CHIT1 interacting molecule(s) that regulate TGF-β1 signaling would be instrumental for further development of therapeutic strategy on SSc patients with ILD.
Figure. Schematic illustration on chitotriosidase (CHIT1) regulation of TGF-β pathway. CHIT1 enhances the effect of TGF-β through the induction of TGF-β receptors expression (both type I and type II) and TGF-β signaling. In the preliminary studies in our laboratory suggest that CHIT1 could bind with a transcription factor such as FoxO3a, or TGF-β receptor associated protein 1 (TGFBRAP1) (unpublished data), modulates TGF-β-stimulated canonical (Smads-dependent) and/or non-canonical MAPK/Erk or Akt signaling. Erk or Akt activation generally increases cell survival or proliferation, that might contribute to the development of tissue fibrosis together with increased expression of profibrotic mediators and extracellular matrix protein accumulation. The exact role and mechanism of CHIT1 in this regulation (such as receptor or interacting proteins of CHIT1) need to be determined in future studies.
COPD
COPD is characterized by chronic airway inflammation and emphysematous alveolar destruction. A dominant hallmark of COPD is an abnormal inflammatory response to inhaled particles which have the potential to produce lung injury.68,69,70 The significantly higher levels of chitinase activity in habitual smokers compared to controls have been shown to result from upregulation of CHIT1 gene expression, especially in macrophages.71 Smokers with COPD have increased CHIT1 in BAL, more CHIT1-positive cells in bronchial biopsies, and an elevated proportion of alveolar macrophages expressing CHIT1 when compared to smokers without COPD or never-smokers.29 CHIT1 levels in BAL correlated with airflow obstruction and emphysema. CHIT1 also correlated with Interleukin (IL)-1β, IL-8, TNF-α, and its type II soluble receptor. In this study, alveolar macrophages from smokers with COPD released CHIT1 after TNF-α-stimulation while alveolar macrophages from controls did not. Further, CHIT1 promoted the release of IL-8, monocyte chemoattractant protein-1 (MCP-1), and matrix metalloproteinase (MMP-9). Finally, CHIT1 overexpression in the lung of normal mice promoted macrophage recruitment and the synthesis of the murine homologue of IL-8, keratinocyte-derived cytokine, and MCP-1. This data demonstrates that CHIT1 is a functional component of the inflammatory pathway produced by cigarette smoke.
Whereas the above studies have shown CHIT1 to be a risk factor for COPD, we have published a study demonstrating that, depending on the clinical population, CHIT1 may represent a potential biomarker for resistance to obstructive, COPD-like lung injury.30 In a nested case-control study involving firefighters from the 9/11 World Trade Center disaster who developed particulate matter-induced lung disease, serum CHIT1 was found not only to reduce the risk of airway obstruction as defined by abnormal forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) and FEV1, but also it was associated with recovery of lung function. The mechanism of CHIT1's potentially protective role against particulate matter-induced airway obstruction is unknown and need to be further defined in future studies.
CHIT1 in other lung disease with inflammation and remodeling: Asthma and cystic fibrosis
The role of chitin, chitinases or chitinase-like proteins in allergy and asthma have been greatly elucidated in recent studies.72 The expression and activity of AMCase were upregulated in an ovalbumin mouse model of asthma independent of IL-13.73
Furthermore, inhibition of AMCase enzymatic activity prevents OVA allergen-induced airway hyperresponsiveness and inflammation present in these mice after challenge. Using a similar acute mouse asthma model, upregulation of both lung AMCase and Ym1 gene expressions was detected by DNA microarray,74 and concurrent elevated levels of AMCase, Ym1 and Ym2 in the BAL fluid was also observed using 2D gel electrophoresis proteomics.75 Studies have additionally demonstrated an association between chitinase-like proteins, such as YKL-40 and asthma.76
While a plethora of investigations have linked AMCase and chitinase-like protein levels to asthma, numerous negative studies have demonstrated lack of association between specific CHIT1 and reactive airway disease.29,77 One study, however, reported that the CHIT1 gene's rs3831317 24-base pair duplication allele, which results in reduced CHIT1 activity, was prevalent in patients with severe asthmatics with fungal sensitization.78 The importance of CHIT1 duplication in cystic fibrosis patients appears less pronounced.78 However, there is an interesting inverse correlation between CHIT1 duplication and W1282 X CFTR mutation that produce few or no function CFTR chloride channel through premature translation termination. It is speculated that the combination of reduced CHIT1 activity, higher rates of Aspergillus colonization, and the use of immune suppressive drug including corticosteroids, may contribute to the high levels of allergic broncho-pulmonary aspergillosis (ABPA) in cystic fibrosis patients.78 The potential protective effect of CHIT1 in fungal infection in cystic fibrosis needs to be further proved through prospective studies including large number of patients and controls.
CONCLUSION AND FUTURE STUDY DIRECTION
Although CHIT1 is an enzyme with chitolytic activity that provides a protective effect on invading pathogens containing chitin, biomarker and animal studies also suggest a role in injury and healing responses in the lung. Through its interaction with TGF-β, CHIT1 could play a pathogenic role in a variety of non-infectious human diseases, including ILD and COPD. As a member of the18-GH family, the expression and activity of CHIT1 are strongly associated with macrophage differentiation and activation status. This macrophage-dominant expression of CHIT1 might confer distinct biologic function of CHIT1 in allergic and infectious diseases from other members of chitinases or chitnase-like proteins such as AMCase or Chi3l1.72,79,80 In this sense, the role of CHIT1 in monocyte or macrophage differentiation and polarization would be important to understand biologic effects of CHIT1 in pathologic tissue responses. CHIT1 likely contributes to tissue repair responses associated with injury produced by adaptive Th2 responses. Enhanced TGF-β receptor expression and signaling in alternative macrophage activation would favor a fibrotic tissue response in overwhelming injury produced by bleomycin. In less severe injury produced by irritant dust exposure, it could promote appropriate tissue repair. This possibility need to be further explored in future studies.
Currently, it is generally a well-accepted concept that ILD as well as COPD are the diseases resulting from abnormal tissue injury and repair response. With the recognition of CHIT1 as a modifier of this tissue remodeling process in addition to an innate immune molecule, the specific role and effect of CHIT1 in the pathogenesis of these diseases need to be further explored in future studies. In this regard, the cellular trafficking mechanism and the receptor or interacting proteins, and the signaling pathway of CHIT1 need to be determined. Additional prospective human population studies together with in vitro and in vivo animal studies using recently developed genetically modified mice will be instrumental in determining whether CHIT1 is a useful biomarker and therapeutic target of various lung diseases with inflammation and tissue remodeling.
ACKNOWLEDGMENTS
This research was supported by NIH grants RO1-HL115813 and PO1-HL114501.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Boot RG, Bussink AP, Verhoek M, de Boer PA, Moorman AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005;53:1283–1292. doi: 10.1369/jhc.4A6547.2005. [DOI] [PubMed] [Google Scholar]
- 2.Fusetti F, von Moeller H, Houston D, Rozeboom HJ, Dijkstra BW, Boot RG, et al. Structure of human chitotriosidase. Implications for specific inhibitor design and function of mammalian chitinase-like lectins. J Biol Chem. 2002;277:25537–25544. doi: 10.1074/jbc.M201636200. [DOI] [PubMed] [Google Scholar]
- 3.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177:959–970. doi: 10.1534/genetics.107.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med. 2005;43:499–502. doi: 10.1515/CCLM.2005.088. [DOI] [PubMed] [Google Scholar]
- 7.Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J Clin Lab Anal. 2005;19:128–132. doi: 10.1002/jcla.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem. 1995;270:2198–2202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 9.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 10.Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. Epithel Biol Pharmacol. 2012;5:1–9. doi: 10.2174/1875044301205010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-beta1 signaling. J Immunol. 2012;189:2635–2644. doi: 10.4049/jimmunol.1201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eijk M, Scheij SS, van Roomen CP, Speijer D, Boot RG, Aerts JM. TLR- and NOD2-dependent regulation of human phagocyte-specific chitotriosidase. FEBS letters. 2007;581:5389–5395. doi: 10.1016/j.febslet.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Artieda M, Cenarro A, Gañán A, Jericó I, Gonzalvo C, Casado JM, et al. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003;23:1645–1652. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- 14.Canudas J, Cenarro A, Civeira F, Garcí-Otín AL, Arístegui R, Díaz C, et al. Chitotriosidase genotype and serum activity in subjects with combined hyperlipidemia: effect of the lipid-lowering agents, atorvastatin and bezafibrate. Metabolism. 2001;50:447–450. doi: 10.1053/meta.2001.21696. [DOI] [PubMed] [Google Scholar]
- 15.Lee P, Waalen J, Crain K, Smargon A, Beutler E. Human chitotriosidase polymorphisms G354R and A442V associated with reduced enzyme activity. Blood Cells Mol Dis. 2007;39:353–360. doi: 10.1016/j.bcmd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaguarnera L, Simporè J, Prodi DA, Angius A, Sassu A, Persico I, et al. A 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: role of parasitic diseases and environmental conditions. Genes Immun. 2003;4:570–574. doi: 10.1038/sj.gene.6364025. [DOI] [PubMed] [Google Scholar]
- 17.Boot RG, Renkema GH, Verhoek M, Strijland A, Bliek J, de Meulemeester TM, et al. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J Biol Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 18.Choi EH, Zimmerman PA, Foster CB, Zhu S, Kumaraswami V, Nutman TB, et al. Genetic polymorphisms in molecules of innate immunity and susceptibility to infection with Wuchereria bancrofti in South India. Genes Immun. 2001;2:248–253. doi: 10.1038/sj.gene.6363767. [DOI] [PubMed] [Google Scholar]
- 19.Labadaridis I, Dimitriou E, Theodorakis M, Kafalidis G, Velegraki A, Michelakakis H. Chitotriosidase in neonates with fungal and bacterial infections. Arch Dis Child Fetal Neonatal Ed. 2005;90:F531–F532. doi: 10.1136/adc.2004.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9:401–408. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehrnbecher T, Bernig T, Hanisch M, Koehl U, Behl M, Reinhardt D, et al. Common genetic variants in the interleukin-6 and chitotriosidase genes are associated with the risk for serious infection in children undergoing therapy for acute myeloid leukemia. Leukemia. 2005;19:1745–1750. doi: 10.1038/sj.leu.2403922. [DOI] [PubMed] [Google Scholar]
- 22.Kim KW, Park J, Lee JH, Lee HS, Lee J, Lee KH, et al. Association of genetic variation in chitotriosidase with atopy in Korean children. Ann Allergy Asthma Immunol. 2013;110:444–449.e1. doi: 10.1016/j.anai.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollak CE, Maas M, Aerts JM. Clinically relevant therapeutic endpoints in type I Gaucher disease. J Inherit Metab Dis. 2001;24(Suppl 2):97–105. doi: 10.1023/a:1012492429191. [DOI] [PubMed] [Google Scholar]
- 25.de Fost M, Hollak CE, Groener JE, Aerts JM, Maas M, Poll LW, et al. Superior effects of high-dose enzyme replacement therapy in type 1 Gaucher disease on bone marrow involvement and chitotriosidase levels: a 2-center retrospective analysis. Blood. 2006;108:830–835. doi: 10.1182/blood-2005-12-5072. [DOI] [PubMed] [Google Scholar]
- 26.Pastores GM, Barnett NL. Current and emerging therapies for the lysosomal storage disorders. Expert Opin Emerg Drugs. 2005;10:891–902. doi: 10.1517/14728214.10.4.891. [DOI] [PubMed] [Google Scholar]
- 27.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 28.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41:379–384. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Létuvé S, Kozhich A, Humbles A, Brewah Y, Dombret MC, Grandsaigne M, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. Am J Pathol. 2010;176:638–649. doi: 10.2353/ajpath.2010.090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho SJ, Nolan A, Echevarria GC, Kwon S, Naveed B, Schenck E, et al. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013;33:1134–1142. doi: 10.1007/s10875-013-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CM, Park JW, Cho WK, Zhou Y, Han B, Yoon PO, et al. Modifiers of TGF-beta1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med. 2014;29:281–290. doi: 10.3904/kjim.2014.29.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salfinger M, Hale YM, Driscoll JR. Diagnostic tools in tuberculosis. Present and future. Respiration. 1998;65:163–170. doi: 10.1159/000029252. [DOI] [PubMed] [Google Scholar]
- 33.Rivas-Santiago B, Vieyra-Reyes P, Araujo Z. Cell immunity response in human pulmonary tuberculosis. Review. Invest Clin. 2005;46:391–412. [PubMed] [Google Scholar]
- 34.Rajavelu P, Das SD. Th2-type immune response observed in healthy individuals to sonicate antigen prepared from the most prevalent Mycobacterium tuberculosis strain with single copy of IS6110. FEMS Immunol Med Microbiol. 2005;45:95–102. doi: 10.1016/j.femsim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Cakir G, Gumus S, Ucar E, Kaya H, Tozkoparan E, Akgul EO, et al. Serum chitotriosidase activity in pulmonary tuberculosis: response to treatment and correlations with clinical parameters. Ann Lab Med. 2012;32:184–189. doi: 10.3343/alm.2012.32.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasci C, Tapan S, Ozkaya S, Demirer E, Deniz O, Balkan A, et al. Efficacy of serum chitotriosidase activity in early treatment of patients with active tuberculosis and a negative sputum smear. Ther Clin Risk Manag. 2012;8:369–372. doi: 10.2147/TCRM.S31752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. American Thoracic Society; European Respiratory Society; World Association of Sarcoidosis and other Granulomatous Disorders. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 38.Lynch JP, 3rd, Kazerooni EA, Gay SE. Pulmonary sarcoidosis. Clin Chest Med. 1997;18:755–785. doi: 10.1016/s0272-5231(05)70417-2. [DOI] [PubMed] [Google Scholar]
- 39.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:204–211. [PubMed] [Google Scholar]
- 40.Corte TJ, Wells AU, Nicholson AG, Hansell DM, Wort SJ. Pulmonary hypertension in sarcoidosis: a review. Respirology. 2011;16:69–77. doi: 10.1111/j.1440-1843.2011.01937_12.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agostini C, Semenzato G. Cytokines in sarcoidosis. Semin Respir Infect. 1998;13:184–196. [PubMed] [Google Scholar]
- 42.Ziegenhagen MW, Rothe ME, Schlaak M, Müller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–413. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 43.Rottoli P, Magi B, Perari MG, Liberatori S, Nikiforakis N, Bargagli E, et al. Cytokine profile and proteome analysis in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics. 2005;5:1423–1430. doi: 10.1002/pmic.200301007. [DOI] [PubMed] [Google Scholar]
- 44.Kantrow SP, Meyer KC, Kidd P, Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J. 1997;10:2716–2721. doi: 10.1183/09031936.97.10122716. [DOI] [PubMed] [Google Scholar]
- 45.Agostini C, Trentin L, Facco M, Sancetta R, Cerutti A, Tassinari C, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J Immunol. 1996;157:910–918. [PubMed] [Google Scholar]
- 46.Straub JP, van Kamp GJ, van Maarsseveen TC, Stam J. Biochemical parameters in BAL of sarcoisosis. Sarcoidosis. 1995;12:51–57. [PubMed] [Google Scholar]
- 47.Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76:234–238. doi: 10.1159/000134009. [DOI] [PubMed] [Google Scholar]
- 48.Dai H, Guzman J, Chen B, Costabel U. Production of soluble tumor necrosis factor receptors and tumor necrosis factor-alpha by alveolar macrophages in sarcoidosis and extrinsic allergic alveolitis. Chest. 2005;127:251–256. doi: 10.1378/chest.127.1.251. [DOI] [PubMed] [Google Scholar]
- 49.Ziegenhagen MW, Rothe ME, Zissel G, Müller-Quernheim J. Exaggerated TNFalpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:185–190. [PubMed] [Google Scholar]
- 50.Grosso S, Margollicci MA, Bargagli E, Buccoliero QR, Perrone A, Galimberti D, et al. Serum levels of chitotriosidase as a marker of disease activity and clinical stage in sarcoidosis. Scand J Clin Lab Invest. 2004;64:57–62. doi: 10.1080/00365510410004092. [DOI] [PubMed] [Google Scholar]
- 51.Bargagli E, Margollicci M, Perrone A, Luddi A, Perari MG, Bianchi N, et al. Chitotriosidase analysis in bronchoalveolar lavage of patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:59–64. doi: 10.1007/s11083-007-9059-z. [DOI] [PubMed] [Google Scholar]
- 52.Bargagli E, Bennett D, Maggiorelli C, Di Sipio P, Margollicci M, Bianchi N, et al. Human chitotriosidase: a sensitive biomarker of sarcoidosis. J Clin Immunol. 2013;33:264–270. doi: 10.1007/s10875-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 53.Zissel G, Prasse A, Müller-Quernheim J. Sarcoidosis--immunopathogenetic concepts. Semin Respir Crit Care Med. 2007;28:3–14. doi: 10.1055/s-2007-970329. [DOI] [PubMed] [Google Scholar]
- 54.Lem VM, Lipscomb MF, Weissler JC, Nunez G, Ball EJ, Stastny P, et al. Bronchoalveolar cells from sarcoid patients demonstrate enhanced antigen presentation. J Immunol. 1985;135:1766–1771. [PubMed] [Google Scholar]
- 55.Ina Y, Takada K, Yamamoto M, Morishita M, Miyachi A. Antigen-presenting capacity in patients with sarcoidosis. Chest. 1990;98:911–916. doi: 10.1378/chest.98.4.911. [DOI] [PubMed] [Google Scholar]
- 56.Venet A, Hance AJ, Saltini C, Robinson BW, Crystal RG. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985;75:293–301. doi: 10.1172/JCI111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tchernev G, Ananiev J, Cardoso JC, Wollina U, Verma SB, Patterson JW, et al. Sarcoidosis and molecular mimicry--important etiopathogenetic aspects: current state and future directions. Wien Klin Wochenschr. 2012;124:227–238. doi: 10.1007/s00508-012-0154-9. [DOI] [PubMed] [Google Scholar]
- 58.Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747–755. doi: 10.1111/j.1468-3083.2009.03517.x. [DOI] [PubMed] [Google Scholar]
- 59.Bargagli E, Bianchi N, Margollicci M, Olivieri C, Luddi A, Coviello G, et al. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest. 2008;68:479–483. doi: 10.1080/00365510701854975. [DOI] [PubMed] [Google Scholar]
- 60.Strausz J, Müller-Quernheim J, Ferlinz R. Secreted interleukin-2 receptor as a parameter of the activity of sarcoidosis. Dtsch Med Wochenschr. 1989;114:744–749. doi: 10.1055/s-2008-1066666. [DOI] [PubMed] [Google Scholar]
- 61.Boot RG, Hollak CE, Verhoek M, Alberts C, Jonkers RE, Aerts JM. Plasma chitotriosidase and CCL18 as surrogate markers for granulomatous macrophages in sarcoidosis. Clin Chim Acta. 2010;411:31–36. doi: 10.1016/j.cca.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 64.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 65.Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 66.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 67.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 69.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 70.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 71.Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot RG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. The Journal of allergy and clinical immunology. 2008;122:944–950.e3. doi: 10.1016/j.jaci.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res. 2010;2:20–27. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 74.Zimmermann N, Mishra A, King NE, Fulkerson PC, Doepker MP, Nikolaidis NM, et al. Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. J Immunol. 2004;172:1815–1824. doi: 10.4049/jimmunol.172.3.1815. [DOI] [PubMed] [Google Scholar]
- 75.Zhao J, Zhu H, Wong CH, Leung KY, Wong WS. Increased lungkine and chitinase levels in allergic airway inflammation: a proteomics approach. Proteomics. 2005;5:2799–2807. doi: 10.1002/pmic.200401169. [DOI] [PubMed] [Google Scholar]
- 76.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149:369–377. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 78.Livnat G, Bar-Yoseph R, Mory A, Dagan E, Elias N, Gershoni R, et al. Duplication in CHIT1 gene and the risk for Aspergillus lung disease in CF patients. Pediatr Pulmonol. 2014;49:21–27. doi: 10.1002/ppul.22749. [DOI] [PubMed] [Google Scholar]
- 79.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009;50:22–30. doi: 10.3349/ymj.2009.50.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]